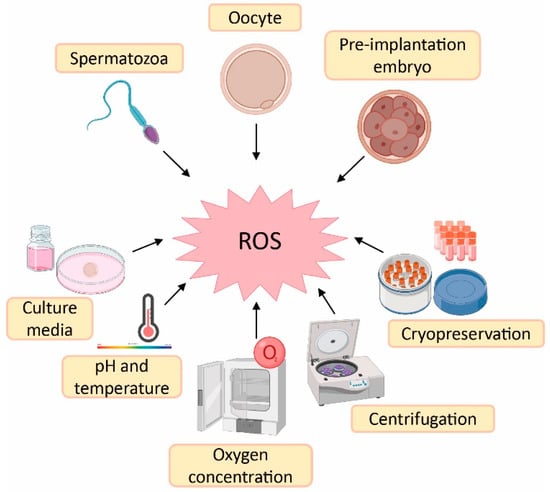
1. Sources of Reactive Oxygen Species (ROS) in Assisted Reproductive Techniques (ART)
Despite the physiological role of ROS on gamete structure and function, an exacerbated production of ROS could be detrimental for gamete physiology
[1][2][12,18]. In this sense, there is a higher risk of oxidative stress during ART procedures compared with in vivo physiological conditions. The reason is the lack of physiological defence mechanisms and the presence of intrinsic sources of ROS such as oocytes, cumulus mass cells, spermatozoa and leukocytes
[3][41]. Likewise, there are extrinsic factors responsible for ROS generation such as culture media, pH, temperature, oxygen concentration, centrifugation and cryopreservation
[3][4][41,42] (
Figure 12).
Figure 12.
Sources of reactive oxygen species (ROS) during assisted reproductive techniques (ART). Created in BioRender.com.
Culture media used during ART have an important impact on embryo quality and, consequently, on treatment success
[3][41]. Some culture media contain metallic ions such as iron and copper. These ions lead to ROS generation, which implies that supplementation of the culture media with antioxidants could be beneficial to reduce ROS formation
[5][43]. The maintenance of pH is also an important variable in culture media, as it influences sperm motility and its binding to oocyte, oocyte maturation and embryo development
[6][44]. The maintenance of culture media pH is highly dependent on levels of CO
2 and temperature, which should remain constant at 5% and 37 °C, respectively. Nevertheless, in the case of those procedures carried out outside an incubator, the pH is maintained by using culture media with reduced levels of bicarbonate or by including a pH buffer
[5][43]. Furthermore, high atmospheric oxygen concentrations can influence embryo quality due to oxidative stress induction. This is the rationale behind the use of low atmospheric oxygen concentrations (5%) in some ART laboratories to mimic in vivo conditions—where others may use an oxygen concentration of 20%
[7][45]. In fact, it has been demonstrated that an atmospheric oxygen concentration of 5% increases embryo quality, implantation and pregnancy rates, as well as live birth rates compared with an oxygen concentration of 20%
[8][46].
Regarding spermatozoa preparation, centrifugation is a common step to separate spermatozoa from the seminal plasma and other components such as death cells, immature spermatozoa and leukocytes
[5][9][43,47]. However, spinning sperm cells for more than 10 min leads to increased levels of ROS
[4][42]. Furthermore, prolonged centrifugation times increase temperature of the sample, which also affects sperm motility
[5][43]. Finally, cryopreservation is an ultra-low-temperature technique to maintain cells and tissues (from −80 °C to −196 °C)
[10][48]. This method is the best option to preserve human gametes; however, freeze–thaw cycles dramatically increase ROS production and reduce antioxidant defences of spermatozoa, thus rendering them more sensitive to oxidative stress. At the same time, this oxidative stress leads to lipid peroxidation of the sperm membrane
[11][49].
