Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Yuriy Demchuk.
The five main theories describing the interaction mechanisms in the bitumen/aggregate system was conducted: theory of weak boundary layers, mechanical theory, electrostatic theory, chemical bonding theory, and thermodynamic theory (adsorption theory). The adhesion assessment methods in the bitumen/aggregate system are described, which can be divided into three main groups: determination of adhesion forces for bitumen with different materials, determination of bitumen resistance to the exfoliating action of water with different materials, and determination of adhesion as a fundamental value (contact angle measurements, interfacial fracture energy, adsorption capacity and others).
- adhesion
- bitumen
- aggregate
1. Introduction
Asphalt pavements include two main ingredients: bitumen and aggregates (crushed stone). Bitumen performs the adhesive function that binds mineral material particles to form an asphalt concrete coating. Being non polar, bitumen has high water resistance properties. It is known that low-paraffinic oil is the most suitable for the bitumen production, but, due to the shortage of this type oil, almost any oil residues are used. This leads to the bitumen materials’ low quality and, as a result, to the asphalt concrete pavement’s low quality.
In general, asphalt concrete appears as a substantially water resistance material, but water can penetrate into the pores in different ways: impregnation (surface water), under the action of capillary forces (water from the bottom of the road base rises to the asphalt concrete), and water vapor (air moisture can penetrate into the asphalt concrete pores and condense). Furthermore, transport wheel pressure accelerates the water penetration into the asphalt concrete pores [1]. Therefore, both passive and active adhesion of bituminous binder to the asphalt concrete must be ensured.
Active adhesion is the ability of the binder to completely surround the particles of the aggregate and adhere to it during the mixing of the components of the asphalt concrete mixture (Figure 1). This type of adhesion arises due to the mutual attraction of positively charged surface-active substances of bitumen molecules to negatively charged aggregate molecules. This type of interaction makes it possible to displace water located at the interface of phases and ensures maximum bitumen coverage of the surface of the mineral material. In case of loss of active adhesion, this can lead to increased movement of moisture in asphalt concrete layers [2,3][2][3].
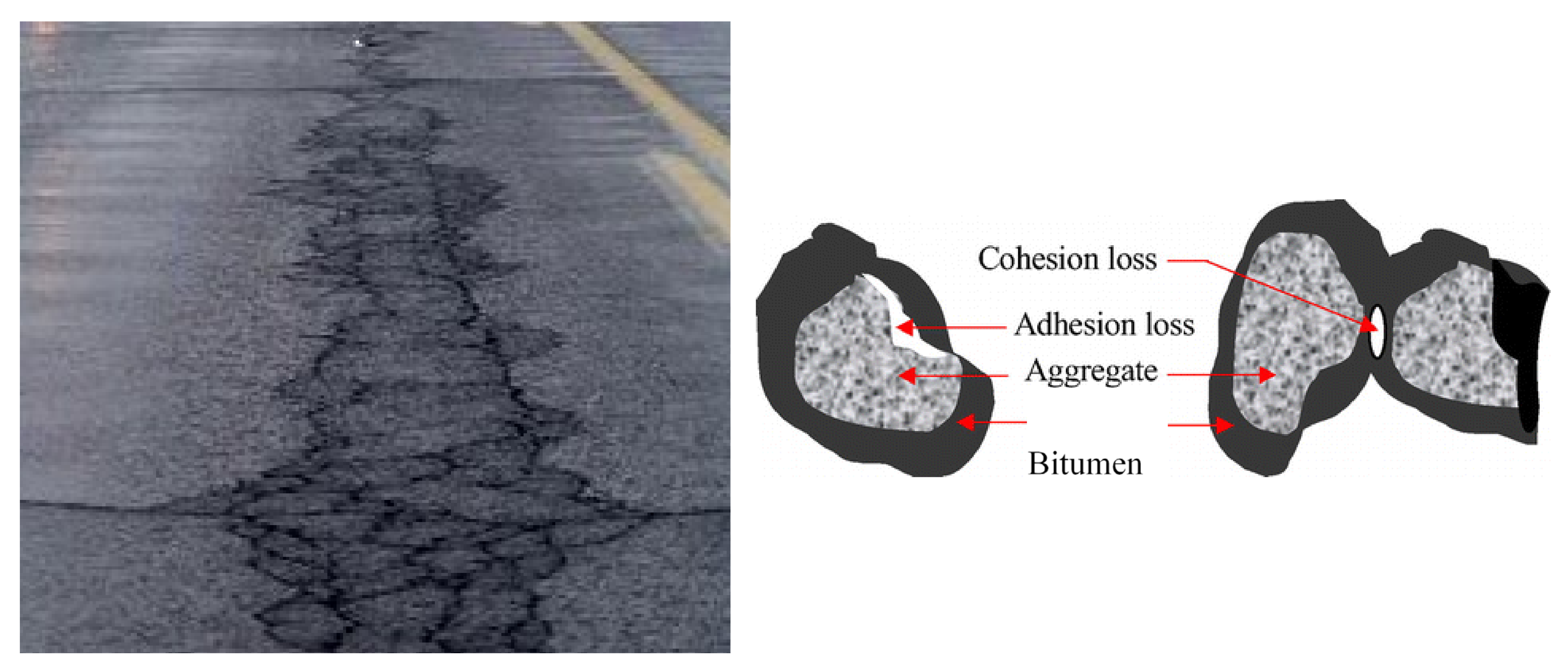
Figure 1. Fatigue cracks caused by low-adhesion in bitumen.
In contrast to active adhesion, passive adhesion occurs as a result of external forces, for example, due to increased pressure in the pores and can be described as the ability to resist the penetration of water into the asphalt mixture. Passive adhesion indicates the ability of bitumen to bind to the surface of the mineral material throughout the further service life of the asphalt concrete without the risk of destruction of these bonds under the action of vehicle wheels or water [4]. Loss or insufficiency of passive adhesion can cause premature appearance of cracks, potholes and ruts [5].
