You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 1 by Lixing Huang and Version 2 by Rita Xu.
It is estimated that vibriosis account for about half of the economic losses in Asian fish culture. Consequently, the prevention and control of vibriosis is one of the priority research topics in the field of Asian fish culture disease. Relevant measures have been proposed to control some
Vibrios that pose a threat to Asian fish culture, there are currently only a few effective vaccines available to combat these
that pose a threat to Asian fish culture, but there are currently only a few effective vaccines available to combat these
Vibrios
.
- Asian
- fish culture
- vibriosis
- prevention
1. Introduction
Twenty years ago, aquatic products played a secondary role in people’s food choices. However, now aquatic products have become one of the mainstream food categories. Looking back on the development of global aquaculture from 1997 to 2017, aquaculture has made a substantial contribution to food production throughout the world, especially in Asia. According to the current consumption, aquaculture production needs to increase from 82,087 kilotons in 2018 to 129,000 kilotons in 2050 to meet global needs [1][2][1,2]. By 2050, aquaculture will dominate the global seafood supply [3].
Vibrio is one of the important pathogenic microorganisms of humans and marine animals. It widely exists in marine and freshwater ecosystems. Because of its high abundance and biomass, Vibrio plays a crucial role in the aquatic environment. More than 80 species of Vibrio have been reported, some of which are pathogenic to animals, especially aquatic animals, some to humans, and some to both animals and humans [4]. The outbreak of vibriosis will not only seriously affect marine biomass but also lead to serious economic losses in Asian fish culture.
With the rapid development of Asian fish culture in recent decades, the cases of Vibrio infection through aquatic products at home and abroad, causing human disease or huge economic losses, are also increasing year by year. At the same time, the prevention and control measures for Vibrio are also developing. At present, the use of antibiotics is the most important treatment for vibriosis in Asian fish culture [5]. At the same time, the overuse of broad-spectrum antibiotics has resulted in an increase in the number of drug-resistant bacteria. The resistance genes of these bacteria can be transferred to other bacteria that have never been exposed to the antibiotic [6]. Therefore, it is necessary to develop some antibiotic-free methods. For example, using vaccines, probiotics, bacteriophages and other technologies.
Before considering the prevention and control of Vibrio, it is essential first to identify the exact pathogen. At present, the mainly used identification methods are still conventional physiological, biochemical analyses, 16S rDNA sequencing and drug sensitivity test. In addition to these widely used assays, some convenient, fast and highly sensitive detection methods have been developed in recent years, for example, the identification of biomarkers based on host genes [7], exosomic miRNAs [8] and so on.
Vaccination in Asian fish culture can prevent or mitigate the spread of disease and is effective against many related pathogens [9]. Vaccination is usually a secure and economic precaution. For this reason, illness prevention based on stimulating the immune system of aquatic animals has proved to be the basis of the development of modern Asian fish culture. Nevertheless, there are only a few Vibrios with vaccine control technology.
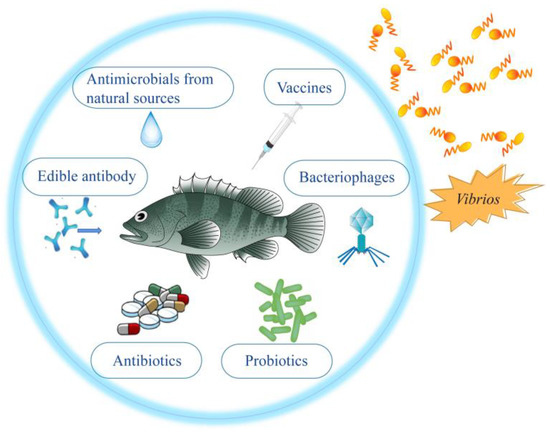
The control and prevention strategies of seven Vibrio species that are seriously harmful to Asian fish culture, including Vibrio harveyi, Vibrio vulnificus, Vibrio parahaemolyticus, Vibrio mimicus, Vibrio anguillarum, Vibrio alginolyticus and Vibrio cholerae. For each Vibrio, rwesearchers describe their prevention and treatment methods (Figure 1), especially vaccine prevention methods, in order to provide views for better prevention and control of vibriosis in Asian fish culture in the future.
[44]. For this reason, using modern methods for understanding and developing new V. parahaemolyticus immunogenic proteins and antibodies are necessary [45][46][45,46].

Figure 1. Strategies for prevention and control of vibriosis in Asian fish culture mentioned in this review.
