You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by Oriana Piermatti.
Various noble metal and nonprecious metal or metal oxide nanoparticles have been utilized to exploit the C–H activation process to promote the formation of a new C–O bond and access alcohols or carbonyls.
- metal nanoparticles
- metal oxide nanoparticles
- C–H activation
1. Carbon-Oxygen (C–O) Bond Formation
Various noble metal and nonprecious metal or metal oxide nanoparticles (MNPs) have been utilized to exploit the C–H activation process to promote the formation of a new C–O bond and access alcohols or carbonyls. The relevance of this method relies in the importance of these products as key intermediates for the production of fine chemicals and pharmaceuticals, as well as agrochemicals [53,54,55,56][1][2][3][4].
Among these processes, it is worthwhile to mention the representative selective oxidation of methane that remains a chemical challenge due to large energy barriers and the prevention of overoxidation to CO2 [36,57,58,59][5][6][7][8]. Generally, high temperatures are required, but relatively mild conditions have been recently obtained by using bimetallic systems, such as AuPd NPs [60][9]. Moreover, methane can form stable adducts with alkaline metal cations in the gas phase [61][10]. In this revisew, wearch, researchers will focus on C(sp3) and the C(sp2)–H activation of alkanes, arenes and alkenes.
2.1. Toluene C(sp3)–H Activation
2. Toluene C(sp3)–H Activation
The selective C–H oxidation of toluene using MNPs has been widely explored [57][6]. Generally, high temperature and high O2 pressure are required, often leading to a poor control of selectivity. Therefore, the search for an efficient catalyst exhibiting high activity and selectivity under mild conditions still represents a challenge.
The C–H oxidation of toluene can lead to the formation of benzyl alcohol, benzaldehyde, benzoic acid or benzyl benzoate (Scheme 1).
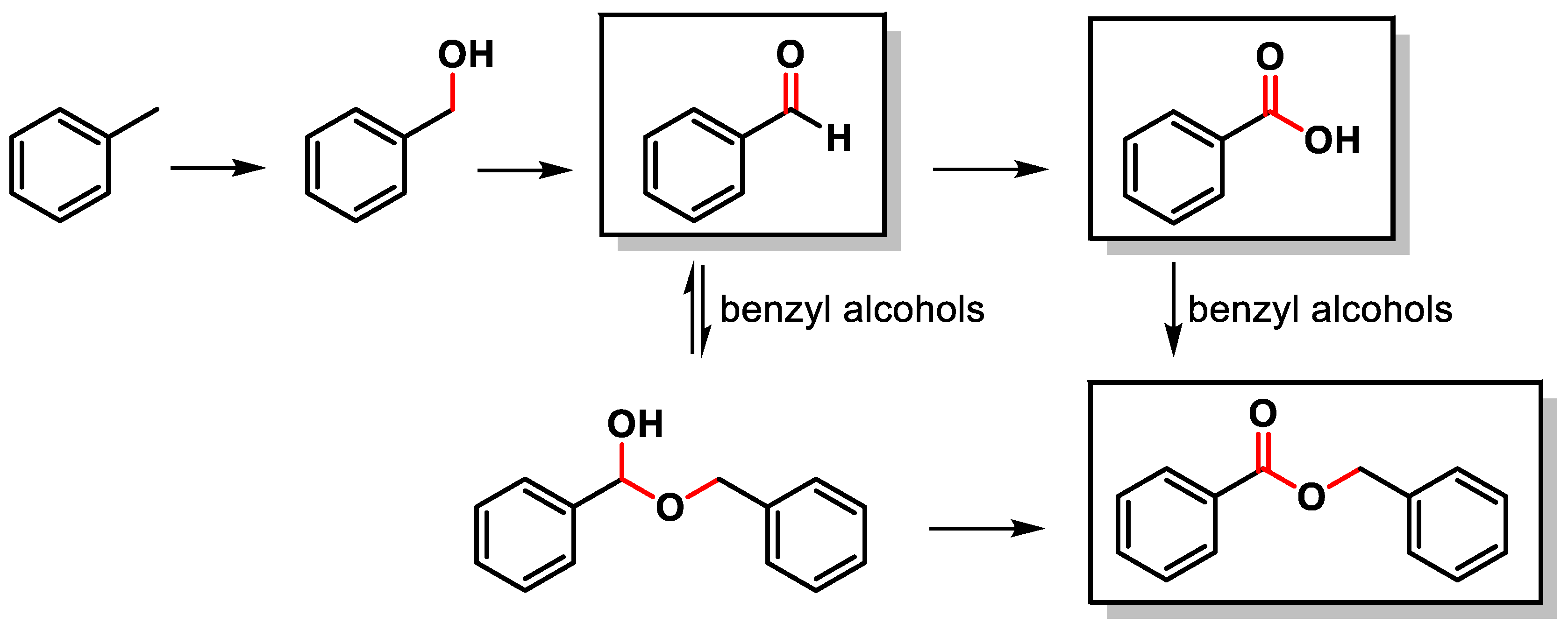
Scheme 1.
