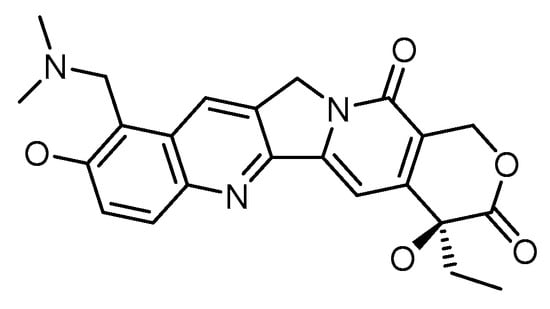
Chemotherapy has been the predominant treatment modality for cancer patients, but its overall performance is still modest. Difficulty in penetration of tumor tissues, a toxic profile in high doses, multidrug resistance in an array of tumor types, and the differential architecture of tumor cells as they grow are some of the bottlenecks associated with the clinical usage of chemotherapeutics. Advances in tumor biology understanding and the emergence of novel targeted drug delivery tools leveraging various nanosystems offer hope for developing effective cancer treatments. Topotecan is a topoisomerase I inhibitor that stabilizes the transient TOPO I-DNA cleavable complex, leading to single-stranded breaks in DNA. Due to its novel mechanism of action, TOPO is reported to be active against various carcinomas, namely small cell lung cancer, cervical cancer, breast cancer, and ovarian cancer. Issues of cross-resistance with numerous drugs, rapid conversion to its inactive form in biological systems, appended adverse effects, and higher water solubility limit its therapeutic efficacy in clinical settings. Topotecan nanoformulations offer several benefits for enhancing the therapeutic action of this significant class of chemotherapeutics. The likelihood that the target cancer cells will be exposed to the chemotherapeutic drug while in the drug-sensitive s-phase is increased due to the slow and sustained release of the chemotherapeutic, which could provide for a sustained duration of exposure of the target cancer cells to the bioavailable drug and result in the desired therapeutic outcome.
- cancer
- topotecan
- side effects
- passive targeting
- active targeting
- combinatorial drug therapy
1. Introduction
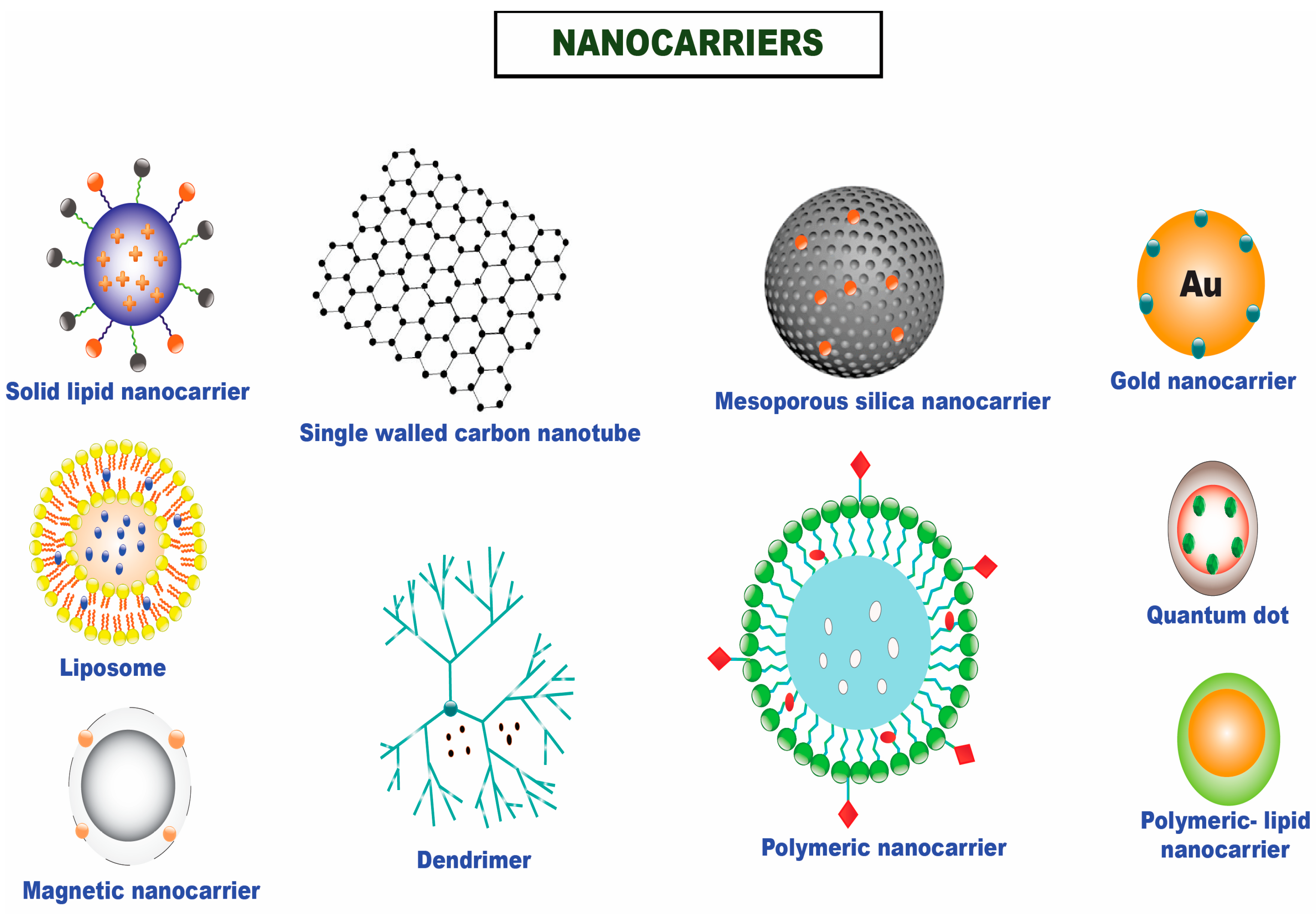
2. Passive Targeted Delivery Approach for Topotecan
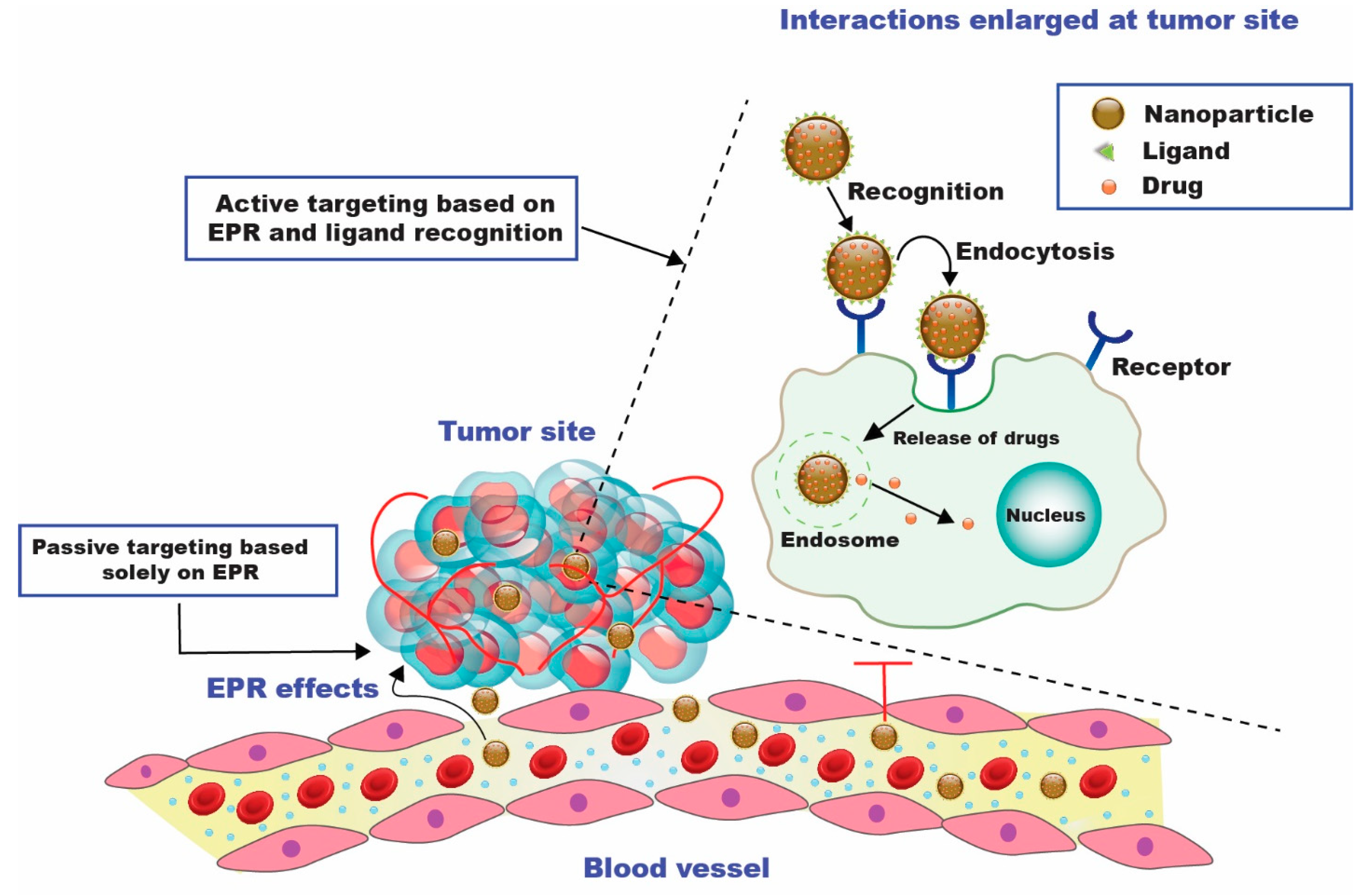
The purpose of passive targeting is to take advantage of the differences between tumor and normal tissues. Chemotherapeutics are efficiently transported to the target site by passive targeting to accomplish a therapeutic function. Substantial cancer cell multiplication causes neovascularization and wide fenestrations in the vascular wall, enhancing the tumor vessels’ permeability relative to healthy vessels [33][23]. Macromolecules, such as NPs, might escape from blood arteries supplying the tumor and amass within tumor tissue due to the fast and deficient angiogenesis. The accumulation of NPs is increased in cancer due to inadequate lymphatic drainage, which enables the nanocarriers to transfer their payloads to tumor cells. These procedures result in the EPR effect, which is one of the drivers behind the passive targeting approach [34][24]. In conjunction with the EPR effect, the tumor milieu plays a significant role in the passive distribution of nanomedicines. One of the metabolic traits of cancer cells is glycolysis, which serves