Poor adhesion between bitumen and aggregate leads to the binder stripping in the presence of water, which ultimately leads to the pothole formations (Figure 1). Moisture damage in asphalt concrete pavements is considered as primary cause of distresses in the asphalt pavement layers. The resistance of bitumen to stripping is determined by its adhesion to the aggregate surface: it must be not only high, but also stable over time, which is one of the conditions for the road surface durability. The situation is exacerbated by the traffic and car numbers constant increase on the roads. Therefore, to obtain high quality coatings, a key factor is high adhesion ensure between bitumen and the road pavement mineral components.
2. Adhesion Mechanism in Bitumen/Aggregate System
Researches in this area were conducted for more than 100 years, but the question about adhesion mechanism between bitumen and aggregate remains debatable. There were proposed many theories to explain the adhesion mechanism between bituminous and aggregate. The main theories explaining the adhesion mechanism in the bitumen/aggregate system are presented in the Table 1 and Figure 2.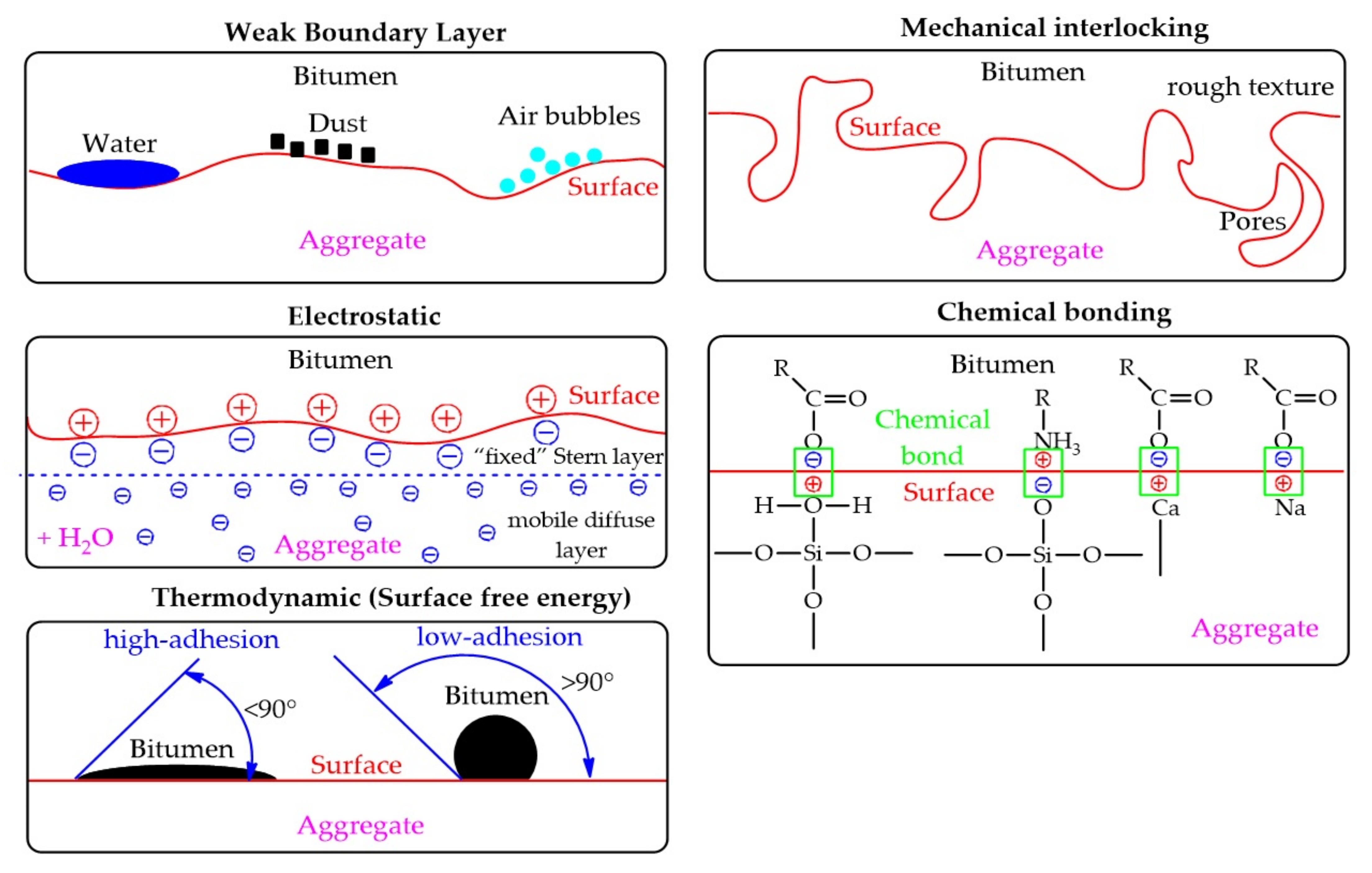
Figure 2. Representation of theories explaining the adhesion mechanism in the bitumen/aggregate system.
Table 1. Theories explaining the adhesion mechanism in the bitumen/aggregate system.
| Theory | Interaction | References | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Weak Boundary Layer | layering | Bikerman, 1961 [6] and other [,11] | Bikerman, 1961 [6] | 7,8,9, | and other [7][8][9] | 10 | [10][11] | ||||||||
| Mechanical interlocking | interlock | McBain and Hopkins, 1925 [12] and other [6,7,8,9,10,11] | McBain and Hopkins, 1925 [12] and other [6][7][8][9][10][11] | ||||||||||||
| Electrostatic | potential | Deryaguin and other, 1948 [7] and other [6,8,9,10,11] | Deryaguin and other, 1948 [7] and other [6][8][9][10][11] | ||||||||||||
| Chemical bonding | covalent and ionic bonds | [6,7,8, | 7][ | 9, | 8][ | 10, | 9][ | 11, | 10][ | 13, | 11][ | 14, | 13][ | 15,16] | [6][14][15][16] |
| Thermodynamic (Surface free energy) | surface free energy | [6,7,8,9,10,11] | [6][7][8][9][10][11] |
2.1. Theory of Weak Boundary Layers
According to the weak boundary layer theory, the breakdown of adhesive bonds occurs either in the adhesive or in the substrate due to the presence of an interfacial region with low cohesive strength. While there is debate as to how common these layers are, they do exist and cannot be ignored. However, certain types of boundary layers, according to some researchers, are important factors for good adhesion. Common causes of weak boundary layers are low molecular weight (and therefore low cohesive strength), and surface contaminants such as organics and water. Dusty substrates can prevent effective wetting and close contact with the adhesive, and dust on aggregates is able to trap air, mixing with bitumen, and thus weaken the bitumen/aggregate bond (Figure 2).2.2. Theory of Mechanical Interlocking