C(sp3)–H oxidation of toluene.
In 2009, Crabtree and coworkers [62][11] showed the use of a recyclable Ir/C catalyst in the selective oxidation of toluene and its derivatives. When the reaction was performed in the presence of Ag2CO3, acting both as base and oxidant, benzyl alcohol was observed as major product, which, forming in the presence of 4-fluorobenzoic acid, gave the corresponding ester and aldehyde as side products. In the absence of Ag2CO3, aldehydes were obtained exclusively (Scheme 2).
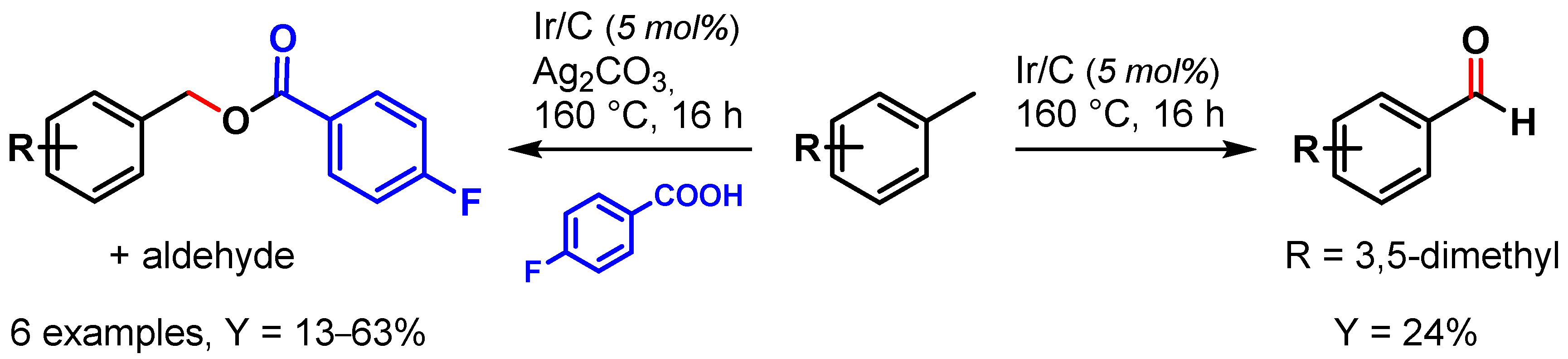
Scheme 2.
Ir/C catalyzed C–H oxidation of toluene.
Aldehydes are formed via a double gem C–H activation of ArCH3, possibly forming a surface-bound ArCH = Schrock carbene. Then water provides the oxygen source for C–O bond formation and α-hydride elimination, followed by the reductive elimination of the product leading to the formation of the aldehydes (Scheme 3).
