Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Magdalena Regel-Rosocka and Version 2 by Conner Chen.
Chromium compounds are used in many chemical processes as industrial catalysts and pigments for glass, porcelain glazes (bright green, yellow, red, and orange). Approximately 90% of all leather is tanned with chrome, and toxic waste tannery effluents are generated.
- chromium(III) removal
- industrial effluents
- chromium(III) applications
- tannery effluents
1. Introduction
The issue of minimizing waste generation and reducing its harmful potential for the environment has become a permanent feature of new industrial solutions. Research focused on this aspect shows a wide range of possibilities, including the choice of techniques that reduce waste generation (e.g., process optimization) or techniques that enable waste sources to be treated, with a consequent reduction in emissions. Although it is, of course, far better to implement the ‘prevention is better than cure’ procedure, this is not always an option. Therefore, the development of waste source treatment techniques should be prioritized.
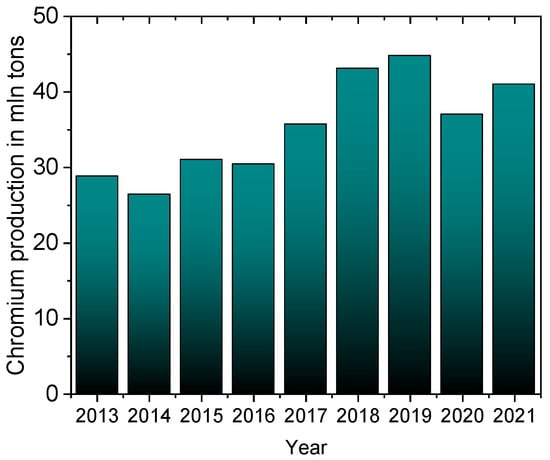
One serious environmental problem is the release of heavy metals, including chromium, into the environment. Chromium occurs in nature in the form of minerals such as crocoite (PbCrO4), chromite (FeCr2O4), or lopesite (Na2Cr2O7). This metal is applied to harden steel and protect it from corrosion, which is why it is a component of stainless steels and different alloys, from which special and everyday products are made, such as airplanes, tanks, machinery, industrial installations, kitchen utensils, surgical tools, etc. [1]. As chromium forms a passivation layer, a fine and solid chromium oxide film, it is used as an external coating to protect the internal metal (steel) from corrosion. Chrome plating is widely used in the leather tanning industry and in the manufacturing of steel products, which has been accelerating the consumption of chromium worldwide for the last 10 years (Figure 1) [2]. In 2021, the global chrome plating market was estimated at $16.6 billion, and is expected to grow to $22.2 billion by 2028 [3].
Chromium compounds are used in many chemical processes as industrial catalysts and pigments for glass, porcelain glazes (bright green, yellow, red, and orange). Approximately 90% of all leather is tanned with chrome [4][5], and toxic waste tannery effluents are generated and must be efficiently purified before release.
In the aquatic environment, chromium is found naturally in rainwater (0.2–1 µg/dm3), seawater (0.04–0.5 µg/dm3), surface waters (0.5–2 µg/dm3) and groundwater (<1 µg/dm3) [6]. Natural water can be contaminated by anthropogenic chromium, especially from tanneries. Chromium is present in aqueous solutions in different forms, trivalent and hexavalent being the prevalent ones. Cr(III) has low solubility and is less dangerous to the aquatic environment than Cr(VI), which shows much better solubility and can easily migrate through the groundwater, mix with it, and contaminate it [7]. The harmfulness of chromium is well known [8][9][10]: toxicity limits are 28–80 mg/dm3 for fish, 0.05 mg/dm3 for drinking water. However, mutations induced by the chromium(III) complex with 2,2′-bipyridyl were found, among others, in oxidation-sensitive Salmonella strains TA2638 and TA102 [11].
