Metal allergy is mainly an environmental disorder which can cause allergic contact dermatitis. Environmental metal exposures include jewelry, everyday metal items, mobile phones, leather, metal-rich food and implants, including stents or anchors. While consumer exposure is liable for the majority of metal hypersensitivity cases, the significance of occupational exposure to metals remains relevant. Although tThe most common metal allergens are nickel, chromium, and cobalt.
- metal hypersensitivity
- metal allergy
- allergic contact dermatitis (ACD)
- patch test
- nickel
- cobalt
- chromium
- implants
- metal sensitivity
1. Introduction
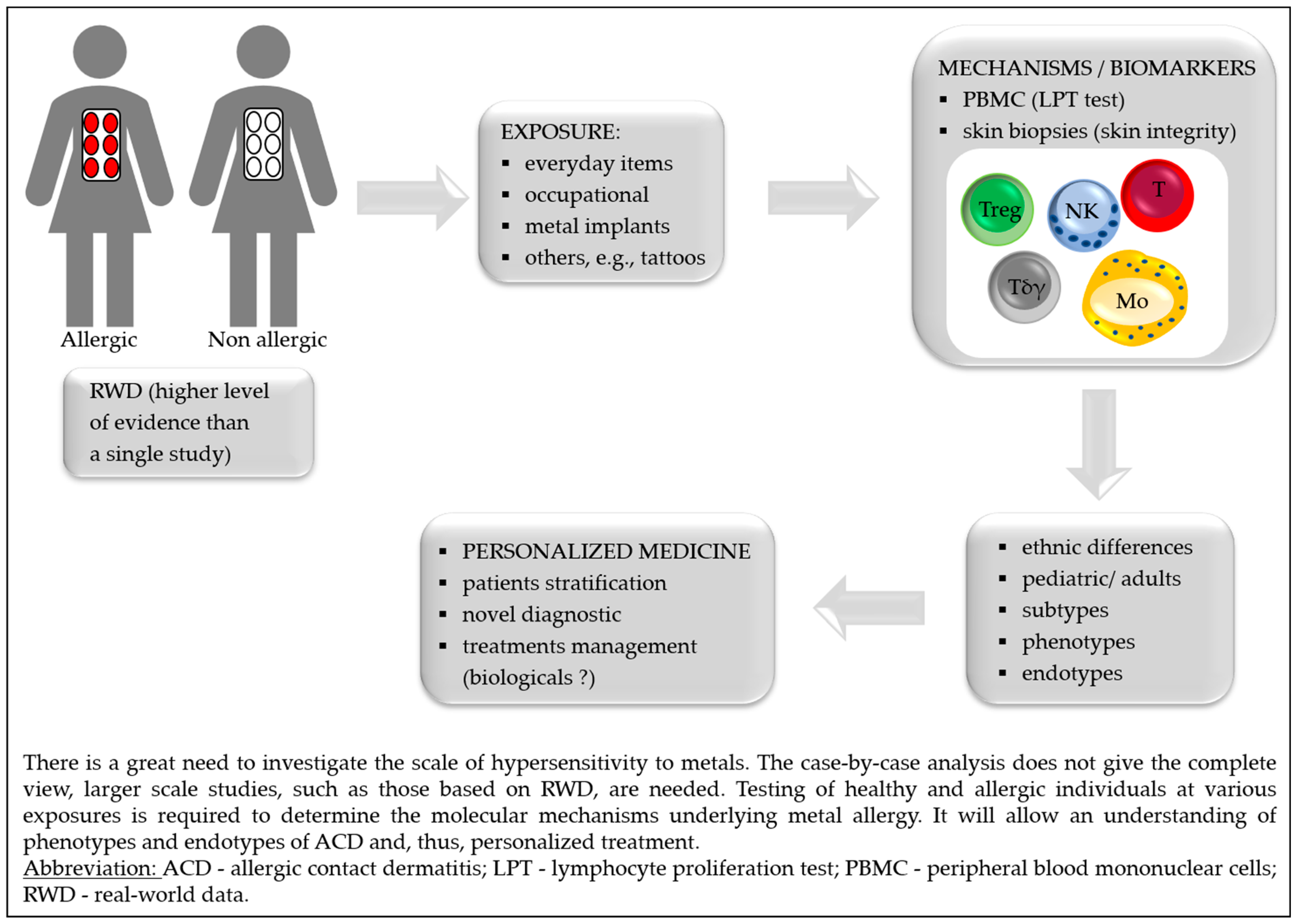
2. Exposure
Humans are constantly exposed to metal ions/salts; allergy to metals is the most prevalent contact allergy in developed societies. More information on the oral, cutaneous, and inhalational bioavailability of metals in humans under different dosing regimens and levels is needed for precise risk assessments. Studies with large numbers of sensitized and non-sensitized individuals, different dosing regimens and dose levels are urgently required. Cutaneous and inhalation exposure: Metal sensitization may cause dermatological disorders. In the Canary Islands, from 1568 patients that underwent PTs, most contact dermatitis patients were older than 40 years, and the main allergen eliciting positive reactions was Ni (36.5%) [7]. Similarly, in the group of 546 patch tested patients, the most common metal allergen in Lithuania was Ni, and women were more often sensitized [8]. In a group of 1919 children, 6% had an allergic reaction to cobalt (Co). ACD triggered by Co should be suspected with dermatitis in a diffused generalized distribution, trunk, or face [9]. Presumably due to their daily exposure to cosmetic products and jewelry, the urine Ni level was significantly higher in females than males on average. Similar occurrence was found in participants who used penetrating jewelry such as earrings and piercings (regardless of gender), compared to subjects not using such jewelry [10]. It was found that potassium dichromate, both in petrolatum and aqua can penetrate the skin, which is important for potential human exposure [11]. Occupational skin diseases appear or exacerbate regarding work and are the second most common type of occupational disease in the world. Nine welding workers had erythematous papules/patches and itching in various areas of the body. The Ni dust was in constant contact with exposed skin, and Ni level exceeded the norm. In two workers, occupational allergic contact dermatitis (OACD) to Ni was confirmed based on a PT [12]. OACD was most frequent in construction workers (45%), the mainly involved area was hands, and the most frequent allergen was chromium (Cr) in cement [13]. It was found that precious metal refinery workers are exposed to non-platinum group metals: lead (Pb), Co, Ni, copper (Cu), arsenic (As) and silver (Ag), with exceeded occupational limits of the South African [14]. The occupational metal ions released at the workplace might entail legal ramifications regarding insurance law. Oral exposure: Ni-rich food is an excellent source of exposure, mainly breakfast cereals, soy products, chocolate spreads and legumes. It demonstrates an evolution in potential risk to human health to Ni exposure due to the shift towards a more plant-based diet [15,16,17,18,19,20][15][16][17][18][19][20]. Ni-allergic contact mucositis (Ni-ACM) is a disorder where Ni-containing food can impact previously sensitized patients and can be diagnosed by a Ni oral mucosa PT (omPT) [21]. Celiac disease patients on a gluten-free diet with positive Ni-omPT displayed a recurrence of gastrointestinal and extraintestinal symptoms, although serological and histological remission has been reached. Relief of symptoms appeared after a gluten-free-low-Ni diet [22]. Irritable bowel syndrome-like disorders are also present in endometriosis. In women with a positive Ni-omTP, a low-Ni diet affected gastrointestinal, extra-intestinal, and gynecological symptoms reduction [23]. An association has also been found between gastroesophageal reflux disease and a low-Ni diet by improving symptoms [24]. Ulcerative colitis (UC) patients often had significant Ni or palladium (Pd) hypersensitivities confirmed by a PT. All subjects had metallic dental implants, implying that exposure to Ni is possible involvement in UC pathogenesis [25]. IgE-mediated reactivity to lipid transfer proteins (LTP) is a group of highly conserved proteins mainly found in fruits. They represent the leading cause of primary food allergy in adults in Mediterranean countries. The prevalence of systemic Ni allergy syndrome (SNAS) in the LTP allergic population is clinically relevant [26]. SNAS and Ni-ACD are very common among overweight/obese patients. Ni exposure leads to abnormal production/release of growth hormone (GH). In addition, Ni-allergic patients show GH-insulin-like growth factor 1 (IGF1) axis impairment, probably by increasing the inflammation in the pituitary gland [27]. Releasing metals from items: Exposure to metals and their sensitization potential is extremely difficult to assess. The exact composition of the objects we encounter is often unknown, and the composition is not uniform, making diagnosis difficult, e.g., the impact of Ni in tattoo inks is unclear. A positive PT is not sufficient to verify the reaction following tattooing. Epidemiologic case–control studies with regular biopsies of healthy and inflamed tattoos and PTs would facilitate comprehending the role of Ni in tattoo ink allergies [28]. Furthermore, the release of metal from objects is not easy to estimate. The gold standard for Ni release assessment is EN1811 test, which has reproducibility limitations [29]. The proposed alternative dimethylglyoxime (DMG) spot test has high specificity but low sensitivity, which undermines its usability, especially when Ni release is low. It was proven that Cu ions could have a masking effect, resulting in an inaccurate reading of the DMG spot test to Ni [30]. The Ni release from everyday products is widespread and often above the DMG test limits. It might be potentially dangerous for Ni-sensitive subjects [31]. Metal parts of laptops and mouses release Ni [32]. Micro-needling made from stainless steel repeatedly puncturing the skin may release Ni [33]. Ni and Co is released in allergology-relevant amounts from beauty tools [34] and metal hairdressers’ tools [35,36][35][36]. The European Union established a limit of 0.2 μg/cm2/week Ni release for the items by the Directive in 2004 [37]. The excessive Ni release from earrings was found in more than 15% of tested earrings [38[38][39],39], also Cr and Co were released [40]. Ni release depends on the solution pH; at pH 4, the release is the greatest, which is essential for the stainless-steel crown in dentistry [41]. Types of metal that can sensitize: Ni is not the only metal that can sensitize, together with Cr and Co are the most common metals that cause allergic reactions. A patient had itchy erythema confined to the bilateral antihelix. A mono-positive PT revealed gold (Au) hypersensitivity, also the headphones he was using contained Au-plated metal parts. After stopping using them, there was no recurrence of symptoms [42]. Iron (Fe) can be a relevant sensitizer, especially in complicated knee arthroscopy [43]. Aluminum (Al) salts are adjuvants found in many vaccines. Although rare, patients have reported cutaneous reactions, including ongoing pruritic subcutaneous nodules at the injection site. In most cases, delayed reactions are not contraindications to further vaccine administration. However, it should be evaluated case-by-case basis offering alternative Al-free vaccines [44,45][44][45]. Allergy from contact exposure to Al, e.g., topical medicaments and deodorants might be more common than thought (7 case reports) [46,47][46][47]. Sunscreens containing Al might lead to ACD in pediatric patients [48]. Unfortunately, Al-PT is only positive when there is a strong Al-allergy [49].3. Diagnostics
Patch tests (PTs): PTs are the gold standard for the diagnosis of allergic hypersensitivity [50]. It is known that PT readings on day seven (D7) may show additional positive reactions. Metal allergens and older age were predictive for late positive reactions. Within the tested allergens, without D7 readings, on average, 12% of sensitizations would have been missed [51,52][51][52]. Long-lasting allergic PT reactions (LLAPTR) are positive PT reactions lasting more than two weeks. A 90-year-old dental patient had a positive PT on D2 and D4 for platinum (Pt), Pd, and indium (In), but for Au the test was negative until D45 [53]. Ideally, the PTs should be performed while the patient is not taking biologics. Unfortunately, this is not always possible, so clinicians need to assess the risks and benefits of testing during therapy [54]. The precision of PTs after dupilumab is under