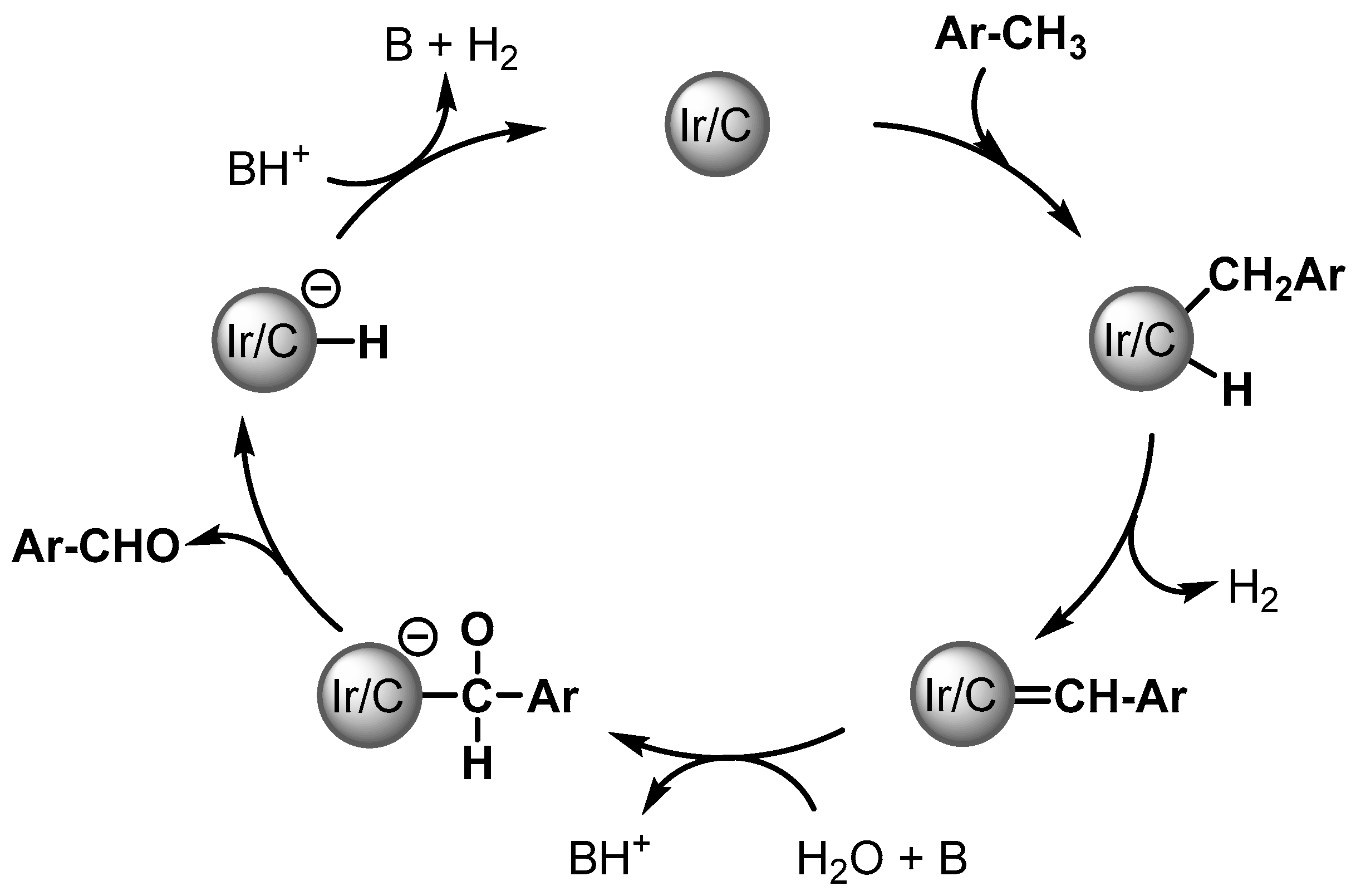
Scheme 3.
Plausible mechanism of Ir/C catalyzed aldehyde formation.