Although Cr(III) is less toxic to living organisms (negative results in most mutagenicity tests) than Cr(VI) and trace quantities of Cr(III) are even essential for the proper functioning of the human organism, discharge of Cr(III) present in large amounts in spent tanning liquors is burdensome for the environment. For example, groundwater contamination with chromium near tanneries around the world must be carefully monitored [7][12][13]. An additional risk is the fact that Cr(III) can be oxidized to hexavalent chromium in natural water and soil [13].
There are no unified discharge limits for Cr(III) or Cr(VI) to the aquatic environment not only in different parts of the world but also within the EU or the World Health Organization. Each country establishes its own standards for the chromium discharge limits to various aquatic systems (marine water, lake, river, and sewer system). The maximum discharge limit to the aquatic environment in the EU is 0.05–2 and 5 mg/dm3 for Cr(VI) and Crtotal, respectively [14][15].
It should be noted that, depending on the industry in which wastewater is generated, the presence of associated components, for example, surfactants, other metal ions, acids, and bases must be taken into account. Therefore, it is not possible to unequivocally identify one single best method for chromium removal. Moreover, as science progresses, new materials, techniques, and solutions can be expected to solve the problem of chromium separation from aqueous systems.
2. Chromium in Spent Industrial Effluents
Chromium-containing waste effluents can originate not only from steel plating and tannery processes but also can be generated as a result of the leaching of various types of steel [16][17][18][19]. The removal of chromium from industrial effluents is important not only for the removal of hazardous metal ions but also for the purification of the effluents before further steps of treatment/recovery of valuable metals. In order to provide an initial indication of the complexity of the chromium effluent problem, the exemplary compositions of Cr(III)-containing industrial effluents are summarized in Table 1.Table 1.
Origin and composition of Cr(III)-containing spent industrial effluents.
| Origin | Composition | Ref. |
|---|---|---|
| Steel leaching | in g/dm3: 20.4–37.2 Ni(II); 11.4–21.4 Co(II); 13.4–24.5 Cr(III); 7.20–8.78 Al(III); 0.02–0.53 Cu(II); 0.04–0.138 Fe(III); 0.11 Na(I); 0.023 Mg(II); 0.023 Zn(II) in mol/dm3: 3.46–4.98 H+; 2.28–3.22 SO42−; 0.16–0.34 Cl− |
[16][17] |
| Ilmenite leaching | in mol/dm3: 2.1×10−3 V(V); 5.41×10−3 Cr(III); 0.627 Ti(IV); 0.39 Fetotal; 2.73×10−2 Mg(II); 1.74×10−2 Al(III); 6.4×10−4 Ln(III); 6 H2SO4 | [20] |
| Chromium sludge leaching | in g/dm3: 20.64 Cr(III); 2.87 V(V); 5.84 Fe(III); 2.01 Si(IV); 0.83 Ca(II); 0.70 Mn ions; 0.54 Mg(II) in H2SO4 | [21] |
| Passivation bath | in g/dm3: 11–20.5 Zn(II); 3–7 Crtot, in mg/dm3: 15–100 Fetot; acidic pH |
[19][22][23] |
| Spent tanning liquor 1 | in mol/dm3: 0.042 Cr(III); 0.201 SO42−; 0.35 Cl−; pH 4.35 | [13] |
| Spent tanning liquor 2 | in mol/dm3: 0.102 Cr(III); 0.324 SO42−; 0.752 Cl−; pH 3.70 | [13] |