2. Control and Prevention Strategies of Vibrios
2.1. V. harveyi
V. harveyi is a luminous marine bacterium and is also a well-recognized and acute pathogen of marine fish [10]. The research on the control and prevention measures of V. harveyi started early, and now there are a variety of control technologies (Table 1).2.1.1. Antibiotics
Antibiotic methods are generally used in the initial stage of prevention and treatment of vibriosis or emergency treatment. In the case of skin ulcer disease of young hybrid groupers, researchers confirm that the pathogen of this sickness is V. harveyi ML01 strain, which is sensitive to minocycline, doxycycline and ceftriaxone. In other words, these three antibiotics can be used for emergency treatment of V. harveyi infection [11]. In 2017, the drug sensitivity test of V. harveyi extracted from the diseased cultured hippocampus was carried out, and the results showed that V. harveyi is highly sensitive to doxycycline and tetracycline. This provides a reference for the prevention strategy of vibriosis in seahorse culture in eastern China [12]. Although antibiotics are widely used, the rapid increase in antibiotic resistance is really puzzling.2.1.2. Bacteriophages
As people gradually realize the risk of using antibiotics in Asian fish culture, probiotics, bacteriophage, antimicrobials from natural sources and so on are gradually replacing antibiotics. In bacteriophage therapy, phages such as lytic Vibrio phage VhKM4 [13] can resist V. harveyi efficiently due to its strong lytic activity. Although several research studies have proven these methods effective, there have not been enough similar studies of each method to prove that they should be promoted in practical application. At the same time, whether these biological control methods have potential threats still needs further study in future research.2.1.3. Vaccines
One of the research hot spots of Vibrio prevention is vaccine development. Most research on V. harveyi vaccine is targeted at fish. Many excellent achievements have been made in the research of V. harveyi vaccine. Vaccine exploration started from the traditional whole-cell inactivation method, followed by the study on the method of purifying subcellular components, making vaccine technology enter the era of modern vaccines represented by DNA vaccine [10]. Whole-cell vaccines can be categorized into two types, attenuated live vaccine and inactivated vaccine. The production cost of these vaccines is not high [14]. This traditional vaccine is the most widely used in the prevention of aquatic animal diseases.-
Inactivated vaccines
-
Attenuated live vaccines
-
Subunit vaccines
-
Anti-idiotypic vaccines
-
DNA vaccines
-
mRNA vaccines
Table 1.
Control and prevention strategies of
V. harveyi.
NR: Not Relevant, None: the method has not been tested in vivo or relevant data has not been found.
| Pathogen | Prevention and Control Technology | Concrete Measure/ Vaccine Type |
Host | Vaccine Antigen Components | Route of Infection | Ref. |
|---|---|---|---|---|---|---|
| V. harveyi | Antibiotics | Ceftriaxone, Doxycycline, Minocycline |
Juvenile hybrid groupers |
NR | Bath, Injection (IP) |
[11] |
| 26 | ||||||
| ] | ||||||
| Juvenile sea bass | ||||||
| Expressed r-OmpK | ||||||
| of | ||||||
| Vibrio | ||||||
| Injection (IP) | ||||||
| [ | 27 | ] | ||||
| Anti-idiotypic | Grouper | Anti-Id IgG (Fab) | Injection (IP) | [28] | ||
| DNA | Japanese flounder | Plasmid pDV | Injection (IP, IM) |
[29] | ||
| Turbot | Plasmid with OmpU | Injection (IP, IM) |
[30] | |||
| mRNA | Fish | and B-cell epitopes in hemolysin protein | None | [32] |
2.2. V. vulnificus
V. vulnificus is a gram-negative bacterium that can cause wound infection and septicemia. Unlike other Vibrios, it is able to ferment lactose. According to genetic, biochemical and serological tests and host infection, V. vulnificus is currently classified into three biotypes. Biotype 1 strains are the source of most human infections, and biotype 2 strains mainly infect eels. The recently discovered biotype 3 has the biochemical characteristics of biotype 2 and 1 and can result in human wound infection [33]. In this era of environmental protection and sustainable development, the biological control strategy of Vibrio is gradually emerging, but there are few examples in fish farming.Vaccines
-
Inactivated vaccines
Table 2. Control and prevention strategies of V. vulnificus. NR: Not Relevant.