as the primary energy supply for the development of cancer cells [16]. The tumor microenvironment’s pH is decreased by glycolysis, which creates an acidic setting. As a result, some pH-sensitive NPs are activated by the lower pH and can release medications close to cancer cells [35][25]. Topotecan (TOPO) was encased in mesoporous silica nanoparticles (MSNs), and the nanosystem allowed the drug’s active form to be delivered in endosomes/lysosomes (pH 5.5) upon the internalization of nanoparticles. A pH-sensitive coating, a multimodal gelatin shell that protected TOPO from hydrolysis and premature release, and several anchorage sites for marking targeted ligands for preferential uptake in tumor cells were the hallmarks of MSNs. The nanosystems effectively destroy tumor cells while not affecting normal cells’ survival. On the other hand, free TOPO could not kill both cell lines due to the drug’s deactivation. This revolutionary nanodevice represents a step ahead in developing new cancer-fighting weaponry [36][26]. A topotecan-entrapped liposomal nanoformulation (LNP) was developed based on a loading process that entails the production of a copper water-soluble camptothecin complex. The same loading process developed for irinotecan was followed to produce an LNP topotecan formulation (Topophore C). At a final drug-to-lipid (D/L) mole ratio of 0.1, the entrapment efficiency of topotecan was noted to be >98%. Greater D/L ratios were possible; however, in vitro drug release tests revealed that the ensuing formulations were less stable. Topotecan plasma half-life and AUC were raised 10- to 22-fold in mice after Topophore C treatment, compared to free topotecan. Topophore C was noted to be 2-to 3-fold more toxic than free topotecan, but it had considerably superior anti-tumor effectiveness with no adverse effects. Based on the inferred findings, it can be inferred that Topophore C is a promising pharmacological candidate for treating platinum-resistant ovarian cancer [37][27]. Topotecan would continue to benefit from the targeted site delivery by utilizing nanocarriers. Anti-epidermal growth factor receptor (EGFR) and anti-human epidermal growth receptor 2 (HER2)-immunoliposome formulations substantially boosted topotecan internalization compared to the non-targeted counterparts and free topotecan, resulting in enhanced cytotoxic activity and superior antitumor efficacy against HER2-overexpressing human breast cancer (BT474) xenografts. Topotecan’s targeting capability and pharmacokinetic properties were considerably improved when it was stabilized in nanoliposomes, enabling potent and effective formulations against solid tumors [38][28]. Topotecan was further reported to have enhanced efficacy in ovarian cancer. An appreciable particle size and entrapment efficiency of 60.9 ± 2.2% were obtained in a nanometric range. The formulated nanoparticles illustrated a sustained release in physiological and acidic