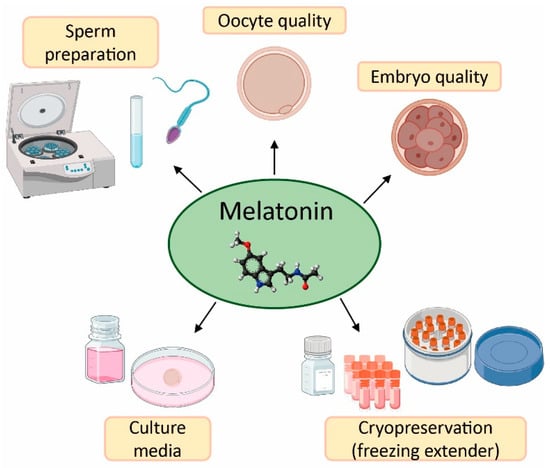
The field of reproductive medicine has achieved remarkable advances in the last few years. However, ongoing research is nowadays focused on enhancing the success rates of infertility treatments. For this purpose, in vitro and in vivo studies have concentrated their efforts on the application of antioxidants (e.g., vitamins C, D, E, resveratrol, and quercetin)
[12][13][14][15][16][17][50,51,52,53,54,55], and particularly melatonin, in ART to counteract the negative effects of oxidative stress due to its free radical scavenging properties (
Figure 23)
[18][19][20,56].
Figure 23.
Applications and benefits of melatonin in assisted reproductive techniques (ART). Created in BioRender.com.
2. Effect of Melatonin in Oocyte Quality and Embryo Quality
Numerous in vitro studies have supplemented culture media with melatonin so as to enhance oocyte maturation, oocyte fertilization and embryo development
[18][20]. This approach assumes that oxidative stress accelerates apoptosis in oocytes and hence influences their capacity for fertilization. In fact, animal studies have shown that oxidative stress occurs after oocyte in vitro incubation for only 8 h; however, supplementation of oocyte culture media with 1 mM melatonin markedly relieved such a stress in a time-dependent manner in mouse oocytes, thereby delaying the onset of apoptosis. Moreover, melatonin supplementation also significant improved embryo quality
[20][57]. The addition of 1 µM melatonin in oocyte culture media has also been proved with a prolonged in vitro incubation time of 52 h. The results demonstrate that the indoleamine was able to enhance the quality and development of porcine oocytes by decreasing ROS generation, apoptosis and DNA damage
[21][58]. Similarly, low melatonin doses, i.e., 1 nM and 0.1 µM, improved the production and quality of bovine blastocysts, substantially increased the expression of important genes related to embryo development such as DNA methyltransferase 3a (DNMT3A), occludin (OCC) and cadherin (CDH1), and decreased the expression of aquaporin 3 (AQP3), which leads to an enhanced resistance to apoptosis
[22][23][59,60].
Ovarian aging is characterized by a gradually depleted number of primordial follicles and a diminished quality of oocytes, thus causing a progressive reduction of fertility
[24][61]. An investigation with 10-week-old female mice has demonstrated that the administration of water containing 100 µg/mL of melatonin delays ovarian aging. This supplementation, kept until mice were 43-weeks old, resulted in a higher number of primordial, primary and antral follicles, as well as better fertilization and blastocysts rates in treated mice in comparison with littermate control mice
[25][62]. Additionally, melatonin significantly enhanced telomere length in old mice, and improved the expression of aging-related genes, such as sirtuins (SIRT1, SIRT3) and the autophagy-related gene microtubule-associated protein light chain 3 (LC3). Melatonin has also been shown to be able to upregulate 40 ribosome-related genes that are commonly downregulated during aging, these results demonstrating the capacity of melatonin to delay ovarian aging
[25][62].
