Cyanobacteria ascribed to the genus Lyngbya (Family Oscillatoriaceae) represent a potential therapeutic gold mine of chemically and biologically diverse natural products that exhibit a wide array of biological properties. Phylogenetic analyses have established the Lyngbya ‘morpho-type’ as a highly polyphyletic group and have resulted in taxonomic revision and description of an additional six new cyanobacterial genera in the same family to date. Among the most prolific marine cyanobacterial producers of biologically active compounds are the species Moorena producens (previously L. majuscula, then Moorea producens), M. bouillonii (previously L. bouillonii), and L. confervoides. Over the years, compounding evidence from in vitro and in vivo studies in support of the significant pharmaceutical potential of ‘Lyngbya’-derived natural products has made the Lyngbya morphotype a significant target for biomedical research and novel drug leads development. RThis comprehesearchers concludednsive review covers compounds with reported anti-infective activities through 2022 from the Lyngbya morphotype, including new genera arising from recent phylogenetic re-classification. So far, 72 anti-infective secondary metabolites have been isolated from various Dapis, Lyngbya, Moorea, and Okeania species.
- marine cyanobacteria
- Lyngbya morphotype
- secondary metabolites
- Antibacterial
1. Introduction
2. Compounds with Antibacterial Activities
Among the diverse bioactivities that Lyngbya secondary metabolites have displayed is the activity against bacteria. In 1979, malyngolide (1) (Figure 1), a δ-lactone was reported from Hawaiian Lyngbya majuscula in Kahala Beach, showed effective antibacterial activity against Mycobacterium smegmatis and Streptococcus pyogenes and was less active against Staphylococcus aureus and Bacillus subtilis, and inactive towards Enterobacter aerogenes, Escherichia coli, Pseudomonas aeruginosa, Salmonella enteritidis, and Staphylococcus marcescens [12].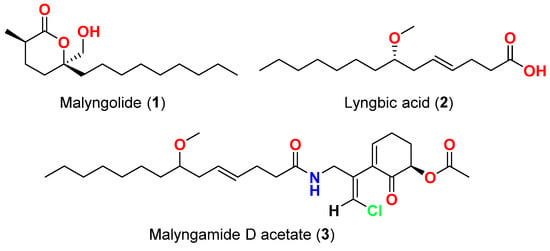
| Compound | Source Organism | Collection Site | Targeted Bacteria | MIC/Inhibition Zone/IC50 | Reference | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Malyngolide (1) | L. majuscula | Hawaii, USA | M. smegmatis, S. pyogenes, S. aureus and B. subtilis | More active against M. smegmatis and S. pyogenes than S. aureus and B. subtilis | |||||||
| Malyngolide (1) | L. majuscula | [ | Hawaii, USA | M. smegmatis, S. pyogenes, S. aureus and B. subtilis | 12] | ||||||