In 2011, Hutchings and coworkers [63][12] studied the catalytic activity of AuPd alloy NPs, prepared by sol-gel immobilization on carbon (Au-Pd/C). They used this catalytic system in the solvent free C–H bond oxidation of toluene at 160 °C in the presence of O2 (10 bar) and obtained benzyl benzoate with high yield and selectivity. It was found that metal nanoparticles significantly enhanced oxygen adsorption and are able to promote the formation of a surface-active superoxide species, such as O2., and O2.− (O2*), that promote C–H abstraction (Scheme 4).
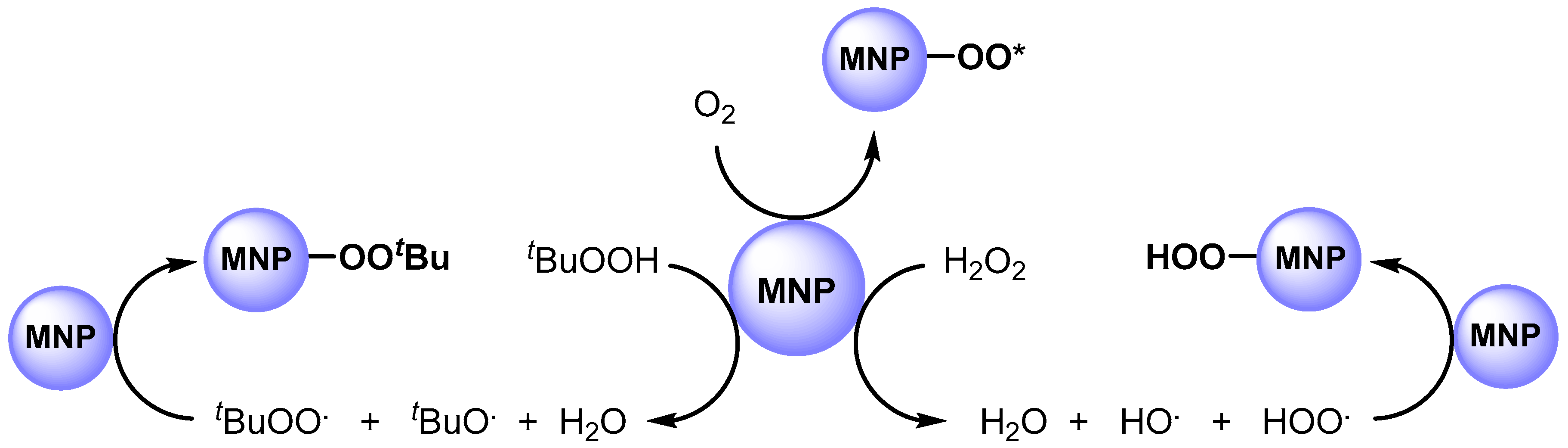
Scheme 4.
Formation of surface-bound oxygen radical species.
In 2012, the same authors [64][13] performed the C–H oxidation of toluene in milder conditions, at 80 °C, by using Au-Pd nanoparticles immobilized on TiO2 (Au-Pd/TiO2) in the presence of tert-butyl hydroperoxide (TBHP). The authors showed, for the first time, that MNPs can efficiently promote the decomposition of peroxides such as TBHP (or H2O2) to generate the tert-butyl peroxy (t-BuOO.) and tert-butoxy (t-BuO.) radical species (i.e., hydroperoxy and hydroxy radical species). The obtained radicals can be adsorbed on the surface of the metal to generate surface-bound radical species that play a key role in C–H bond activation (Scheme 4).
More recently, a wide variety of metal and metal oxide nanoparticles have been used for the selective oxidation of toluene via C–H activation, and examples of the selective formation of benzaldehyde are listed in Scheme 5 [65,66,67,68,69][14][15][16][17][18].