As for in vivo human studies, several trials have investigated the efficacy of melatonin administration to patients who underwent in vitro fertilization and embryo transfer (IVF-ET) procedure with the idea of rising follicular melatonin concentrations and hence improving oocyte quality
[26][40]. In this sense, oral supplementation with 3 mg/day melatonin in women undergoing IVF-ET increased the percentage of mature oocytes and the number of top-quality embryos, although no significant differences were observed in fertilization rates and clinical pregnancy rates compared to a control group
[27][63]. The same melatonin dosage was also tested in patients with poor oocyte and embryo quality and resulted in a better fertilization rate in the second cycle of IVF-ET in comparison with the first cycle without melatonin supplementation
[28][64]. Other studies have been carried out in women with diminished ovarian reserve who received 3 mg/day melatonin commencing the fifth day of their menstrual cycle till the day of follicular puncture. The number of mature oocytes and top-quality embryos were higher in melatonin treated women than in the control group; however, no statistically significant differences were found in clinical pregnancy and spontaneous miscarriage rates between both groups
[29][65]. In relation to unexplained infertility, oral supplementation with 3 mg/day or 6 mg/day of melatonin for 40 days rebalanced intrafollicular oxidative state, and enhanced oocyte quality and IVF success rates
[30][66]. Furthermore, melatonin supplementation (5 mg/day) in IVF cycles was also effective in women aged over 40, and raised intrafollicular levels of indolamine, the number of mature oocytes and embryo quality
[31][67]. Nevertheless, all these positive findings differ from other studies in which melatonin was unable to ameliorate oocyte quality. For example, in a clinical trial with oral administration of different antioxidants, including 0.975 mg of melatonin, there was an observed improvement in embryo quality upon melatonin treatment, but with no significant differences in terms of the number of follicles, mature oocytes and clinical pregnancy rates
[32][68]. Similarly, doses of 2, 4 and 8 mg of melatonin administered twice a day enhanced neither the number of mature oocytes and embryos nor clinical pregnancy rates, even though the dose of 8 mg resulted in higher concentrations of intrafollicular melatonin compared with the placebo group
[19][56].
As in female patients, melatonin administration was also studied in infertile men to investigate its effects on sperm quality and the quality of the embryos retrieved from their couples when undergoing an IVF cycle. The results demonstrate that supplementation for 45 days of 6 mg melatonin/day promoted a remarkable increase of seminal total antioxidant activity and a reduction in sperm DNA oxidative damage. Moreover, embryos obtained from women whose male couple was taking melatonin experienced significant increment in the percentage of grade A (top quality), B (good quality) and C (impaired quality) embryos, but a decrease in grade D (poor quality, not recommended for ET) embryos, according to the Spanish Association for the Study of Reproductive Biology (ASEBIR) criteria
[33][69].
3. Application of Melatonin in Sperm Preparation for ART
Sperm preparation for ART aim at the selection and enrichment of motile and functionally competent spermatozoa from the ejaculate
[34][70]. Starting from simple washing of spermatozoa, conventional techniques for the separation of spermatozoa from seminal plasma are based on different principles such as migration (which relies on the presence of motile spermatozoa within the semen sample, e.g., swim-up procedure), filtration (relies on sperm motility and the propensity of sperm to adhere to filtration matrices, e.g., glass wool filtration) and density gradient centrifugation (relies on sperm motility and the property of sperm to collect at the border between liquid phases, e.g., continuous density gradient with different media)
[35][71]. Different studies carried out in diverse animal models have proved the effects of melatonin supplementation, at different doses, during sperm preparation
[36][37][38][39][40][41][42][43][72,73,74,75,76,77,78,79]. In this regard, thawed bovine sperm samples were treated with 1 mM melatonin. The results demonstrate that the indolamine decreased the expression of pro-apoptotic genes such as caspase-3 and BAX and caused a dramatic rise in the expression of both the anti-apoptotic genes Bcl-2 and X-linked inhibitor of apoptosis protein (XIAP), and the antioxidant enzyme catalase (CAT). Likewise, in the same study, a concentration of 10 µM melatonin enhanced plasma membrane integrity and acrosome integrity, along with a reduction of the intracellular ROS levels
[44][80]. The very same dose (10 µM) also proved to be efficient in sex-sorted bull semen as it protected semen samples against oxidative stress by increasing the activity of endogenous antioxidants such as Gpx, superoxide dismutase (SOD) and CAT, while inhibiting phosphatidylserine externalization and lipid peroxidation (measured as MDA levels), which are events related to apoptosis and acrosomal membrane integrity, respectively. Moreover, it was found that the dose of 10 µM melatonin led to an increment of the fertilization capacity and an enhancement in embryo development with respect to both the untreated control group and the different doses of the indolamine (1 nM, 0.1 µM and 1 mM)
[45][81].