consideration. Reactions may stay positive (with no dampening effect), change to negative (false-negative reactions), or become newly positive after its administration, indicating that patient-specific factors should be taken into consideration [55,56,57][55][56][57]. Secukinumab (anti-IL-17 mAb) showed no significant reduction in Ni-PT in patients with ACD (with confirmed Ni allergy before) [58]. When hypersensitivity to metal implant is suspected, the PT should be done for confirmation. Additionally, the identification of implant compositions should be accomplished before potential removal. The patient underwent a skin grafting that was covered with a tattoo. An allergic reaction to anchors or tattoo inks was suspected. The DMG spot test result was negative for metal devices. Finally, an inductively coupled plasma-mass spectrometry analysis revealed the release of Ni from the anchor [59]. Ag is widely used topically because of its anti-microbial properties. It should be considered to include Ag as an extension of PTs of dermatitis in subjects with skin ulcers since it is not present in some of the commercial series. Hereby, a greater number of cases of ACD to Ag could be identified earlier [60]. Ni allergen is present in different PT lines, e.g., ICDRG baseline, European or Swedish baseline, but it occurs as Ni sulfate 200 μg/cm2 in TRUE Test, or Ni sulfate 2.5% petrolatum (pet.) and 5% pet. The reactions vary, and most positive reactions were found in 5% pet., TRUE Test and 2.5% pet. shown similar responses [61]. In vitro tests: The in vitro lymphocyte proliferation test (LPT) can be an additional method in case the skin is not the primary organ of exposure. The classical LPT uses tritiated thymidine, so a radioactive-free LPT test with carboxyfluorescein succinimidyl ester (CFSE) was proposed, which furthermore can distinguish T cell subsets and assess the released cytokines [62,63][62][63]. However, the read-out method can affect the sensitivity of the LPT; it was confirmed that ELISA or flow cytometry provides the best detection of sensitization in the context of allergies [64]. In the culture of PBMC performed by LPT, the metal-reactive (Cr, Ni, and Co) T helper lymphocytes (Th cells) with high CD45RO expression and co-expression of cutaneous lymphocyte-associated antigen (CLA) and C-C Motif Chemokine Receptor 6 (CCR6) were identified. Th cells identified individuals with a positive Ni-PT with 100% sensitivity and 92% specificity [65]. Other tests: Urine Ni concentration is an effective predictor of Ni exposure but not of an allergy. The urine Ni concentrations were not statistically different in allergic patients with Ni-positive PT compared to negative controls. However, the urine level of Ni correlated with lifestyle [10].4. Mechanisms and Biomarkers
The discovery of the molecular mechanisms by which contact allergens cause skin sensitization has a potential implication for treatment decisions. The biomarkers might facilitate the diagnosis of metal hypersensitivities and enable patient stratification for potential treatment strategies [66]. Key advances in the understanding of metal allergy mechanisms and biomarkers are presented in Table 1.Outcomes | Exposition to Metal | Ref. | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
↑ metal-specific CD154+ CD4+ Tmem | (overexpression of TRAV9-2 and CDR3 histidine) | Allergic and non-allergic subjects stimulated with Ni, | Co or Pd (PBMC) | ||||||||
↑ IL-5 | Ni-allergic patients (PBMC) |
Abbreviations: ↑ = increase; ↓ = decrease; + = positive; CCL = C-C motif ligand; CD = cluster of differentiation; CDR3 = complementarity determining region 3; CLDN = claudin; Co = cobalt; Cr = chromium; CXCL = C-X-C motif chemokine ligand; DC = dendritic cell; FA2H = fatty acid 2-hydroxylase; FABP7 = fatty acid binding protein 7, brain; FLG = filaggrin; FOXP3 = forkhead box P3; IL = interleukin; LAMP = lysosomal associated membrane glycoprotein; LCEs = late cornified envelope proteins; LOR = loricrin; M1/M2 = proinflammatory/anti-inflammatory macrophages; MAG/DAG = mono/diacylglycerols; MAPK = mitogen-activated protein kinases; MBP = myelin basic protein; Ni = nickel; NK = natural killer cells; OLP = oral lichen planus; PBMC = peripheral blood mononuclear cells; Pd = palladium; RHS = reconstructed human skin; SBNS = suprabasin; Sema3A = semaphorin; Tmem = memory lymphocytes T; TNF-α = tumor necrosis factor-alpha; TRAV9-2 = α-chain V-segment; TSLP = thymic stromal lymphopoietin; Tγδ = T gamma-delta cells.