tumor microenvironmental conditions. The nanometric size enabled ideal internalization in SKOV3 (ovarian cancer) cell lines over time compared to topotecan in a soluble form, with a 13.05-fold rise in bioavailability when evaluated for pharmacokinetic attributes [39][29]. The polylactic-co-glycolic acid (PLGA) nanocarrier was also used to formulate topotecan (TPT), which improved the drug’s efficacy by reducing the accelerated conversion of the bioactive lactone form to the inactive carboxylate form. TPT’s stability was ensured by maintaining the drug-containing phase at an acidic pH. The drug maintained its active lactone form by lowering the pH of the inside of nanoparticles, which led to a 15-day biphasic release profile. Furthermore, compared to a neat drug, the cytotoxicity screening and in vivo antitumor effectiveness revealed considerable potential for greater proliferation inhibition [14]. Nanostructured lipid carriers (NLC) incorporating topotecan (TPT-NLC) were fabricated in hydrogels with hydroxyethyl cellulose and chitosan (TPT-NLC-HEC and TPT-NLC-Ch). For around 30 days, the said formulations retained the drug and nanoparticle dispersions stably. TPT release was dramatically reduced when nanoparticles were added to gels. TPT-NLC-HEC boosted permeability by 2.37 times compared to TPT-HEC (11.9 and 5.0 g/cm2, respectively). Nanoencapsulation significantly increased TPT cytotoxicity when analyzed in B16F10 melanoma cells. With an IC50 value of 5.74 g/mL, TPT-NLC was noted to be more toxic than free TPT, whereas free TPT had an IC50 of >20 g/mL. Because the skin penetrated values of TPT from the established formulation (TPT-NLC) were higher than the melanoma IC50, it may be stated that chemotherapeutic permeated quantities may be adequate for a therapeutic impact [40][30]. TPT-SLNs were integrated into a thermoresponsive hydrogel system (TRHS) to create TPT-SLNs-TRHS, which allowed for controlled drug release and reduced drug-associated toxicity. When TPT-SLNs-TRHS was injected into the rat’s rectum, it showed good gelation capabilities. Furthermore, drug release was demonstrated to be controlled over an extended period for the integrated TPT. TPT bioavailability was improved with enhanced plasma concentration and area under the curve (AUC) in pharmacokinetic investigations. Furthermore, compared to the test formulations, it significantly improved antitumor impact in tumor-bearing animals. The study inferred that SLNs combined with TRHS could be a potential source of antitumor drug delivery with improved control over drug release, with no associated toxicity [41][31]. To augment topotecan’s transport to the lymphatic system, a primary conduit for cancer metastasis, and further enhance topotecan’s bioavailability and retention in target organs such as lung and brain, a research group formulated topotecan-loaded polymeric nanoparticles [42][32]. The cumulative percentage of topotecan release