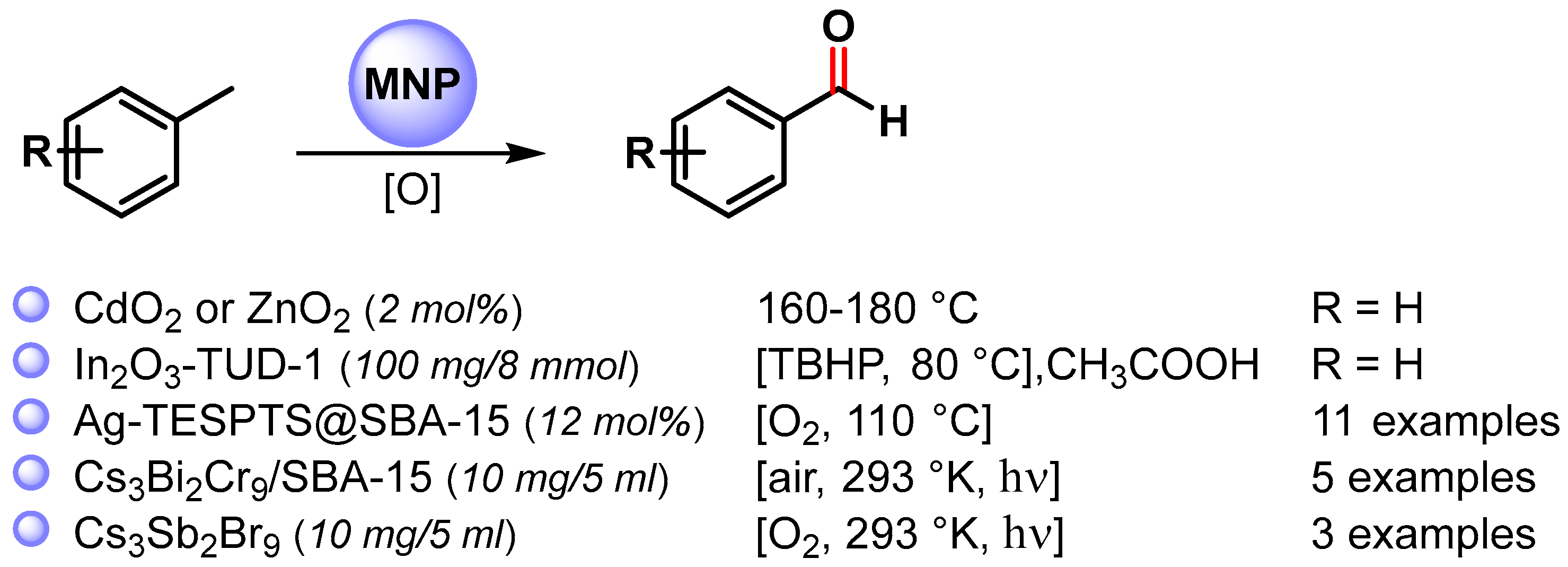
Scheme 5.
C(sp3)–H bond activation of toluene to benzaldehyde.
In 2013, Rao and coworkers [65][14] reported the C–H bond activation of toluene by using metal peroxide nanoparticles, CdO2 and ZnO2, prepared by a reaction of the corresponding oxide with H2O2. These metal peroxides are able to oxidize toluene primarily to benzaldehyde in the range of 160–180 °C. At this temperature, the peroxide nanoparticles decompose to give the corresponding oxides. Although CdO2 nanoparticles showed higher yields, more environmentally benign ZnO2 is better in terms of selectivity. A benzyl hydroperoxide intermediate has been detected during the transformation of toluene to benzaldehyde.
In 2020, Das and Chowdhury [66][15] described the use of indium oxide nanoparticles embedded in mesoporous silica TUD-1 (In-TUD-1) for the green synthesis of benzaldehyde from toluene using TBHP as oxygen donor and acetic acid as solvent, under very mild reaction conditions (80 °C, 5 h). The catalyst showed high catalytic activity with 48% toluene conversion and 83% benzaldehyde selectivity. Moreover, the catalyst was recyclable up to five times (from 48 to 36%). The use of a radical scavenger, such as quinhydrone, stopped the reaction, suggesting that it proceeds through a radical chain mechanism. The coordination of TBHP on the surface of In2O3 generates the tert-butyl peroxy (t-BuOO.) and tert-butoxy (t-BuO.) radical species. Toluene reacted with surface radical species to form the benzyl radical that combined with the metal surface and tert-butyl peroxy radical to produce benzaldehyde and t-BuOH as a by-product.