The role of melatonin in sperm capacitation has also been studied, this process being necessary for the sperm cells to acquire fertilizing capacity
[46][82]. In this sense, low melatonin concentrations (100 pM) have been shown to modulate sperm capacitation by rising motile spermatozoa subpopulation in ram samples, thereby leading to better oocyte fertilization rates after IVF
[47][48][83,84]. In fact, one of the first investigations reporting the involvement of melatonin in sperm motility modulation revealed that melatonin concentrations ranging from 1 pM to 1 µM, when added to supernatant after swim-up sperm selection, enhanced sperm hyperactivation, this action being dependent on MT1 receptor
[49][85].
In relation to human studies, it has been observed that preincubation with 6 mM melatonin during sperm capacitation readily improved progressive motility and membrane integrity of asthenoteratozoospermic men samples
[50][86]. Moreover, it has been reported that the use of lower doses, i.e., 1 mM melatonin, also produced good results since it provoked a significant increment of spermatozoa suitable for oocyte fertilization. Additionally, melatonin (1 mM) enhanced migration of spermatozoa with compacted DNA in oligozoospermic human samples and also avoided DNA fragmentation in normozoospermic human samples
[51][52][87,88]. Likewise, a concentration of 2 mM melatonin also displayed beneficial effects in human sperm motility. Thus, the addition of melatonin after swim-up resulted in an increase in the number of fast and progressively motile spermatozoa, along with an improved sperm viability
[53][89]. On the other hand, it has been demonstrated that a concentration of 1 mM melatonin exerted an anti-apoptotic effect in human spermatozoa treated with H
2O
2 or progesterone due to the inhibition of caspase-3 and the activation of caspase-9, and also prevented phosphatidylserine externalization, which is one of the main hallmarks of apoptosis
[54][90]. Subsequent experiments have demonstrated that 1 mM melatonin managed to revert H
2O
2-induced DNA fragmentation and suggested that the protective effect of melatonin on sperm apoptosis is dependent on MT1 receptor and ERK signalling
[55][14]. Interestingly, the fact that the indoleamine prevents DNA oxidative damage is not only dependent on its antioxidant effect but also relies on its ability to regulate diverse DNA repair pathways, including, but not limited to, base excision repair, homologous recombination and mismatch mediated repair, by different mechanisms (for a detailed review, see
[56][91]).
4. Melatonin as Protective Agent in Gametes Cryopreservation
Sperm cryopreservation is the most commonly used method in cancer patients as they undergo aggressive treatments that can affect sperm quality and ultimately lead to azoospermia
[57][92]. The main drawback of cryopreservation is that sperm quality can be negatively affected due to the freeze–thaw cycles, which may result in oxidative stress, lipid peroxidation increase and loss of plasma membrane integrity, hence disturbing the capacity of sperm–oocyte fertilization
[58][59][60][61][62][63][64][93,94,95,96,97,98,99]. For this reason, the scientific community has focused its attention on the possible protective effects of melatonin on spermatozoa during cryopreservation given its powerful antioxidant action. This protection is associated with a reduction of lipid peroxidation events in sperm cells, which is related to the melatonin-evoked increase in both total antioxidant capacity and activity of antioxidant enzymes
[65][100].