from the nanoformulation after a time period of 120 h were 91.56 and 92.02%, respectively, according to the results of in vitro release assays for the nanoformulation and free drug as a reference standard. PLGA nanoparticles with topotecan loading displayed a protracted release pattern in the studied time frame. Following 6 h after treatment, topotecan distribution was noted to be larger in each target organ after administration of the topotecan nanoformulation at a dose of 4 mg/kg than following delivery of the free drug. A similar pattern was seen following oral administration. The substantial intensity of luminescence was demonstrated for six hours following the injection of the nanoformulation. Higher luminescence in lymphoid tissues was noted, which was coherent with the quantitative observation of significant topotecan in the said tissues after intravenous administration of these nanoparticles. The outcomes of this study imply that topotecan NPs may produce superior therapeutic outcomes because they have a better pharmacokinetic profile and are efficient enough to distribute the drug more effectively to lymphoid tissues, the lung, and the brain as contrasted to the free drug [42][32].3. Active Targeting
When the nanocarriers reach the tumoral zone, they face a challenging situation. Tumoral aggregates comprise various cell types, ranging from tumoral cells to immunological, supporting, and healthy cells from the extracellular matrix [47][33]. Consequently, nanocarriers must be able to distinguish malignant cells and localize their action on them to accomplish an effective therapeutic effect. By binding targeting moieties to the particle surface, this capability can effectively be included in the nanocarriers [48][34]. These targeting components are small compounds or macromolecules that engage specific receptors on tumor cells’ surfaces. These cellular receptors are found in healthy cells in many cases, such as for the extensively used targeting moieties folic acid [49][35], transferrin [38][28], and sugars [50][36]. However, because tumor cells have a larger nutrition need, their population is much higher than healthy cells. As a result, this receptor overexpression can be leveraged to selectively deliver therapeutic medications to tumor cells. Another option is to produce synthetic targeting components that are more selective and efficacious at binding to specific receptors [51][37]. Mesoporous silica nanoparticles laden with topotecan were synthesized and then surface-conjugated with folic acid (FTMN) to increase the drug’s effectiveness in treating retinoblastoma (RB) cancers. In physiological settings, the particles were nanosized and showed a controlled release of the entrapped drug. Compared to non-targeted nanoparticles, the folic acid-conjugated nanoformulations had a phenomenal absorption in RB cells. These findings strongly suggest that cellular uptake was regulated by receptor-mediated