5. Implants
Metal that Caused Hypersensitivity/Implant Type | Symptoms | Ref. | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Multiple metals/orthopedic implants: hip, knee shoulder joint | Different; the most common—delayed wound healing | and/or recurrent wound issues after the implantation, | joint failure or loosening | |||||||||||||
[ | ||||||||||||||||
Ni/endovascular implants (stents) | In-stent restenosis or prominent eczematous reaction overlying | 69] |
||||||||||||||
the endovascular implant, eosinophilia | Ni-allergy patients, differentiation between independent Ni or cross-reactivity of Ni/Pd allergy (PBMC) |
[71] | ||||||||||||||
Co, Ni/drug-eluting stents | [70] |
|||||||||||||||
Pruritic rash with hypereosinophilia |
[108] |
[89] |
Cross reactivity between Ni/Cr and Pd | Sensitization with Ni or Cr, challenge with Pd | (mice model) |
[70] |
[71] |
|||||||||
Au and Pd/dental implant | Oral lichenoid contact lesion (OLCLs) |
[103] |
[90] |
Skin barrier defects: ↓ terminal differentiation—FLG, FLG2, LOR, LCEs, tight junction—CLDN1/CLDN8, lipid metabolism—FA2H, FABP7 | Biopsies from healthy subjects after Ni-topical application | |||||||||||
Ti/dental implants | Rash, urticaria, pruritus, redness, dermatitis and facial eczema, pain, hyperaemia of soft tissues, swelling in submental and labial sulcus, gingival hyperplasia acne-like facial inflammation |
[73] |
[72] |
|||||||||||||
[ | 105] |
[91] |
Cellular infiltrates: ↑ CD3+ T, | CD11c + myeloid DC, DC-LAMP+ mature DC, | MBP + eosinophils, FOXP3+ Treg | |||||||||||
Ni/metal anchors | Erythematous and vesicular lesions around the grafted tattoo skin, but the tattoo was not affected (placed with clips or anchors) |
[59] |
↑ M1, mast cells, neutrophils, NK, | CD4 + Tmem, CD8+ T | Biopsies from Ni-allergic patients | |||||||||||
Ni/stainless-steel skull pins | Erythema on sites of the head where the skull pins inserted |
[74] |
[73] |
|||||||||||||
[ | 109] |
[92] |
↓ M2, resting mast cells, Tγδ, Treg | |||||||||||||
Ti/cervical implant | Persistent refractory neck pain; subsequently, after eight years, | a planter rush |
[110] |
[93] |
↓ SBSN | Ni-allergic patients (serum) | ||||||||||
Ti/metal clips for cholecystectomy | Right upper quadrant pain, diarrhea, and nausea | [75] |
[74] |
|||||||||||||
[ | 111] |
[94] |
↑ Sema3A (activates MAPK and TNF-α) | Ni-induced allergy (mouse ear tissue) | ||||||||||||
Low-grade fever, nausea, vomiting, joint pain, | bloody diarrhea |
[112] |
[95] |
[76] |
[75] |
|||||||||||
↑ TSLP in keratinocytes | and TNF-α in epithelium | |||||||||||||||
Co, Ni, Hg/metal clips for cholecystectomy | OLPs metal-allergy patients | Myalgia, joint pain and tenderness, mental fogginess, mild forgetfulness, irritable bowel syndrome, stomach cramps, | dry skin and hair, hair loss |
[77] |
[76] |
|||||||||||
[ | 113] |
[96] |
↑ IL-6, CXCL8, CCL2, CCL5, and CCL20 | |||||||||||||
Cu/intrauterine device | RHS exposure to Ni and Streptococcus mitis exposure | Cutaneous eruption |
[78] |
[77] |
||||||||||||
[ | 114] |
[97] |
Lipid profile: ↑ cholesterol, DAG, MAG | Non-allergic skin exposed to Co |
[79] |
[78] |
||||||||||
Multiple-metals/dental implant | Palmoplantar pustulosis (PPP) and periodontitis | Lipid profile: ↑ DAG | ||||||||||||||
Au/dental implants | Non-allergic skin exposed to Cr | |||||||||||||||
Oral lesions with characteristic Wickham’s striae |
[116] |
[100] |
Lipid profile: ↓ DAG and MAG | |||||||||||||
Ti/temporary tissue expander | Non-allergic skin exposed to Ni | |||||||||||||||
Well-demarcated, erythematous plaque over the left breast reconstructive breast surgery |
[117] |
[101] |
LC emigration of the epidermis | (in an IL-10 but not IL-1B dependent way) | RHS exposure to Ti |
[80] |
[79] |
Abbreviations: Au = gold; Co = cobalt; Cu = copper, Hg = mercury; Ni = nickel; Pd = palladium; Ti = titanium.
References
- Johansen, J.D.; Bonefeld, C.M.; Schwensen, J.F.B.; Thyssen, J.P.; Uter, W. Novel insights into contact dermatitis. J. Allergy Clin. Immunol. 2022, 149, 1162–1171.
- Luger, T.; Amagai, M.; Dreno, B.; Dagnelie, M.-A.; Liao, W.; Kabashima, K.; Schikowski, T.; Proksch, E.; Elias, P.M.; Simon, M.; et al. Atopic dermatitis: Role of the skin barrier, environment, microbiome, and therapeutic agents. J. Dermatol. Sci. 2021, 102, 142–157.
- Fonacier, L.; Frankel, D.; Mawhirt, S. Contact allergens for the allergist. Ann. Allergy Asthma Immunol. 2022, 128, 629–644.
- Agache, I.; Akdis, C.A. Precision medicine and phenotypes, endotypes, genotypes, regiotypes, and theratypes of allergic diseases. J. Clin. Investig. 2019, 129, 1493–1503.
- Tokura, Y.; Hayano, S. Subtypes of atopic dermatitis: From phenotype to endotype. Allergol. Int. 2022, 71, 14–24.
- Wang, T.; Yin, L.; Ma, Z.; Zhang, Y. Chlorogenic Acid-Loaded Mesoporous Silica Nanoparticles Modified with Hexa-Histidine Peptides Reduce Skin Allergies by Capturing Nickel. Molecules 2022, 27, 1430.
- Quintana, B.R.; Hernández, A.F.; Guedes, A.S.; Borrego, L. Contact Dermatitis to Allergens in the Spanish Standard Series: PT Findings in the South of Gran Canaria. Actas Dermosifiliogr. 2022, 113, T555–T562.
- Linauskienė, K.; Malinauskienė, L.; Blažienė, A. Metals Are Important Contact Sensitizers: An Experience from Lithuania. BioMed Res. Int. 2017, 2017, 3964041.
- Silverberg, J.I.; Patel, N.; Warshaw, E.M.; Maibach, H.I.; Belsito, D.V.; DeKoven, J.G.; Zug, K.A.; Taylor, J.S.; Sasseville, D.; DeLeo, V.A.; et al. Patch testing with cobalt in children and adolescents: North American contact dermatitis group experience, 2001–2018. Contact Dermat. 2022, 87, 420–429.
- Mercan, S.; Vehid, H.; Semen, S.; Celik, U.; Yayla, M.; Engin, B. An ICP-MS Study for Quantitation of Nickel and Other Inorganic Elements in Urine Samples: Correlation of Patch Test Results with Lifestyle Habits. Biol. Trace Element Res. 2021, 200, 49–58.