| Tannery effluents (six different leather industries in Bara and Parsa districts (Nepal)) | in mg/dm3: Cr 0.7–345 | [10] |
| Tannery spent effluent collected from CSIR-CLRI (Central Leather Research Institute), Chennai | in mg/dm3: total Cr 2481; Cl− 36,000; SO42− 28,480; protein 570; lipid 981; pH 4.4 | [24] |
| Tannery wastewater after chemical treatment | in mg/dm3: total Cr 2007.08; Ca 755.3; Fe 1.998; Na 31,030; Ni 0.3054; Zn 20.69; SO42− 60,414.61; CN− 2; pH 4.13 | [25] |
| Tannery wastewater from Kombolcha Tannery Share Company, Ethiopia | in mg/dm3: total Cr 200; dissolved solid 3000; suspended solid 2100; pH 5.3 | [26] |
| Tannery effluent from Mexico | in mg/dm3: 2760 Cr(III); 0.023 Cr(VI); 19,080 Na(I); 832.7 Ca(II); 0.14 Cu(II); 0.029 Pb ions; 0.014 Ni(II), pH 4 | [27] |
| Tannery effluent from Mexico | in mg/dm3: 5061 Cr(III); 0.023 Cr(VI); pH 5.23 | [28] |
| Tannery effluent from Old Cairo, Egypt | in mg/dm3: 2131 Cr(III); 821 Cr(VI); 249 SO42−, pH 3.6 | [29] |
| Chromite ore processing waste (Hackensack River (NJ, USA) | in mg/kg: Cr total 199–3970; Cr(VI) 0.3–19; As 8.9–59.6; Cd 0.7–9.6; Fe 11,100–47,500; Pb 44.7–281; Mn 232–585; Hg 0.08–2.45; Zn 95.3–597 | [30] |
| Textile mill effluents (Eight textile industries in Delhi NCR, India) | in mg/dm3: Cr 0.11–0.21; Cu 0.17–0.28; Fe 0.39–0.90; Pb 0.02–0.10; Ni 0.11–0.22; Zn 0.11–0.51; Cd 0.01 | [31] |
| Chrome plating industry wastewater | in mg/dm3: Cr(VI) 5721.95; Fe 79.5; Pb 1.095; Cu 28.3, pH 2.09 | [32] |
| Steel industry slags | in mg/kg: Cr 2915; Zn 1084; Ba 380; Sr 266; Cu 175; Zr 109; V 92; Nb 62; Pb 59; Ni 26; Sn 15; Mo 11; Rb 11; As 10; Cd 8; U 4; Br 5; Ce, Co, La < 5; Y, Th, Bi, Ga < 3 | [33] |
| Chromium slag from Chemical Holdings Co., Ltd. (Fuzhou, China) during chromium salt production | in mg/kg: Cr(III) 112; Cr(VI) 464; Ca 26,600; Mg 3160; Fe 4550; Al 64.9; Cd 1.3; Ni 3.2; Cu 5.8; Mn 10.2; As 4.6; Co 1.5 | [34] |
References
- Liu, Z.; Li, S.; Wang, W. Review: Research Progress on Liquid–Liquid Extraction of Chromium. JOM 2021, 73, 1371–1385.
- Statista Mine Production of Chromium Worldwide from 2010 to 2021. Available online: https://www.statista.com/statistics/587342/mine-production-of-mercury-worldwide/ (accessed on 20 November 2022).
- Insider_Strategy&Stats, S. Report Id: SNS/C&M/2557: Chrome Plating Market Research Report. Global Forecast from 2022 to 2030. Available online: https://dataintelo.com/report/chrome-plating-market-report/ (accessed on 20 November 2022).
- Royal Society of Chemistry Periodic Table. Chromium. Available online: https://www.rsc.org/periodic-table/element/24/chromium (accessed on 20 November 2022).
- Nur-E-Alam, M.; Mia, M.A.S.; Ahmad, F.; Rahman, M.M. An Overview of Chromium Removal Techniques from Tannery Effluent. Appl. Water Sci. 2020, 10, 1–22.
- World Health Organization. Chromium in Drinking-Water: A Background Document for Development of World Health Organisation Guidelines for Drinking Water (WHO/HEP/ECH/WSH/2020.3); World Health Organization: Geneva, Switzerland, 2020.
- Mani Tripathi, S.; Chaurasia, S.R. Detection of Chromium in Surface and Groundwater and Its Bio-Absorption Using Bio-Wastes and Vermiculite. Eng. Sci. Technol. Int. J. 2020, 23, 1153–1161.