In the same year, Biswas and coworkers [67][16] described the preparation of highly dispersed silver nanoparticles supported on 2D hexagonally ordered functionalized mesoporous silica (Ag-TESPTS@SBA-15, AgMS) and their catalytic application in the C–H activation of toluene and its derivatives to produce the corresponding aldehydes under solvent-free conditions using a flow of oxygen at 110 °C. Moderate to good yields (59–79%) and high selectivities (89–96%) were achieved with both electron-rich and electron-poor groups. A significant decrease in the reaction yield was observed in the presence of TEMPO, confirming a radical pathway for the C–H oxidation wherein AgNPs promote the activation of O2 molecules with the formation on the surface of active oxygen species and of a benzyl peroxy radical intermediate (Scheme 6).
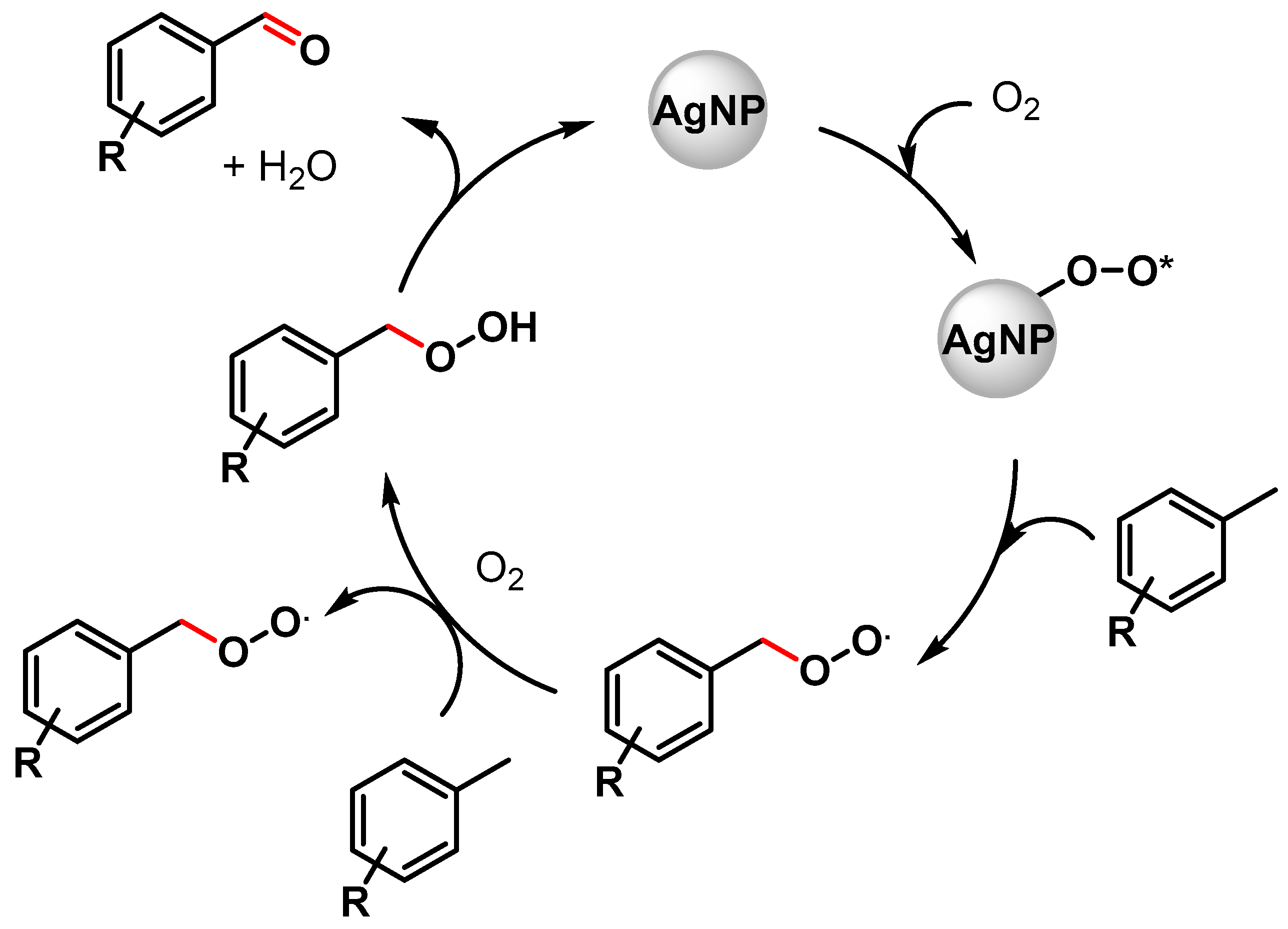
Scheme 6.