| More active against | M. smegmatis | and | S. pyogenes | than S. aureus and B. subtilis | [12] | Lyngbic acid (2) | M. producens | Red Sea | M. tuberculosis H37Rv | 65% inhibition at 12.5 μg/mL | [13] |
| Lyngbic acid ( | |||||||||||
| Lyngbic acid (2) | M. producens | Red Sea | M. tuberculosis H37Rv | 65% inhibition at 12.5 μg/mL | [13] | 2) | L. majuscula | Caribbean region | S. aureus and B. subtilis | Antibacterial activity | [14] |
| Lyngbic acid (2) | L. majuscula | Caribbean region | S. aureus and B. subtilis | Antibacterial activity | [14] | Malyngamide D acetate (3) | L. majuscula | Caribbean region | S. aureus | Slight activity | [14] |
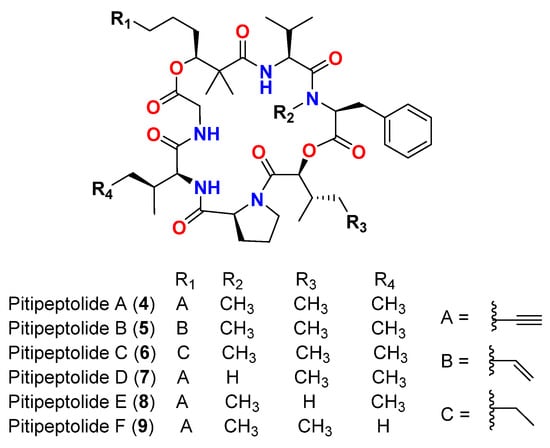
| Malyngamide D acetate (3) | L. majuscula | Caribbean region | S. aureus | Slight activity | [14] | Pitipeptolide A (4) | L. majuscula | Guam | M. tuberculosis ATCC 25177 | ||
| Pitipeptolide A (4) | 25 mm at 100 µg | L. majuscula | Guam | M. tuberculosis ATCC 25177 10 mm at 25 µg |
[15] | ||||||
| 25 mm at 100 µg | 10 mm at 25 µg | [ | 15 | ] | Pitipeptolide A (4) | L. majuscula | Guam | M. tuberculosis ATCC 35818 | 15 mm at 100 µg 9 mm at 25 µg |
[15] | |
| Pitipeptolide B ( | |||||||||||
| Pitipeptolide A (4) | L. majuscula | Guam | M. tuberculosis ATCC 35818 | 15 mm at 100 µg 9 mm at 25 µg |
[15] | 5) | L. majuscula | ||||
| Pitipeptolide B (5 | Guam | ) | L. majusculaM. tuberculosis ATCC 25177 | Guam30 mm at 100 µg |
M. tuberculosis ATCC 2517715 mm at 25 µg | 30 mm at 100 µg [15] |
|||||
| 15 mm at 25 µg | [ | 15 | ] | Pitipeptolide B (5) | L. majuscula | Guam | M. tuberculosis ATCC 35818 | 15 mm at 100 µg | |||
| Pitipeptolide B (5) | 10 mm at 25 µg | L. majuscula | [ | Guam | M. tuberculosis ATCC 3581815] | ||||||
| 15 mm at 100 µg | 10 mm at 25 µg | [ | 15 | ] | Pitipeptolide A (4) | L. majuscula | Guam | ||||
| Pitipeptolide A (4) | M | L. majuscula | . tuberculosis ATCC 25177 | Guam | M. tuberculosis ATCC 2517728 mm at 100 µg 23 mm at 50 µg 9 mm at 10 µg |
28 mm at 100 µg 23 mm at 50 µg 9 mm at 10 µg[16] |
|||||
| [ | 16 | ] | Pitipeptolide B (5) | L. majuscula | Guam | ||||||
| Pitipeptolide B (5) | M | L. majuscula | . tuberculosis ATCC 25177 | Guam | M. tuberculosis30 mm at 100 µg 24 mm at 50 µg 14 mm at 10 µg |
[16] | |||||
| ATCC 25177 | 30 mm at 100 µg | 24 mm at 50 µg | 14 mm at 10 µg |
[16] | Pitipeptolide C (6) | L. majuscula | Guam | ||||
| Pitipeptolide C (6) | M | L. majuscula | . tuberculosis ATCC 25177 | Guam | M. tuberculosis ATCC 2517726 mm at 100 µg 21 mm at 50 µg 18 mm at 10 µg |