- Singh, D.; Singh, C.K.; Singh, D.; Sarkar, S.K.; Prasad, S.K.; Sharma, N.L.; Singh, I. Glycine Betaine Modulates Chromium (VI)-Induced Morpho-Physiological and Biochemical Responses to Mitigate Chromium Toxicity in Chickpea (Cicer arietinum, L.) Cultivars. Sci. Rep. 2022, 12, 1–17.
- Kocaoba, S.; Cetin, G.; Akcin, G. Chromium Removal from Tannery Wastewaters with a Strong Cation Exchange Resin and Species Analysis of Chromium by MINEQL+. Sci. Rep. 2022, 12, 9618.
- Prasad, S.; Yadav, K.K.; Kumar, S.; Gupta, N.; Cabral-Pinto, M.M.S.; Rezania, S.; Radwan, N.; Alam, J. Chromium Contamination and Effect on Environmental Health and Its Remediation: A Sustainable Approaches. J. Environ. Manag. 2021, 285, 112174.
- Casadevall, M.; Kortenkamp, A. Chromium and Cancer. In Heavy Metals in The Environment; CRC Press: Boca Raton, FL, USA, 2002; pp. 250–283. ISBN 9780429221767.
- Khan, A.; Michelsen, N.; Marandi, A.; Hossain, R.; Hossain, M.A.; Roehl, K.E.; Zahid, A.; Hassan, M.Q.; Schüth, C. Processes Controlling the Extent of Groundwater Pollution with Chromium from Tanneries in the Hazaribagh Area, Dhaka, Bangladesh. Sci. Total Environ. 2020, 710, 136213.
- Wionczyk, B.; Apostoluk, W.; Charewicz, W.A. Solvent Extraction of Chromium (III) from Spent Tanning Liquors with Aliquat 336. Hydrometallurgy 2006, 82, 83–92.
- Vaiopoulou, E.; Gikas, P. Regulations for Chromium Emissions to the Aquatic Environment in Europe and Elsewhere. Chemosphere 2020, 254, 126876.
- Tumolo, M.; Ancona, V.; De Paola, D.; Losacco, D.; Campanale, C.; Massarelli, C.; Uricchio, V.F. Chromium Pollution in European Water, Sources, Health Risk, and Remediation Strategies: An Overview. Int. J. Environ. Res. Public Health 2020, 17, 5438.
- Kadłubowicz, A.; Janiszewska, M.; Baraniak, M.; Lota, G.; Staszak, K.; Regel-Rosocka, M. Diffusion Dialysis and Extraction Integrated System for Recovery of Cobalt(II) from Industrial Effluent. J. Water Process Eng. 2021, 39, 101754.
- Kostrzewa, M.; Staszak, K.; Ginter-Kramarczyk, D.; Kruszelnicka, I.; Góra, W.; Baraniak, M.; Lota, G.; Regel-Rosocka, M. Chromium(III) Removal from Nickel(II)-Containing Waste Solutions as a Pretreatment Step in a Hydrometallurgical Process. Materials 2022, 15, 6217.
- Ishfaq, A.; Ilyas, S.; Yaseen, A.; Farhan, M. Hydrometallurgical Valorization of Chromium, Iron, and Zinc from an Electroplating Effluent. Sep. Purif. Technol. 2019, 209, 964–971.
- Alguacil, F.J.; Diban, N.; Urtiaga, A. Zinc and Iron Removal from Chromium(III) Passivation Baths by Solvent Extraction with Cyanex 272. Desalin. Water Treat. 2018, 133, 252–256.
- Nayl, A.A.; Aly, H.F. Solvent Extraction of V(V) and Cr(III) from Acidic Leach Liquors of Ilmenite Using Aliquat 336. Trans. Nonferrous Met. Soc. China 2015, 25, 4183–4191.
- Guo, Y.; Li, H.Y.; Shen, S.; Cheng, J.; Diao, J.; Xie, B. A Novel Process for Comprehensive Resource Utilization of Hazardous Chromium Sludge: Progressive Recovery of Si, V, Fe and Cr. J. Hazard. Mater. 2021, 405, 124669.