Adhesion occurs through the binder material penetration into the aggregate surface irregularities (pores and cavities). Adhesion occurs due to the “mechanical connection” between the binder and aggregate (Figure 2). Rough surfaces provide greater adhesion compared to a smooth surface. It should also be noted that rough surfaces are not the only reason for better adhesion. This may also be due to other factors (clean surfaces, highly reactive surface formation, or a contact area increase). The surface texture of an aggregate also affects its coat-ability, or wettability, in that a smoother surface coats easier than a rough surface. In addition, wettability, including pore filling, also depends on the viscosity of the bitumen and the chemical composition of the surface of both aggregate and bitumen. While many researchers claim that aggregate surface texture is the primary factor affecting adhesion, others report that chemical and electrochemical effects dominate. This theory simply explains the mechanical adhesive bond without taking into account the chemical interaction that can occur between the bitumen and the surface of the aggregate, so this theory has not found wide support among scientists.2.3. Electrostatic Theory
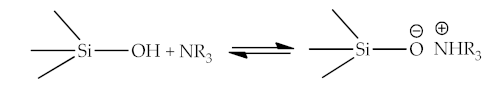
Electrostatic theory is based on the presence of free charges in any material, where the opposite charges attract each other (Figure 2). The presence of free charges in the materials causes an electrochemical potential difference between two contacting materials and, thus, establish an electrical double layer. Most surfaces are charged in the presence of water. This is due to the high dielectric permeability of water, which makes it a good solvent for ions. When the bitumen/aggregate surface system is exposed to water, the mineral structure of the aggregate easily collapses and ionizes, and thus the adhesive structure of the bitumen film on the aggregate can be broken and ionized. Two layers are formed (“fixed” Stern layer and mobile diffuse layer), together they form an electric double layer. The electric potential at the shear plane between the fixed and mobile layers is measurable, and called the zeta potential (ζ). The zeta potential value depends on two main factors: diffusion of external water to the bitumen/aggregate interface (the amount of moisture in the system) and the pH value of the water. The introduction of various substances can change the pH value of water into the bitumen/aggregate system (for example, mineral powders), which leads to a decrease in zeta potential. Researchers consider this approach to be of fundamental importance for quantifying adhesion in the presence of water, with knowledge of the pH effect. Determination of zeta potential can serve to quantify adhesion in the presence of water, especially taking into account the pH effect.2.4. Chemical Bonding Theory