endocytosis. Compared to other formulations, FTMN had a considerably larger cytotoxic effect in Y79 cancer cells due to its higher cellular absorption. FTMN successfully triggered cancer cell death with a 58% effectiveness. The anticancer efficiency of TPT nanoformulation in Y79 cancer cells was superior to that of native drugs or unconjugated nanoparticles, according to the findings. FTMN revealed a decrease in overall tumor volume compared to the other group and fewer tumor cells in histological staining. Consequently, a folic acid-conjugated nanocarrier system could be a promising therapy option for RB [52][38]. Transferrin-decorated multifunctional nanoparticles (NPs) based on γ-cyclodextrin for tumor-targeted therapy were reported. The formulated NPs were found to cause a considerable increase in cellular uptake in MDA-MB-231 tumor cells leading to cell death. The transferrin-targeted NPs were proven effective carriers of TPT in vivo experiments using an MDA-MB-231 tumor xenografted mice model. TPT has the preferential ability to deliver chemotherapeutics to Tf receptor-positive MDA-MB-231 tumor cells, increasing drug uptake into the tumor cells and intensifying their toxicity [53][39]. Irrespective of the availability of numerous nanocarriers, researchers worldwide have explored liposomes as a suitable carrier system for the targeted delivery of topotecan employing specific ligands.4. Combinatorial Drug Therapy Employing Topotecan
One of the cornerstones of cancer therapy is combinatorial therapy, which utilizes two or more chemotherapeutics to target cancer-inducing or cell-sustaining mechanisms [56][40] selectively. Even though the mono-therapy technique is still a prevalent type of cancer treatment, it is typically thought to be less efficient than the combination therapy strategy. Typical mono-therapeutic approaches non-selectively target actively multiplying cells, inevitably resulting in the death of both malignant and healthy cells. Chemotherapy can harm the patient and comes with several hazards and side effects. It often leads to drug resistance and cancer cell survival (Figure 4). It can also significantly lower the patient’s immune system, by weakening bone marrow cells and making them more vulnerable to host illnesses. The toxicity aspect of combinatorial therapy is greatly reduced because diverse channels will be targeted, even if it can still be harmful if one of the medications is a chemotherapeutic. Combinatorial therapy has a synergistic effect, necessitating a reduced therapeutic dosage of each chemotherapeutic separately [57][41]. Combinatorial therapy may also provide cytotoxic effects on cancer cells while preventing harmful effects on healthy cells.
References
- Garg, P. Selective Preference of Antibody Mimetics over Antibody, as Binding Molecules, for Diagnostic and Therapeutic Applications in Cancer Therapy. Biointerface Res. Appl. Chem. 2021, 11, 10679–10689.