Proposed mechanism for C(sp3)–H bond activation of toluene with AgNPs.
In 2020, Cs3Bi2Br9 perovskite nanoparticles immobilized in the pore channels of mesoporous silica SBA-15 were used as a photocatalyst in the C–H activation of toluene and its derivatives by Tgysgz and coworkers [68][17]. The reactions were performed under visible-light irradiation (>420 nm) in air at 293 K, obtaining the corresponding aldehydes with a high conversion rate and selectivity (80–90%). A control experiment with TEMPO and TEMPO-H verified the formation of a carbon-centered radical as an intermediate, and isotopic labeling experiments (KH/KD = 4.4) showed that the C(sp3)–H bond activation was the rate-determining step.
Similarly, in the same year, Xu and coworkers [69][18] reported the use of Cs3Sb2Br9 perovskite nanoparticles as a photocatalyst under visible-light irradiation (>420 nm), in an O2 atmosphere at 293 K for the oxidation of toluene to benzaldehyde.
In a recent paper, Fan and coworkers [70][19] described the selective oxidation of benzyl alcohol to benzaldehyde via C–H activation under solvent-free conditions at 120 °C. The protocol implies the use of O2 as oxidant and electron-rich Pd atoms consisting of AuPd bimetallic NPs immobilized on TiO2 as catalyst. Extremely high turnover frequency was observed for AuPdx/TiO2 with x = 0.001. Moreover, kinetic studies reveal that the electron rich Pd atoms accelerate the oxidation of benzyl alcohols compared to net Pd atoms and that the cleavage of C–H bond is the rate-determining step (KH/KD = 11.34).
Very few examples deal with the use of MNPs as a catalyst for the selective C–H oxidation of toluene to benzoic acid (Scheme 7) [71,72,73][20][21][22].
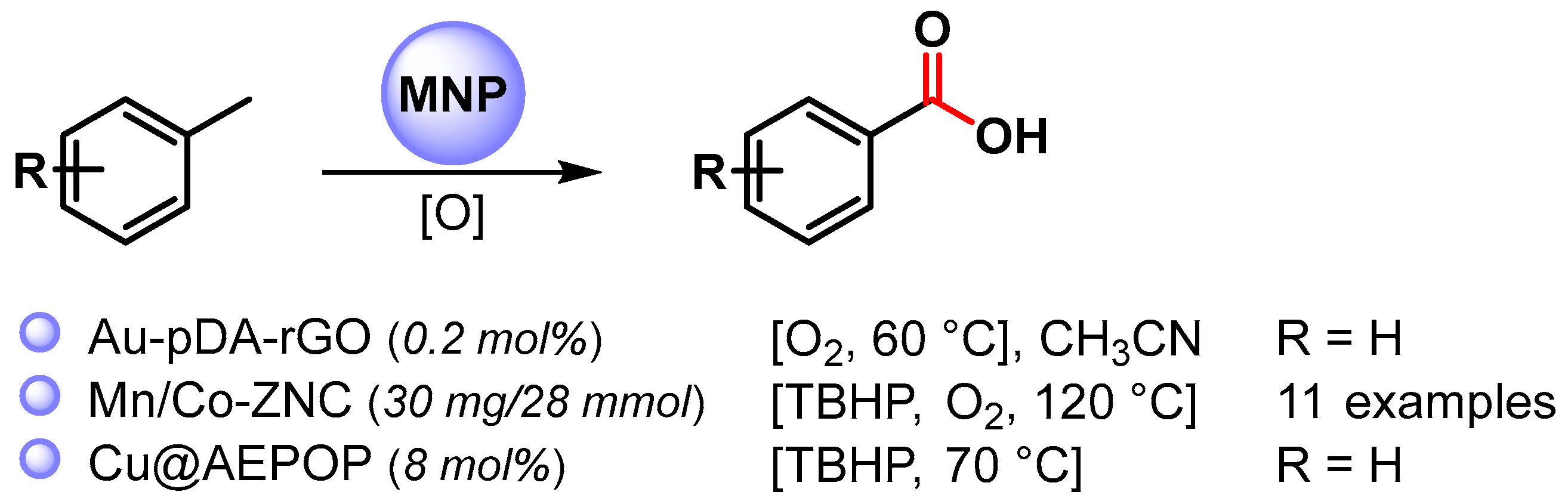
Scheme 7.
C(sp3)–H bond activation of toluene to benzoic acid.
In 2016, Sama and coworkers [71][20] reported the preparation of Au NPs supported on polydopamine-reduced graphene oxide (Au-pDA-rGO) and its use as catalyst for the selective oxidation of benzylic C–H bonds in CH3CN at 60 °C under an O2 atmosphere (10 bar) in the presence of N-hydroxyphthalimide (NHPI). Benzoic acid was obtained with good conversion (60%) and very high selectivity (99.6%). Detailed mechanistic studies showed that the reaction followed a free radical pathway in which O2 activation takes place on AuNPs’ surfaces, generating a superoxide species that, in the presence of NHPI, generates a phthalimide N-oxyl radical (PINO) responsible for C–H breaking to generate benzyl radicals.