- García, V.; Steeghs, W.; Bouten, M.; Ortiz, I.; Urtiaga, A. Implementation of an Eco-Innovative Separation Process for a Cleaner Chromium Passivation in the Galvanic Industry. J. Clean. Prod. 2013, 59, 274–283.
- García-Antón, J.; Fernández-Domene, R.M.; Sánchez-Tovar, R.; Escrivà-Cerdán, C.; Leiva-García, R.; García, V.; Urtiaga, A. Improvement of the Electrochemical Behaviour of Zn-Electroplated Steel Using Regenerated Cr (III) Passivation Baths. Chem. Eng. Sci. 2014, 111, 402–409.
- Selvaraj, R.; Santhanam, M.; Selvamani, V.; Sundaramoorthy, S.; Sundaram, M. A Membrane Electroflotation Process for Recovery of Recyclable Chromium(III) from Tannery Spent Liquor Effluent. J. Hazard. Mater. 2018, 346, 133–139.
- Reyes-Serrano, A.; López-Alejo, J.E.; Hernández-Cortázar, M.A.; Elizalde, I. Removing Contaminants from Tannery Wastewater by Chemical Precipitation Using CaO and Ca(OH)2. Chin. J. Chem. Eng. 2020, 28, 1107–1111.
- Mohammed, K.; Sahu, O. Recovery of Chromium from Tannery Industry Waste Water by Membrane Separation Technology: Health and Engineering Aspects. Sci. Afr. 2019, 4, e00096.
- Reyes-Romero, B.; Gutiérrez-López, A.N.; Hernández-Altamirano, R.; Mena-Cervantes, V.Y.; Ruiz-Baca, E.; Neri-Torres, E.E.; Chairez, I.; García-Solares, S.M.; Vazquez-Arenas, J. Removal of Concentrated Cr(III) from Real Tannery Wastewater Using Abiotic and Anaerobic Processes with Native Microbial Consortia. J. Environ. Chem. Eng. 2021, 9, 104626.
- Moreno-García, A.F.; Neri-Torres, E.E.; Mena-Cervantes, V.Y.; Altamirano, R.H.; Pineda-Flores, G.; Luna-Sánchez, R.; García-Solares, M.; Vazquez-Arenas, J.; Suastes-Rivas, J.K. Sustainable Biorefinery Associated with Wastewater Treatment of Cr (III) Using a Native Microalgae Consortium. Fuel 2021, 290, 119040.
- Ahmed, E.; Abdulla, H.M.; Mohamed, A.H.; El-Bassuony, A.D. Remediation and Recycling of Chromium from Tannery Wastewater Using Combined Chemical–Biological Treatment System. Process Saf. Environ. Prot. 2016, 104, 1–10.
- Becker, D.S.; Long, E.R.; Proctor, D.M.; Ginn, T.C. Evaluation of Potential Toxicity and Bioavailability of Chromium in Sediments Associated with Chromite Ore Processing Residue. Environ. Toxicol. Chem. 2006, 25, 2576–2583.
- Bhardwaj, V.; Kumar, P.; Singhal, G. Toxicity of Heavy Metals Pollutants in Textile Mills Effluents. Int. J. Sci. Eng. Res. 2014, 5, 664–666.
- Karegar, S.; Bhargavi, M.; Divekar, S.V. Treatmet of Wastewater from Chrome Plating Industry by Ion Exchange Method. Int. J. Res. Eng. Technol. 2015, 4, 393–401.
- Sas, W.; Głuchowski, A.; Radziemska, M.; Dzięcioł, J.; Szymański, A. Environmental and Geotechnical Assessment of the Steel Slags as a Material for Road Structure. Materials 2015, 8, 4857–4875.
- Liu, Y.; Ding, J.; Zhu, H.; Wu, X.; Dai, L.; Chen, R.; Van der Bruggen, B. Recovery of Trivalent and Hexavalent Chromium from Chromium Slag Using a Bipolar Membrane System Combined with Oxidation. J. Colloid Interface Sci. 2022, 619, 280–288.
More