26 mm at 100 µg 21 mm at 50 µg 18 mm at 10 µg[16] |
|||||
| [ | 16 | ] | Pitipeptolide D (7) | L. majuscula | Guam | ||||||
| Pitipeptolide D (7) | M | L. majuscula | . tuberculosis ATCC 25177 | Guam | M. tuberculosis10 mm at 100 µg 0 mm at 50 µg 0 mm at 10 µg |
[16] | |||||
| ATCC 25177 | 10 mm at 100 µg | 0 mm at 50 µg | 0 mm at 10 µg |
[16] | Pitipeptolide E (8) | L. majuscula | Guam | ||||
| Pitipeptolide E (8) | M | L. majuscula | . tuberculosis ATCC 25177 | Guam | M. tuberculosis ATCC 2517721 mm at 100 µg 15 mm at 50 µg 0 mm at 10 µg |
21 mm at 100 µg 15 mm at 50 µg 0 mm at 10 µg[16] |
|||||
| [ | 16 | ] | Pitipeptolide F (9) | L. majuscula | Guam | ||||||
| Pitipeptolide F (9) | M | L. majuscula | . tuberculosis ATCC 25177 | Guam | M. tuberculosis40 mm at 100 µg 30 mm at 50 µg 10 mm at 10 µg |
[16] | |||||
| ATCC 25177 | 40 mm at 100 µg | 30 mm at 50 µg | 10 mm at 10 µg |
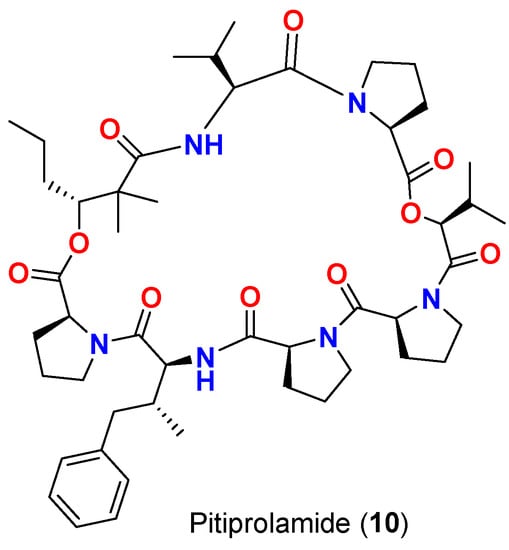
[16] | Pitiprolamide (10) | L. majuscula | Guam | M. tuberculosis ATCC 25177 | |||
| Pitiprolamide (10) | 23 mm at 100 µg | L. majuscula | 13 mm at 50 µg | Guam |
M. tuberculosis ATCC 251770 mm at 10 µg | 23 mm at 100 µg 13 mm at 50 µg[17] |
|||||
| 0 mm at 10 µg | [ | 17 | ] | Pitiprolamide (10) | L. majuscula | Guam | B. cereus ATCC 10987 | ||||
| Pitiprolamide (10) | IC | 50 | = 70 μM at 1 μM | L. majuscula | Guam | B. cereus ATCC 10987 | IC50 = 70 μM at 1 μM[17] | ||||
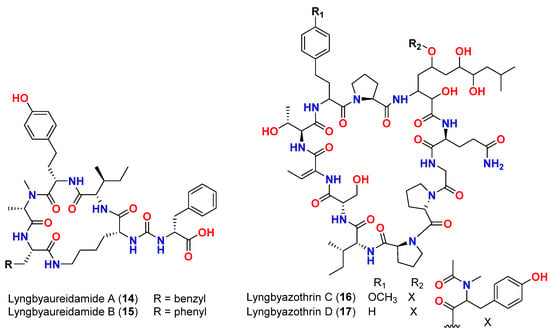
| [ | 17 | ] | Mixture of lyngbyazothrins A and B (14 and 15) | Lyngbya sp. | Germany (Culture) | M. flaVus SBUG 16 | |||||
| Mixture of lyngbyazothrins A and B (14 and | 8 mm at 100 μg/disk | [ | 18 | ] | |||||||
| 15) | Lyngbya sp. | Germany (Culture) | M. flaVus SBUG 16 | 8 mm at 100 μg/disk | [18] | Mixture of lyngbyazothrins C (16) and D (17) | |||||
| Mixture of lyngbyazothrins C (16) and D ( | Lyngbya sp. | Germany (Culture) | B. subtilis SBUG 14 E. coli ATCC 11229 E. coli SBUG 13 P. aeruginosa ATCC 27853 S. marcescens SBUG 9 |
18 mm at 25 μg/disk 18 mm at 100 μg/disk 15 mm at 100 μg/disk 8 mm at 100 μg/disk 8 mm at 200 μg/disk |
[18] | ||||||
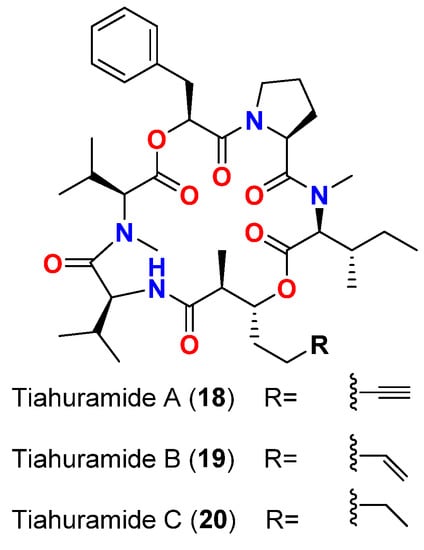
| Tiahuramide A (18) | L. majuscula | French Polynesia | A. salmonicida (CIP 103209T strain), V. anguillarum (CIP 63.36T), S. baltica (CIP 105850T), E. coli (CIP 54.8) and M. luteus (CIP A270) | MIC = 27, 33, >50, 35 and 47 μM, respectively | [19] | ||||||