- Linauskiene, K.; Dahlin, J.; Ezerinskis, Z.; Isaksson, M.; Sapolaite, J.; Malinauskiene, L. The Penetration of Chromium: An Up-To-Date 0.5% Potassium Dichromate Vehicle Comparison. Dermatitis 2022, 33, 368–372.
- Kim, D.; Kim, A.R.; Kim, H.; Lee, S.; Seo, B.; Suh, H.S.; Sim, C.S.; Lee, H.; Yoo, C. Nickel dust-induced occupational contact dermatitis by welding and grinding work in shipyard workers: A report of nine cases. Ann. Occup. Environ. Med. 2022, 34, e7.
- Özkaya, E.; Aslan, M.S.E. Occupational allergic contact dermatitis: A 24-year, retrospective cohort study from Turkey. Contact Dermat. 2021, 85, 503–513.
- Linde, S.J.L.; Franken, A.; du Plessis, J.L. Skin and respiratory exposure to soluble lead, cobalt, nickel, copper, arsenic and silver at two South African precious metals refineries. Int. Arch. Occup. Environ. Health 2022, 1–12.
- Babaahmadifooladi, M.; Jacxsens, L.; De Meulenaer, B.; Du Laing, G. Nickel in foods sampled on the Belgian market: Identification of potential contamination sources. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2020, 37, 607–621.
- Babaahmadifooladi, M.; Jacxsens, L. Chronic dietary exposure to nickel from selected foods consumed in Belgium. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2021, 38, 95–112.
- Cubadda, F.; Iacoponi, F.; Ferraris, F.; D’Amato, M.; Aureli, F.; Raggi, A.; Sette, S.; Turrini, A.; Mantovani, A. Dietary exposure of the Italian population to nickel: The national Total Diet Study. Food Chem. Toxicol. 2020, 146, 111813.
- Mania, M.; Rebeniak, M.; Orshulyak, O.; Postupolski, J. Assessment of exposure to nickel intake with selected cereal grains and cereal-based products. Rocz. Panstw. Zakl. Hig. 2020, 71, 371–376.
- EFSA Panel on Contaminants in the Food Chain (CONTAM); Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; Del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.; Leblanc, J.; et al. Update of the risk assessment of nickel in food and drinking water. EFSA J. 2020, 18, e06268.
- Pearson, A.J.; Ashmore, E. Risk assessment of antimony, barium, beryllium, boron, bromine, lithium, nickel, strontium, thallium and uranium concentrations in the New Zealand diet. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2020, 37, 451–464.
- Picarelli, A.; Greco, N.; Sciuttini, F.; Marini, C.; Meacci, A. High consumption of Nickel-containing foods and IBS-like disorders: Late events in a gluten-free diet. Ecotoxicol. Environ. Saf. 2021, 222, 112492.
- Borghini, R.; De Amicis, N.; Bella, A.; Greco, N.; Donato, G.; Picarelli, A. Beneficial Effects of a Low-Nickel Diet on Relapsing IBS-Like and Extraintestinal Symptoms of Celiac Patients during a Proper Gluten-Free Diet: Nickel Allergic Contact Mucositis in Suspected Non-Responsive Celiac Disease. Nutrients 2020, 12, 2277.
- Borghini, R.; Porpora, M.G.; Casale, R.; Marino, M.; Palmieri, E.; Greco, N.; Donato, G.; Picarelli, A. Irritable Bowel Syndrome-Like Disorders in Endometriosis: Prevalence of Nickel Sensitivity and Effects of a Low-Nickel Diet. An Open-Label Pilot Study. Nutrients 2020, 12, 341.
- Yousaf, A.; Hagen, R.; Mitchell, M.; Ghareeb, E.; Fang, W.; Correa, R.; Zinn, Z.; Gayam, S. The effect of a low-nickel diet and nickel sensitization on gastroesophageal reflux disease: A pilot study. Indian J. Gastroenterol. 2021, 40, 137–143.
- Kageyama, Y.; Shimokawa, Y.; Kawauchi, K.; Morimoto, M.; Aida, K.; Akiyama, T.; Nakamura, T. Higher Prevalence of Nickel and Palladium Hypersensitivity in Patients with Ulcerative Colitis. Int. Arch. Allergy Immunol. 2020, 181, 456–461.
- Rizzi, A.; Chini, R.; Inchingolo, R.; Carusi, V.; Pandolfi, F.; Gasbarrini, A.; Nucera, E. Nickel allergy in lipid transfer protein sensitized patients: Prevalence and clinical features. Int. J. Immunopathol. Pharmacol. 2020, 34, 2058738420974895.
- Risi, R.; Masieri, S.; Poggiogalle, E.; Watanabe, M.; Caputi, A.; Tozzi, R.; Gangitano, E.; Masi, D.; Mariani, S.; Gnessi, L.; et al. Nickel Sensitivity Is Associated with GH-IGF1 Axis Impairment and Pituitary Abnormalities on MRI in Overweight and Obese Subjects. Int. J. Mol. Sci. 2020, 21, 9733.
- Kluger, N. Nickel and tattoos: Where are we? Contact Dermat. 2021, 85, 136–140.
- Blaser, P.; Rothmund, B.; Schmid, P.; Stadler, R.; Gemperle, C.; McCombie, G. Nickel release from metal items in contact with skin: A comparison of methods and practical implications for regulation in Europe. J. Environ. Sci. Health Part A Tox/Hazard. Subst. Environ. Eng. 2022, 57, 45–51.
- Wennervaldt, M.; Ahlström, M.G.; Menné, T.; Thyssen, J.P.; Johansen, J.D. Copper release from metals may mask positive nickel spot test results. Contact Dermat. 2022, 86, 431–433.
- Pavesi, T.; Moreira, J.C. A comprehensive study of nickel levels in everyday items in Brazil. Contact Dermat. 2020, 83, 88–93.