- Arifin, M.Z.; Parikesit, A.A.; Agustriawan, D. Molecular Simulation of MDM2 and E6AP Proteins as P53 Regulator in Cervical Cancer. Biointerface Res. Appl. Chem. 2020, 10, 5875–5879.
- Alavi, M.; Nokhodchi, A. Micro- and Nanoformulations of Paclitaxel Based on Micelles, Liposomes, Cubosomes, and Lipid Nanoparticles: Recent Advances and Challenges. Drug Discov. Today 2022, 27, 576–584.
- Abdelaziz, H.M.; Gaber, M.; Abd-Elwakil, M.M.; Mabrouk, M.T.; Elgohary, M.M.; Kamel, N.M.; Kabary, D.M.; Freag, M.S.; Samaha, M.W.; Mortada, S.M.; et al. Inhalable Particulate Drug Delivery Systems for Lung Cancer Therapy: Nanoparticles, Microparticles, Nanocomposites and Nanoaggregates. J. Control. Release 2018, 269, 374–392.
- Verma, D.; Thakur, P.S.; Padhi, S.; Khuroo, T.; Talegaonkar, S.; Iqbal, Z. Design Expert Assisted Nanoformulation Design for Co-Delivery of Topotecan and Thymoquinone: Optimization, in Vitro Characterization and Stability Assessment. J. Mol. Liq. 2017, 242, 382–394.
- Curcio, M.; Diaz-Gomez, L.; Cirillo, G.; Nicoletta, F.P.; Leggio, A.; Iemma, F. Dual-Targeted Hyaluronic Acid/Albumin Micelle-Like Nanoparticles for the Vectorization of Doxorubicin. Pharmaceutics 2021, 13, 304.
- Padhi, S.; Behera, A. Nanotechnology Based Targeting Strategies for the Delivery of Camptothecin. Sustain. Agric. Rev. 2020, 44, 243–272.
- Kundu, A.; Padhi, S.; Behera, A.; Hasnain, M.S.; Nayak, A.K. Tumor Targeting Strategies by Chitosan-Based Nanocarriers. In Chitosan in Biomedical Applications; Academic Press: Cambridge, MA, USA, 2022; pp. 163–188.
- Behera, A.; Mittu, B.; Padhi, S.; Singh, A. Antimicrobial Efficacy of Essential Oil Nanoemulsions. In Nanotechnological Approaches in Food Microbiology; CRC Press: Boca Raton, FL, USA, 2020; pp. 293–309.
- Hassan, N.; Firdaus, S.; Padhi, S.; Ali, A.; Iqbal, Z. Investigating Natural Antibiofilm Components: A New Therapeutic Perspective against Candidal Vulvovaginitis. Med. Hypotheses 2021, 148, 110515.
- Behera, A.; Patra, N.; Mittu, B.; Padhi, S.; Singh, J. Bimetallic Nanoparticles: Green Synthesis, Applications, and Future Perspectives. In Multifunctional Hybrid Nanomaterials for Sustainable Agri-food and Ecosystems; Elsevier: Amsterdam, The Netherlands, 2020; pp. 639–682.
- Padhi, S.; Behera, A. Advanced Drug Delivery Systems in the Treatment of Ovarian Cancer. In Advanced Drug Delivery Systems in the Management of Cancer; Academic Press: Cambridge, MA, USA, 2021; pp. 127–139.
- Behera, A.; Padhi, S.; Nayak, A.K. Engineered Liposomes as Drug Delivery and Imaging Agents. Des. Appl. Theranostic. Nanomed. 2022, 75.
- Padhi, S.; Mirza, M.A.; Verma, D.; Khuroo, T.; Panda, A.K.; Talegaonkar, S.; Khar, R.K.; Iqbal, Z. Revisiting the Nanoformulation Design Approach for Effective Delivery of Topotecan in Its Stable Form: An Appraisal of Its in Vitro Behavior and Tumor Amelioration Potential. Drug Deliv. 2015, 23, 2827–2837.
- Patnaik, S.; Gorain, B.; Padhi, S.; Choudhury, H.; Gabr, G.A.; Md, S.; Kumar, M.D.; Kesharwani, P. Recent Update of Toxicity Aspects of Nanoparticulate Systems for Drug Delivery. Eur. J. Pharm. Biopharm. 2021, 161, 100–119.