Several studies have proved the role of melatonin as an effective cryoprotectant in sperm cryopreservation. For instance, it has been reported that the supplementation with 2 mM or 3 mM melatonin in the semen extender counteracted the adverse effects of freeze–thaw cycles in bull sperm, as it lessened lipid peroxidation and boosted total antioxidant capacity and activity of antioxidant enzymes
[66][101]. Similarly, the use of melatonin at a concentration of 100 μM or 1 mM improved both motility and viability parameters in cryopreserved buffalo semen samples, which was positively reflected in their in vitro fertilization capacity and the percentage of embryos obtained
[67][68][69][102,103,104]. Moreover, it has also been documented that the addition of 1 mM melatonin in the cryoprotectant was efficient in various animal models such as rabbit, ram, horse, and dog. These studies proved that melatonin enhanced sperm DNA and acrosome integrity
[70][71][72][105,106,107], which led to increased total cleavage rates and, hence, to higher fertilization and birth rates
[70][73][105,108]. Furthermore, the indolamine managed to decrease oxidative stress by ameliorating antioxidant enzymes activation and, therefore, reducing ROS concentration during cryopreservation process
[74][109]. Furthermore, the supplementation of the extender with 500 μM melatonin has been shown to improve the viability of post-thaw mouse sperm samples because of an increased expression of the anti-apoptotic gene B-cell lymphoma-extra-large Bcl-xL and a reduction in the percentage of viable spermatozoa with ROS overproduction
[75][110].
On the other hand, studies carried out with human sperm samples have demonstrated that the use of 100 μM melatonin added as cryoprotectant was able to significantly raise sperm viability and membrane integrity, while diminishing intracellular ROS levels and lipid peroxidation. Of note, this supplementation did not have any detrimental effect on human sperm during cryopreservation
[58][76][11,93]. Likewise, other authors have also reported that different melatonin concentrations (10 μM and 3 mM) resulted in higher viability and motility of cryopreserved spermatozoa, and lower intracellular ROS levels
[77][78][111,112]. Finally, a recent study investigated the effect of 2 mM caffeine added before cryopreservation in normozoospermic semen samples previously treated with 2 mM melatonin. The findings showed that the combination of caffeine and melatonin ameliorated sperm motility and mitochondrial activity compared with samples treated only with melatonin
[79][113].
Regarding mature oocyte cryopreservation, this technique can be used for women facing anticipated fertility decline for various reasons, including gonadotoxic cancer therapies, surgeries with risk of damage to ovary or oophorectomies, and women with increased risk of primary ovarian insufficiency
[80][81][82][114,115,116]. With the idea of minimizing cellular osmotic and/or oxidative stresses during this procedure, the use of antioxidants such as melatonin has been implemented in the last few years. In this sense, it has been reported that loading porcine cumulus–oocyte complexes with melatonin plus glycine (1 μM and 6 mM, respectively) during vitrification (an ultra-rapid method of cryopreservation) enhanced the developmental competency of vitrified porcine oocytes, while lessening levels of ROS and apoptotic occurrence in mature oocytes
[83][117]. Similarly, it has been demonstrated that the addition into vitrification media of melatonin and resveratrol (1 pM and 0.5 μM, respectively) co-encapsulated by solid lipid nanocarriers synergistically improved maturation, fertilization, and embryo development rates and decreased extra/intracellular ROS levels in mature oocytes
[84][118]. Importantly, the indoleamine can also improve the effect of cryopreservation in human oocytes, as has been recently demonstrated
[85][119]. Apart from protecting oocytes during cryopreservation, melatonin has also been proven to foster in vitro maturation of vitrified mouse
[86][87][88][89][90][91][92][93][94][95][120,121,122,123,124,125,126,127,128,129] and equine
[96][130] oocytes. Nevertheless, other studies found no effect of exogenous melatonin on development of cryopreserved oocytes in mouse
[97][131].
Altogether, these studies indicate that melatonin can be used as an effective cryoprotectant, which would acquire special clinical relevance in cryopreserved samples from oncological patients that choose this method for preserving their fertility.