- Nayak, S.U.K.; Amala, D.; Shenoi, S.D. Nickel release from laptop detected by dimethylglyoxime (DMG) test. Indian J. Dermatol. 2021, 66, 696–697.
- Margulies, S.; Samia, A.M.; Montañez-Wiscovich, M.; Saikaly, S.K. Microneedling in the nickel-allergic patient. JAAD Int. 2022, 9, 48–49.
- Symanzik, C.; Uter, W.; Becker, S.; Skudlik, C.; John, S.M. Nickel and cobalt release from beauty tools: A field study in the German cosmetics trade. Contact Dermat. 2022, 87, 162–169.
- Symanzik, C.; Skudlik, C.; John, S.M. Nickel and cobalt: Underestimated contact allergens in hairdressers? Allergol. Sel. 2022, 6, 98–103.
- Symanzik, C.; Skudlik, C.; John, S.M. Experimental evaluation of nickel and cobalt release from tools and self-reported prevalence of nickel and cobalt allergy in the German hairdressing trade. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 965–972.
- Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2004:301:0051:0052:EN:PDF (accessed on 14 October 2022).
- Wennervaldt, M.; Ahlström, M.G.; Menné, T.; Thyssen, J.P.; Johansen, J.D. Nickel release from metallic earrings: A survey of the Danish market and validation of the nickel spot test. Contact Dermat. 2021, 85, 178–185.
- Mercan, S. A Comprehensive Artificial Sweat Study for Quantitation of Nickel and Other Inorganic Elements Released from Imitation Earrings Purchased in Istanbul Market. Biol. Trace Element Res. 2020, 194, 303–312.
- Wennervaldt, M.; Ahlström, M.G.; Menné, T.; Haulrig, M.B.; Alinaghi, F.; Thyssen, J.P.; Johansen, J.D. Chromium and cobalt release from metallic earrings from the Danish market. Contact Dermat. 2021, 85, 523–530.
- Saxena, S.; Tiwari, S. Effects of pH and time on Nickel Ion release from pediatric stainless-steel crowns: An In-Vitro Comparative Study. J. Pharm. Bioallied Sci. 2022, 14 (Suppl. S1), S545–S549.
- Hayakawa, M.; Suzuki, C.; Zhu, Y.; Anzai, H. Allergic contact dermatitis to gold in the parts of in-ear headphones. Contact Dermat. 2022, 86, 328–330.
- Oppel, E.; Kapp, F.; Böhm, A.S.; Pohl, R.; Thomas, P.; Summer, B. Contact sensitization to iron: A potentially underestimated metal allergen and elicitor of complications in patients with metal implants. Contact Dermat. 2022, 86, 531–538.
- Mistry, B.D.; DeKoven, J.G. Widespread cutaneous eruption after aluminum-containing vaccination: A case report and review of current literature. Pediatr. Dermatol. 2021, 38, 872–874.
- Aquino, M.R.; Bingemann, T.A.; Nanda, A.; Maples, K.M. Delayed allergic skin reactions to vaccines. Allergy Asthma Proc. 2022, 43, 20–29.
- Hoffmann, S.S.; Wennervaldt, M.; Alinaghi, F.; Simonsen, A.B.; Johansen, J.D. Aluminium contact allergy without vaccination granulomas: A systematic review and meta-analysis. Contact Dermat. 2021.
- Novack, D.E.; Yu, J.; Adler, B.L. Aluminum: The 2022 American Contact Dermatitis Society Allergen of the Year. Cutis 2022, 110, 21–24.
- Hoffmann, S.S.; Elberling, J.; Thyssen, J.P.; Hansen, K.S.; Johansen, J.D. Does aluminium in sunscreens cause dermatitis in children with aluminium contact allergy: A repeated open application test study. Contact Dermat. 2021, 86, 9–14.
- Bruze, M.; Netterlid, E.; Siemund, I. Aluminum—Allergen of the Year 2022. Dermatitis 2022, 33, 10–15.
- Fonacier, L.; Noor, I. Contact dermatitis and patch testing for the allergist. Ann. Allergy Asthma Immunol. 2018, 120, 592–598.
- Tupker, R.A.; Stapper, W.G.C.; Kelder, J.C. Predictive factors for Day 7 positive patch test readings at a secondary referral centre. Ski. Health Dis. 2021, 2, e79.
- Forkel, S.; Schubert, S.; Dickel, H.; Gina, M.; Schröder-Kraft, C.; Vieluf, D.; Brans, R.; Kreft, B.; Wurpts, G.; Geier, J.; et al. The benefit of late readings in patch testing depends both on allergen and patient characteristics. Allergy 2022, 77, 1477–1485.
- Kato, M.; Oiso, N.; Yanagihara, S.; Kawada, A. Long-lasting allergic patch test reactions to dental metal allergens in a patient with palmoplantar pustulosis and pustulotic arthro-osteitis. J. Dermatol. 2020, 47, e324–e325.
- Mufti, A.; Lu, J.D.; Sachdeva, M.; Zaaroura, H.; Kashetsky, N.; Yeung, J.; Maibach, H.I.; DeKoven, J. Patch Testing During Immunosuppressive Therapy: A Systematic Review. Dermatitis 2021, 32, 365–374.
- de Wijs, L.E.M.; van der Waa, J.D.; Nijsten, T.; Silverberg, J.I.; Kunkeler, A.C.M.; Hijnen, D.J. Effects of dupilumab treatment on patch test reactions: A retrospective evaluation. Clin. Exp. Allergy 2021, 51, 959–967.
- Raffi, J.; Suresh, R.; Botto, N.; Murase, J.E. The impact of dupilumab on patch testing and the prevalence of comorbid allergic contact dermatitis in recalcitrant atopic dermatitis: A retrospective chart review. J. Am. Acad. Dermatol. 2020, 82, 132–138.
- Johnson, H.; Adler, B.L.; Yu, J. Dupilumab for Allergic Contact Dermatitis: An Overview of Its Use and Impact on patch testing. Cutis 2022, 109, 265–267.