- Padhi, S.; Behera, A.; Hasnain, M.S.; Nayak, A.K. Chitosan-Based Drug Delivery Systems in Cancer Therapeutics. Chitosan. Drug Deliv. 2022, 159–193.
- Padhi, S.; Dash, M.; Behera, A. Nanophytochemicals for the Treatment of Type II Diabetes Mellitus: A Review. Environ. Chem. Lett. 2021, 19, 4349–4373.
- Padhi, S.; Nayak, A.K.; Behera, A. Type II Diabetes Mellitus: A Review on Recent Drug Based Therapeutics. Biomed. Pharmacother. 2020, 131, 110708.
- De La Torre, P.; Jesús Pérez-Lorenzo, M.; Alcázar-Garrido, Á.; Flores, A.I.; Baeza, A.; Novio, F.; Paris, J.L. Cell-Based Nanoparticles Delivery Systems for Targeted Cancer Therapy: Lessons from Anti-Angiogenesis Treatments. Molecules 2020, 25, 715.
- Navya, P.N.; Kaphle, A.; Srinivas, S.P.; Bhargava, S.K.; Rotello, V.M.; Daima, H.K. Current Trends and Challenges in Cancer Management and Therapy Using Designer Nanomaterials. Nano Converg. 2019, 6, 1–30.
- Dehshahri, A.; Ashrafizadeh, M.; Ghasemipour, A.E.; Pardakhty, A.; Mandegary, A.; Mohammadinejad, R.; Sethi, G. Topoisomerase Inhibitors: Pharmacology and Emerging Nanoscale Delivery Systems. Pharmacol. Res. 2020, 151, 104551.
- Chen, A.Y.; Choy, H.; Rothenberg, M.L. DNA Topoisomerase I-Targeting Drugs as Radiation Sensitizers. Oncology 1999, 13, 39–46.
- Carmeliet, P.; Jain, R.K. Angiogenesis in Cancer and Other Diseases. Nature 2000, 407, 249–257.
- Behera, A.; Padhi, S. Passive and Active Targeting Strategies for the Delivery of the Camptothecin Anticancer Drug: A Review. Environ. Chem. Lett. 2020, 18, 1557–1567.
- Lim, E.-K.; Chung, B.H.; Chung, S.J. Recent Advances in PH-Sensitive Polymeric Nanoparticles for Smart Drug Delivery in Cancer Therapy. Curr. Drug Targets 2016, 19, 300–317.
- Martínez-Carmona, M.; Lozano, D.; Colilla, M.; Vallet-Regí, M. Selective Topotecan Delivery to Cancer Cells by Targeted PH-Sensitive Mesoporous Silica Nanoparticles. RSC Adv. 2016, 6, 50923–50932.
- Patankar, N.A.; Waterhouse, D.; Strutt, D.; Anantha, M.; Bally, M.B. Topophore C: A Liposomal Nanoparticle Formulation of Topotecan for Treatment of Ovarian Cancer. Investig. New Drugs 2012, 31, 46–58.
- Drummond, D.C.; Noble, C.O.; Guo, Z.; Hayes, M.E.; Connolly-Ingram, C.; Gabriel, B.S.; Hann, B.; Liu, B.; Park, J.W.; Hong, K.; et al. Development of a Highly Stable and Targetable Nanoliposomal Formulation of Topotecan. J. Control. Release 2010, 141, 13–21.
- Padhi, S.; Kapoor, R.; Verma, D.; Panda, A.K.; Iqbal, Z. Formulation and Optimization of Topotecan Nanoparticles: In Vitro Characterization, Cytotoxicity, Cellular Uptake and Pharmacokinetic Outcomes. J. Photochem. Photobiol. B Biol. 2018, 183, 222–232.
- Venâncio, J.H.; Andrade, L.M.; Esteves, N.L.S.; Brito, L.B.; Valadares, M.C.; Oliveira, G.A.R.; Lima, E.M.; Marreto, R.N.; Gratieri, T.; Taveira, S.F. Topotecan-Loaded Lipid Nanoparticles as a Viable Tool for the Topical Treatment of Skin Cancers. J. Pharm. Pharmacol. 2017, 69, 1318–1326.