This theory is based on the formation chemical bonds in the system of bitumen and aggregate components. Although bitumen mainly consists non-polar and low-reactive hydrocarbons in relation to the aggregate, it also contains polar compounds that contain heteroatoms (O, S, N and others). The study of adsorption of bituminous model compounds containing specific functional groups is presented in the works [13,14,15,16][13][14][15][16]. Relative affinity of functional groups for aggregate surfaces and their relative displacement by water are presented in these studies. Tests involving the adsorption affinity of the model compounds representing the various functional groups present in asphalt averaged over a series of aggregates (including granites, limestone, gravels, and greywacke) gave the following ranking [7]:sulfoxide > carboxylic acid > nitrogen base > phenol >ketone > pyrrole > 4-ring aromatic > 2-ring aromatic
sulfoxide > carboxylic acid > pyrrole >ketone > nitrogen base > phenol
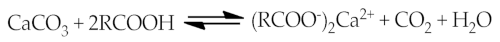

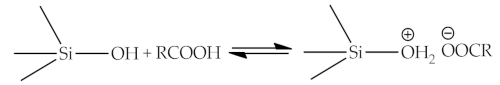
2.5. Thermodynamic Theory (Surface Free Energy)
The physical and chemical adhesion between bitumen and aggregate can be seen as a thermodynamic phenomenon, which requires the removal of bitumen from the aggregate surface (bond failure), that is, the work or energy required to produce new units of area in a vacuum. The physical and chemical adhesion between bitumen and aggregate depends on the surface free energy (SFE) of the material. The higher surface free energy (adhesion work) indicates that the adhesion strength between the bitumen and aggregate interface is better. This theory, is the most widely used approach in adhesion science as indicated by most comprehensive references on this subject. SFE is directly proportional to the contact angle (θ). The theory explains the adhesion phenomenon on base of wettability. The contact angle is the angle formed by a liquid with a solid surface when both materials are in contact. If the contact angle is >90°, the wettability is bad, and if <90° then wettability is good (Figure 2). Thus, if the wetting decreases, it leads to the adhesion decrease. The aggregate wetting remains an important condition for good adhesion. Wetting is necessary for contact between materials, and thus the basic physical and chemical forces establishment that are ultimately responsible for adhesion. Without wetting, it is unlikely to establish the surface texture favorable impact by mechanical adhesion.References
- Ma, L.; Varveri, A.; Jing, R.; Erkens, S. Comprehensive review on the transport and reaction of oxygen and moisture towards coupled oxidative ageing and moisture damage of bitumen. Constr. Build. Mater. 2021, 283, 122632.