- Todberg, T.; Zachariae, C.; Krustrup, D.; Skov, L. The effect of anti-IL-17 treatment on the reaction to a nickel patch test in patients with allergic contact dermatitis. Int. J. Dermatol. 2019, 58, e58–e61.
- Marcant, P.; Alcaraz, I.; Beauval, N.; de Lassalle, E.M.; Chantelot, C.; Staumont-Sallé, D. Metal implant allergy: A diagnostic challenge illustrating the limits of the nickel spot test. Contact Dermat. 2021, 85, 251–253.
- Rodriguez, R.L.; Bujan, J.G. Silver: An underdiagnosed allergen? Contact Dermat. 2021, 84, 464–466.
- Bach, R.O.; Svendsen, M.T.; Mose, K.F.; Bruze, M.; Svedman, C.; Andersen, K.E. A comparison of patch testing with nickel sulfate in TRUE Test and in petrolatum at 2.5% and 5% concentrations. Contact Dermat. 2022, 86, 233–234.
- De Graaf, N.P.J.; Bontkes, H.J.; Roffel, S.; Kleverlaan, C.J.; Rustemeyer, T.; Gibbs, S.; Feilzer, A.J. Non–heat inactivated autologous serum increases accuracy of in vitro CFSE lymphocyte proliferation test (LPT) for nickel. Clin. Exp. Allergy 2020, 50, 722–732.
- Sachs, B.; Fatangare, A.; Sickmann, A.; Glässner, A. Lymphocyte transformation test: History and current approaches. J. Immunol. Methods 2021, 493, 113036.
- Glässner, A.; Dubrall, D.; Weinhold, L.; Schmid, M.; Sachs, B. Lymphocyte transformation test for drug allergy detection: When does it work? Ann. Allergy Asthma Immunol. 2022, 129, 497–506.e3.
- Blom, L.H.; Elrefaii, S.A.; Zachariae, C.; Thyssen, J.P.; Poulsen, L.K.; Johansen, J.D. Memory T helper cells identify patients with nickel, cobalt, and chromium metal allergy. Contact Dermat. 2021, 85, 7–16.
- Renert-Yuval, Y.; Thyssen, J.P.; Bissonnette, R.; Bieber, T.; Kabashima, K.; Hijnen, D.; Guttman-Yassky, E. Biomarkers in atopic dermatitis—A review on behalf of the International Eczema Council. J. Allergy Clin. Immunol. 2021, 147, 1174–1190.e1.
- Aparicio-Soto, M.; Riedel, F.; Leddermann, M.; Bacher, P.; Scheffold, A.; Kuhl, H.; Timmermann, B.; Chudakov, D.M.; Molin, S.; Worm, M.; et al. TCRs with segment TRAV9-2 or a CDR3 histidine are overrepresented among nickel-specific CD4+ T cells. Allergy 2020, 75, 2574–2586.
- Riedel, F.; Aparicio-Soto, M.; Curato, C.; Münch, L.; Abbas, A.; Thierse, H.; Peitsch, W.K.; Luch, A.; Siewert, K. Unique and common TCR repertoire features of Ni2+-, Co2+-, and Pd2+-specific human CD154+ CD4+ T cells. Allergy 2022.
- De Graaf, N.P.J.; Roffel, S.; Gibbs, S.; Kleverlaan, C.J.; Gonzalez, M.L.; Rustemeyer, T.; Feilzer, A.J.; Bontkes, H.J. Nickel allergy is associated with a broad spectrum cytokine response. Contact Dermat. 2022.
- Kapp, F.; Summer, B.; Thomas, P. Usefulness of lymphocyte transformation test and in vitro cytokine release in differentiating between independent and cross-reacting nickel/palladium allergy. Immun. Inflamm. Dis. 2020, 8, 483–492.
- Shigematsu, H.; Kumagai, K.; Suzuki, M.; Eguchi, T.; Matsubara, R.; Nakasone, Y.; Nasu, K.; Yoshizawa, T.; Ichikawa, H.; Mori, T.; et al. Cross-Reactivity of Palladium in a Murine Model of Metal-induced Allergic Contact Dermatitis. Int. J. Mol. Sci. 2020, 21, 4061.
- Pavel, A.B.; Del Duca, E.; Cheng, J.; Wu, J.; Ungar, B.; Estrada, Y.D.; Jack, C.; Maari, C.; Proulx, E.S.-C.; Ramirez-Valle, F.; et al. Delayed type hypersensitivity reactions to various allergens may differently model inflammatory skin diseases. Allergy 2022.
- Wisgrill, L.; Werner, P.; Jalonen, E.; Berger, A.; Lauerma, A.; Alenius, H.; Fyhrquist, N. Integrative transcriptome analysis deciphers mechanisms of nickel contact dermatitis. Allergy 2021, 76, 804–815.
- Nakazawa, S.; Phadungsakswasdi, P.; Kageyama, H.; Fukuchi, K.; Shimauchi, T.; Fujiyama, T.; Ito, T.; Honda, T. Decreased serum level of suprabasin in patients with nickel allergy. J. Dermatol. 2022, 49, e189–e190.
- Liu, L.; Watanabe, M.; Minami, N.; Yunizar, M.F.; Ichikawa, T. Semaphorin 3A: A potential target for prevention and treatment of nickel allergy. Commun. Biol. 2022, 5, 671.
- Yunizar, M.F.; Watanabe, M.; Liu, L.; Minami, N.; Ichikawa, T. Metal Allergy Mediates the Development of Oral Lichen Planus via TSLP-TSLPR Signaling. J. Clin. Med. 2022, 11, 519.
- Shang, L.; Deng, D.; Roffel, S.; Gibbs, S. Differential influence of Streptococcus mitis on host response to metals in reconstructed human skin and oral mucosa. Contact Dermat. 2020, 83, 347–360.