| Tiahuramide B (19) | L. majuscula | French Polynesia | A. salmonicida (CIP 103209T strain), V. anguillarum (CIP 63.36T), S. baltica (CIP 105850T), E. coli (CIP 54.8) and M. luteus (CIP A270) | MIC = 9.4, 8.5, 22, 12 and 29 μM, respectively | [19] | ||||||
| Tiahuramide C (20) | L. majuscula | French Polynesia | A. salmonicida (CIP 103209T strain), V. anguillarum (CIP 63.36T), S. baltica (CIP 105850T), E. coli (CIP 54.8) and M. luteus (CIP A270) | MIC = 6.7, 7.4, 16, 14 and 17 μM, respectively | [19] |
| Compound | Source Organism | Collection Site | Targeted Bacteria | MIC/Inhibition Zone/IC50 | Reference |
|---|---|---|---|---|---|
| 17 | |||||
| ) | |||||
| Lyngbya | |||||
| sp. | |||||
| Germany (Culture) | |||||
| B. subtilis | |||||
| SBUG 14 | |||||
| E. coli | |||||
| ATCC 11229 | |||||
| E. coli | |||||
| SBUG 13 | |||||
| P. aeruginosa | ATCC 27853 | S. marcescens SBUG 9 | 18 mm at 25 μg/disk 18 mm at 100 μg/disk 15 mm at 100 μg/disk 8 mm at 100 μg/disk 8 mm at 200 μg/disk |
[18] | |
| Tiahuramide A (18) | L. majuscula | French Polynesia | A. salmonicida (CIP 103209T strain), V. anguillarum (CIP 63.36T), S. baltica (CIP 105850T), E. coli (CIP 54.8) and M. luteus (CIP A270) | MIC = 27, 33, >50, 35 and 47 μM, respectively | [19] |
| Tiahuramide B (19) | L. majuscula | French Polynesia | A. salmonicida (CIP 103209T strain), V. anguillarum (CIP 63.36T), S. baltica (CIP 105850T), E. coli (CIP 54.8) and M. luteus (CIP A270) | MIC = 9.4, 8.5, 22, 12 and 29 μM, respectively | [19] |
| Tiahuramide C (20) | L. majuscula | French Polynesia | A. salmonicida (CIP 103209T strain), V. anguillarum (CIP 63.36T), S. baltica (CIP 105850T), E. coli (CIP 54.8) and M. luteus (CIP A270) | MIC = 6.7, 7.4, 16, 14 and 17 μM, respectively | [19] |
References
- Schopf, J.W.; Packer, B.M. Early Archean (3.3-billion to 3.5-billion-yearold) microfossils from Warrawoona Group, Australia. Science 1987, 237, 70–73.
- Whitton, B.A.; Potts, M. The Ecology of Cyanobacteria: Their Diversity in Time and Space; Springer: Dordrecht, The Netherlands, 2000.
- Nagle, D.G.; Paul, V.J. Production of secondary metabolites by filamentous tropical marine cyanobacteria: Ecological functions of the compounds. J. Phycol. 1999, 35, 1412–1421.
- Berry, J.P.; Gantar, M.; Perez, M.H.; Berry, G.; Noriega, F.G. Cyanobacterial toxins as allelochemicals with potential applications as algaecides, herbicides and insecticides. Mar. Drugs 2008, 6, 117–146.
- Burja, A.M.; Banaigs, B.; Abou-Mansour, E.; Burgess, J.G.; Wright, P.C. Marine cyanobacteria—A prolific source of natural products. Tetrahedron 2001, 57, 9347–9377.