- Oliviero Rossi, C.; Teltayev, B.; Angelico, R. Adhesion promoters in bituminous road materials: A review. Appl. Sci. 2017, 7, 524.
- Tarefder, R.A.; Zaman, A.M. Nanoscale evaluation of moisture damage in polymer modified asphalts. J. Mater. Civ. Eng. 2010, 22, 714–725.
- Cihlářová, D.; Fencl, I.; Cápayová, S.; Pospíšil, P. Use of adhesion promoters in asphalt mixtures. Slovak J. Civ. Eng. 2018, 26, 19–24.
- Choudhary, J.; Kumar, B.; Gupta, A. Effect of filler on the bitumen-aggregate adhesion in asphalt mix. Int. J. Pavement Eng. 2020, 21, 1482–1490.
- Gardner, D.J.; Blumentritt, M.; Wang, L.; Yildirim, N. Adhesion Theories in Wood Adhesive Bonding. Prog. Adhes. Adhes. 2015, 2, 125–168.
- Hefer, A.W.; Little, D.N.; Lytton, R.L. A synthesis of theories and mechanisms of bitumen-aggregate adhesion including recent advances in quantifying the effects of water. J. Assoc. Asph. Paving Technol. 2005, 74, 139–196.
- Omar, H.A.; Yusoff, N.I.M.; Mubaraki, M.; Ceylan, H. Effects of moisture damage on asphalt mixtures. J. Traffic Transp. Eng. (Engl. Ed.) 2020, 7, 600–628.
- Nardin; Schultz, J.M. Theories and mechanisms of adhesion. In Handbook of Adhesive Technology; Marcel Dekker, Inc.: New York, NY, USA, 1994.
- Shi, N. Interface Bonding Mechanism of Recycled Asphalt Mixture. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2019; Volume 300, p. 032052.
- Little, D.N.; Jones, D.R. Chemical and mechanical processes of moisture damage in hot-mix asphalt pavements. In Moisture Sensitivity of Asphalt Pavements; The National Academies Press: Washington, DC, USA, 2003; pp. 43–76.
- McBain, J.; Hopkins, D. On adhesives and adhesive action. J. Phys. Chem. 1925, 29, 188–204.
- Plancher, H.; Dorrence, S.M.; Petersen, J.C. Identification of Chemical Types in Asphalts Strongly Adsorbed at the Asphalt-Aggregate Interface and Their Relative Displacement by Water ; No. CONF-770216-1; Energy Research and Development Administration: Washington, DC, USA, 1977.
- Curtis, C.W.; Ensley, K.; Epps, J. Fundamental Properties of Asphalt-Aggregate Interactions Including Adhesion and Absorption; No. SHRP-A-341; Strategic Highway Research Program: Washington, DC, USA, 1993.
- Park, S.; Jo, M.C.; Park, J.B. Adsorption and thermal desorption behaviour of asphalt-like functionalities on silica. Adsorpt. Sci. Technol. 2000, 18, 675–684.
- Wistuba, M.P.; Grothe, H.; Grönniger, J.; Handle, F. Adhesion of bitumen: Screening and evaluating laboratory testing techniques. In Proceedings of the 5th Eurasphalt & Eurobitume Congress, Istanbul, Turkey, 13–15 June 2012.
More