- Xing, R.; Mustapha, O.; Ali, T.; Rehman, M.; Zaidi, S.S.; Baseer, A.; Batool, S.; Mukhtiar, M.; Shafique, S.; Malik, M.; et al. Development, Characterization, and Evaluation of SLN-Loaded Thermoresponsive Hydrogel System of Topotecan as Biological Macromolecule for Colorectal Delivery. Biomed. Res. Int. 2021, 2021, 1–14.
- Jeong, S.H.; Jang, J.H.; Lee, Y.B. Oral Delivery of Topotecan in Polymeric Nanoparticles: Lymphatic Distribution and Pharmacokinetics. J. Control. Release 2021, 335, 86–102.
- Egeblad, M.; Nakasone, E.S.; Werb, Z. Tumors as Organs: Complex Tissues That Interface with the Entire Organism. Dev. Cell 2010, 18, 884–901.
- Ediriwickrema, A.; Saltzman, W.M. Nanotherapy for Cancer: Targeting and Multifunctionality in the Future of Cancer Therapies. ACS Biomater. Sci. Eng. 2015, 1, 64–78.
- Lee, K.Y.; Seow, E.; Zhang, Y.; Lim, Y.C. Targeting CCL21-Folic Acid-Upconversion Nanoparticles Conjugates to Folate Receptor-α Expressing Tumor Cells in an Endothelial-Tumor Cell Bilayer Model. Biomaterials 2013, 34, 4860–4871.
- Vaillant, O.; Cheikh, K.E.; Warther, D.; Brevet, D.; Maynadier, M.; Bouffard, E.; Salgues, F.; Jeanjean, A.; Puche, P.; Mazerolles, C.; et al. Mannose-6-Phosphate Receptor: A Target for Theranostics of Prostate Cancer. Angew. Chem. Int. Ed. 2015, 54, 5952–5956.
- Yang, K.S.; Budin, G.; Tassa, C.; Kister, O.; Weissleder, R. Bioorthogonal Approach to Identify Unsuspected Drug Targets in Live Cells. Angew. Chem. 2013, 125, 10787–10791.
- Qu, W.; Meng, B.; Yu, Y.; Wang, S. Folic Acid-Conjugated Mesoporous Silica Nanoparticles for Enhanced Therapeutic Efficacy of Topotecan in Retina Cancers. Int. J. Nanomed. 2018, 13, 4379.
- Yoon, S.; Kim, Y.; Youn, Y.S.; Oh, K.T.; Kim, D.; Lee, E.S. Transferrin-Conjugated PH-Responsive γ-Cyclodextrin Nanoparticles for Antitumoral Topotecan Delivery. Pharmaceutics 2020, 12, 1109.
- Mokhtari, R.B.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination Therapy in Combating Cancer. Oncotarget 2017, 8, 38022.
- Mokhtari, R.B.; Kumar, S.; Islam, S.S.; Yazdanpanah, M.; Adeli, K.; Cutz, E.; Yeger, H. Combination of Carbonic Anhydrase Inhibitor, Acetazolamide, and Sulforaphane, Reduces the Viability and Growth of Bronchial Carcinoid Cell Lines. BMC Cancer 2013, 13, 1–18.
- Kumar, R.; Chaudhary, K.; Gupta, S.; Singh, H.; Kumar, S.; Gautam, A.; Kapoor, P.; Raghava, G.P.S. CancerDR: Cancer drug resistance database. Sci. Rep. 2013, 3, 1445.
- Du, J.; Lu, W.L.; Ying, X.; Liu, Y.; Du, P.; Tian, W.; Men, Y.; Guo, J.; Zhang, Y.; Li, R.J.; et al. Dual-Targeting Topotecan Liposomes Modified with Tamoxifen and Wheat Germ Agglutinin Significantly Improve Drug Transport across the Blood-Brain Barrier and Survival of Brain Tumor-Bearing Animals. Mol. Pharm. 2009, 6, 905–917.
- Jain, A.; Jain, S.K. Multipronged, Strategic Delivery of Paclitaxel-Topotecan Using Engineered Liposomes to Ovarian Cancer. Drug Dev. Ind. Pharm. 2015, 42, 136–149.
- Khuroo, T.; Verma, D.; Talegaonkar, S.; Padhi, S.; Panda, A.K.; Iqbal, Z. Topotecan–Tamoxifen Duple PLGA Polymeric Nanoparticles: Investigation of in Vitro, in Vivo and Cellular Uptake Potential. Int. J. Pharm. 2014, 473, 384–394.