- Knox, S.; Hagvall, L.; Malmberg, P.; O’Boyle, N.M. Topical Application of Metal Allergens Induces Changes to Lipid Composition of Human Skin. Front. Toxicol. 2022, 4, 867163.
- Neves, C.T.R.; Spiekstra, S.W.; de Graaf, N.P.; Rustemeyer, T.; Feilzer, A.J.; Kleverlaan, C.J.; Gibbs, S. Titanium salts tested in reconstructed human skin with integrated MUTZ-3-derived Langerhans cells show an irritant rather than a sensitizing potential. Contact Dermat. 2020, 83, 337–346.
- Schalock, P.C.; Thyssen, J.P. Patch Testers’ Opinions Regarding Diagnostic Criteria for Metal Hypersensitivity Reactions to Metallic Implants. Dermatitis 2013, 24, 183–185.
- Shahid, M.; Vassileva, S.; Drenovska, K. Nickel and Skin: From Allergy to Autoimmunity. Endocr. Metab. Immune Disord.-Drug Targets 2020, 20, 1032–1040.
- Peacock, C.J.H.; Fu, H.; Asopa, V.; Clement, N.D.; Kader, D.; Sochart, D.H. The effect of Nickel hypersensitivity on the outcome of total knee arthroplasty and the value of skin patch testing: A systematic review. Arthroplasty 2022, 4, 40.
- Baumann, C.A.; Crist, B.D. Nickel allergy to orthopaedic implants: A review and case series. J. Clin. Orthop. Trauma 2020, 11 (Suppl. S4), S596–S603.
- Yang, S.; Choi, E.; Ng, Y.H. Cutaneous Metal Hypersensitivity Reaction. Case Rep. Dermatol. 2022, 14, 61–65.
- Matar, H.E.; Porter, P.J.; Porter, M.L. Metal allergy in primary and revision total knee arthroplasty. Bone Jt. Open 2021, 2, 785–795.
- Lamb, L.; Dass, R.; Dass, K. M311 An unusual presentation of metal hypersensitivity symptom recurrence during omalizumab treatment successfully treated with dupilumab. Ann. Allergy Asthma Immunol. 2021, 127, S128–S129.
- Guéroult, A.M.; Al-Balah, A.; Davies, A.H.; Shalhoub, J. Nickel hypersensitivity and endovascular devices: A systematic review and meta-analysis. Heart 2021, 108, 1707–1715.
- Joshi, S.R.; Khan, D.A. Effective Use of Dupilumab in Managing Systemic Allergic Contact Dermatitis. Dermatitis 2018, 29, 282–284.
- Faybusovich, P.; Lim, J.; Ioffreda, M.D.; Al-Shaikhly, T. Mepolizumab for treating systemic allergic dermatitis with hypereosinophilia likely secondary to a nickel/cobalt-containing coronary artery stent. Contact Dermat. 2022, 86, 123–125.
- Tsushima, F.; Sakurai, J.; Shimizu, R.; Kayamori, K.; Harada, H. Oral lichenoid contact lesions related to dental metal allergy may resolve after allergen removal. J. Dent. Sci. 2022, 17, 1300–1306.
- Comino-Garayoa, R.; Brinkmann, J.C.-B.; Peláez, J.; López-Suárez, C.; Martínez-González, J.M.; Suárez, M.J. Allergies to Titanium Dental Implants: What Do We Really Know about Them? A Scoping Review. Biology 2020, 9, 404.
- Matsudate, Y. Case of allergic contact dermatitis due to nickel contained in stainless steel skull pins. J. Dermatol. 2022, 49, e307–e308.
- Aoyama, R.; Anazawa, U.; Hotta, H.; Watanabe, I.; Takahashi, Y.; Matsumoto, S. Cervical Implant Allergy With Chronic Neck Pain: A Case Report. Cureus 2022, 14, e28293.
- Yabit, F.; Hughes, L.; Sylvester, B.; Tiesenga, F. Hypersensitivity Reaction Post Laparoscopic Cholecystectomy Due to Retained Titanium Clips. Cureus 2022, 14, e26167.
- Jain, M.S.; Lingarajah, S.; Luvsannyam, E.; Somagutta, M.R.; Jagani, R.P.; Sanni, J.; Ebose, E.; Tiesenga, F.M.; Jorge, J.M. Delayed Titanium Hypersensitivity and Retained Foreign Body Causing Late Abdominal Complications. Case Rep. Surg. 2021, 2021, 5515401.
- Shah, R.N.; Tiesenga, F.; Jorge, J.; Chaudhry, A.F. Surgical clips metal allergy postlaparoscopic cholecystectomy. Int. J. Surgery Glob. Health 2020, 4, e48.
- Gara, S.; Litaiem, N.; Bacha, T.; Jones, M.; Houas, A.; Zeglaoui, F. Systemic allergic dermatitis caused by a copper-containing intra-uterine device. Contact Dermat. 2021, 84, 132–134.
- Takaoka, Y.; Akiba, Y.; Nagasawa, M.; Ito, A.; Masui, Y.; Akiba, N.; Eguchi, K.; Miyazawa, H.; Tabeta, K.; Uoshima, K. The relationship between dental metal allergy, periodontitis, and palmoplantar pustulosis: An observational study. J. Prosthodont. Res. 2022, 66, 438–444.
- Nagura, S.; Sakai, M.; Obi, H.; Fukahara, K. Aortic valve replacement in a patient with self-reported systemic multiple metal allergy. Gen. Thorac. Cardiovasc. Surg. 2021, 70, 79–82.
- Rasul, T.F.; Anderson, J.; Bergholz, D.R.; Faiz, A.; Prasad, R.R. Gold Dental Implant-Induced Oral Lichen Planus. Cureus 2022, 14, e21852.
- Buonomo, M.; Ruggiero, J.L.; Hylwa, S. Titanium allergy as a likely cause of post-reconstruction dermatitis of the breast. Contact Dermat. 2022, 86, 142–143.