| Pathogen | Prevention and Control Technology | Concrete Measure/ Vaccine Type |
Host | Vaccine Antigen Components | Route of Infection | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|
| V. vulnificus | Vaccines | Inactivated | Tilapia (Sex reversed hybrid) |
Atypical V. vulnificus (Formalin killed cells) |
Injection (IP) | [34] | |||
| Doxycycline, Tetracycline | Hippocampus | NR | Injection (IP) | [12] | |||||
| E. coioides | Vh + Vv + Va inactive vaccine and ISKNV whole cell inactive vaccine |
Injection (IP) | [16 | Bacteriophages | Phage VhKM4 | Finfish | NR | [13] | |
| Vaccines | Inactivated | Marine Red Hybrid Tilapia |
V. harveyi strain Vh1 (Formalin-Inactivated) |
Injection (IP) | [15] | ||||
| E. coioides | VICV | Injection (IP) | [16] | ||||||
| Orange-spotted grouper | V. Harveyi (formalin-killed, Adjuvant: ISA763 AVG) |
Injection (IP) | [17] | ||||||
| Turbot | V. Harveyi (formalin-killed, Adjuvant: TMISA763 AVG) |
Injection (IP) | [18] | ||||||
| Pearl gentian grouper |
V. harveyi ZJ0603 (Formalin-killed, combine with β-glucanhas) |
Injection (IP) | [20] | ||||||
| Attenuated | Grouper | Non-toxic V. harveyi | Bath, Injection (IP) |
[22] | |||||
| Japanese flounder | Attenuated mutant V. Harveyi T4DM |
Bath, Injection (IP) |
[24] | ||||||
| ] | |||||||||
| Subunit | Japanese eel | Expressed OmpU of V. vulnificus |
Injection (IP) | [35] | |||||
| Multivalent | Japanese eel | Recombinant Omp containing both OmpA and OmpU | Injection (IP) | [36] | |||||
| European eel | Trivalent outer membrane protein (OmpⅡ-U-A) | Injection (IP) | [37] | Subunit | Japanese flounder | Recombinant Vhp1 | Injection (IP) | [21] | |
| Golden pompano | Antigen encoding TssJ | Injection (IP) | [25] | ||||||
| Orange-spotted grouper |
VirB11 | Injection (IP) | [ |
-
Subunit vaccines
-
Multivalent vaccines
2.3. V. parahaemolyticus
V. parahaemolyticus is a Gram-negative, slightly halophilic bacterium that inhabits brackish aquatic environments such as coastal and estuarine waters. Apart from being pathogenic to aquatic organisms, V. parahaemolyticus is also known as a global food-borne pathogen and one of the most common causes of gastroenteritis in East Asia due to the local dietary habit of eating raw fish and shellfish [38][39][38,39]. V. parahaemolyticus is antibiotic-resistant, so it cannot be treated with antibiotics which is currently the most commonly used measure in Asian fish culture [40]. Consequently, there is a pressing need to exploit fresh, effective alternatives for antibiotics against V. parahaemolyticus (Table 3), while the vaccine is the most promising approach due to its economy, efficacy and safety in public awareness [40][41][42][40,41,42].Table 3. Control and prevention strategies of V. parahaemolyticus. NR: Not Relevant.
| Pathogen | Prevention and Control Technology | Concrete Measure/ Vaccine Type |
Host | Vaccine Antigen Components | Route of Infection | Ref. |
|---|
2.4. V. cholerae
V. cholerae is a Gram-negative motile bacterium that can cause fatal pandemic diseases. There are millions of cholera cases worldwide every year, and the mortality rate is extremely high [47]. Consuming contaminated seafood by mistake is one of the reasons why people are infected with V. cholerae. As an important food-borne pathogen, V. cholerae is widely distributed in fish, which brings serious safety hazards to human and aquatic animal health [48].2.4.1. Antibiotics
V. cholerae is one of the important pathogens related to fish vibriosis. In the bluegill sunfish that died in the farms of Guangdong around 2018, the pathogen identified was non-O1/non-O139 V. cholerae. The antibiotic sensitivity displayed that the isolated strain was sensitive to azithromycin, chloramphenicol, neomycin, norfloxacin, doxycycline, etc. The possible method to prevent infection of bluegill sunfish is to give neomycin or doxycycline for seven days [49].2.4.2. Vaccines
After consulting a large number of data, rwesearchers found that the current prevention and control of V. cholerae in Asian fish culture is still based on antibiotics, and no V. cholerae vaccine for aquatic animals has been found yet. Nevertheless, in recent decades, the misuse of antibiotics has resulted in the emergence and spread of drug-resistant bacteria in the environment, which is likely to pose a threat to public health [50]. Therefore, people are also constantly exploring new methods for the prevention and control of V. cholerae (Table 4).Table 4.
Control and prevention strategies of
V. cholerae.
NR: Not Relevant, None: the method has not been tested in vivo or relevant data has not been found.
Vaccines
-
Inactivated vaccines
-
Recombinant vaccines