- Engene, N.; Rottacker, E.C.; Kas, J.; Byrum, T.; Gerwick, W.H.; Choi, H.; Ellisman, M.H. Moorea producens Gen. Nov., Sp. Nov. and Moorea bouillonii Comb. Nov., Tropical marine cyanobacteria rich in bioactive secondary metabolites. Int. J. Syst. Evol. Microbiol. 2012, 62 Pt 5, 1171–1178.
- Engene, N.; Tronholm, A.; Paul, V.J. Uncovering cryptic diversity of Lyngbya: The new tropical marine cyanobacterial genus Dapis (Oscillatoriales). J. Phycol. 2018, 54, 435–446.
- Jiří, K.; Eliška, Z.; Jan, Š.; Jiří, K.; Jason, W.; Brett, A.N.; Jaroslava, K. Polyphasic evaluation of Limnoraphis robusta, a water—Bloom forming cyanobacterium from Lake Atitlán, Guatemala, with a description of Limnoraphis gen. nov. J. Czech Phycol. Soc. 2013, 13, 39–52.
- Tronholm, A.; Engene, N. Moorena Gen. Nov., a valid name for “Moorea Engene & Al.” Nom. Inval. (Oscillatoriaceae, Cyanobacteria). Not. Algarum 2019, 122, 1–2.
- Mcgregor, G.B.; Sendall, B.C. Phylogeny and toxicology of Lyngbya wollei (Cyanobacteria, Oscillatoriales) from North-Eastern Australia, with description of Microseira gen. nov. J. Phycol. 2015, 51, 109–119.
- Engene, N.; Paul, V.J.; Byrum, T.; Gerwick, W.H.; Thor, A.; Ellisman, M.H. Five chemically rich species of tropical marine cyanobacteria of the genus Okeania gen. nov. (Oscillatoriales, Cyanoprokaryota). J. Phycol. 2013, 49, 1095–1106.
- Cardllina, J.H.C.; Moore, R.E.; Arnold, E.V.; Clardy, J. Structure and absolute configuration of malyngolide, an antibiotic from the marine blue-green alga Lyngbya majuscula Gomont. J. Org. Chem. 1979, 44, 4039–4042.
- Shaala, L.A.; Youssef, D.T.A.; McPhail, K.L.; Elbandy, M. Malyngamide 4, a new lipopeptide from the Red Sea marine cyanobacterium Moorea producens (Formerly Lyngbya majuscula). Phytochem. Lett. 2013, 6, 183–188.
- Gekwick, W.H.; Reyes, S.; Alvarado, B. Two malyngamides from the Caribbean cyanobacterium Lyngbya majuscula. Phytochemistry 1987, 26, 1701–1704.
- Luesch, H.; Pangilinan, R.; Yoshida, W.Y.; Moore, R.E.; Paul, V.J. Pitipeptolides A and B, new cyclodepsipeptides from the marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2001, 64, 304–307.
- Montaser, R.; Paul, V.J.; Luesch, H. Pitipeptolides C-F, antimycobacterial cyclodepsipeptides from the marine cyanobacterium Lyngbya majuscula from Guam. Phytochemistry 2011, 72, 2068–2074.
- Montaser, R.; Abboud, K.A.; Paul, V.J.; Luesch, H. Pitiprolamide, a proline-rich dolastatin 16 analogue from the marine cyanobacterium Lyngbya majuscula from Guam. J. Nat. Prod. 2011, 74, 109–112.
- Zainuddin, E.N.; Jansen, R.; Nimtz, M.; Wray, V.; Preisitsch, M.; Lalk, M.; Mundt, S. Lyngbyazothrins A-D, antimicrobial cyclic undecapeptides from the cultured cyanobacterium Lyngbya sp. J. Nat. Prod. 2009, 72, 2080.
- Levert, A.; Alvarin, R.; Bornancin, L.; Mansour, E.A.; Burja, A.M.; Genevie, A.; Bonnard, I.; Alonso, E.; Botana, L.; Banaigs, B. Structures and activities of tiahuramides A−C, cyclic depsipeptides from a Tahitian collection of the marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2018, 81, 1301–1310.