In 2021, bimetallic Mn/Co oxide nanoparticles confined in zeolite imidazolate frameworks (ZIFs) were described by Huang and coworkers [72][21]. The C–H oxidation of toluene and substituted toluenes to corresponding carboxylic acids were performed at 120 °C, in the presence of O2 (6 bar) and TBHP as oxidants. Moderate to good conversion rates (39–57%) were obtained with high selectivity (up to 98.6%). A controlled experiment with TEMPO indicates a radical mechanism in which the activation of O2 by metal oxide generate the superoxide radical species that react with TBHP, generating tert-butyl peroxide species responsible for C–H activation by chain-propagating steps. Moreover, the catalyst was recovered and reused for six replicate runs with conversion and selectivity almost unchanged (from 57 to 43% conversion, up to 98% selectivity). A slight drop in reactivity and selectivity were observed thereafter.
In 2022, Wang and coworkers [73][22] reported the use of a porous organic polymer immobilized copper nanoparticles (Cu@AEPOP) as an efficient catalyst for benzylic C–H bond oxidation. Toluene oxidation to benzoic acid was easily performed at 70 °C in the presence of 70% in water TBHP as the sole oxidant (90% yield). A radical mechanism was hypothesized in which TBHP decomposes on the surface of Cu NPs to give the t-BuO. and t-BuOO. radical species responsible for benzylic hydrogen abstraction.
The direct oxidative esterification of methyl arenes with alcohols was reported by Liu and coworker in 2012 [74][23]. Bimetallic AuPd NPs supported on a metal–organic framework (MIL-101) promoted C–H activation at 120 °C in the presence of O2, producing the corresponding esters with good yields and high selectivities (Scheme 8). The catalyst was recovered and reused for 3 runs without a significant loss of catalytic activity (from 34.3 to 35.2% conversion and from 87.8 to 88.5% selectivity).
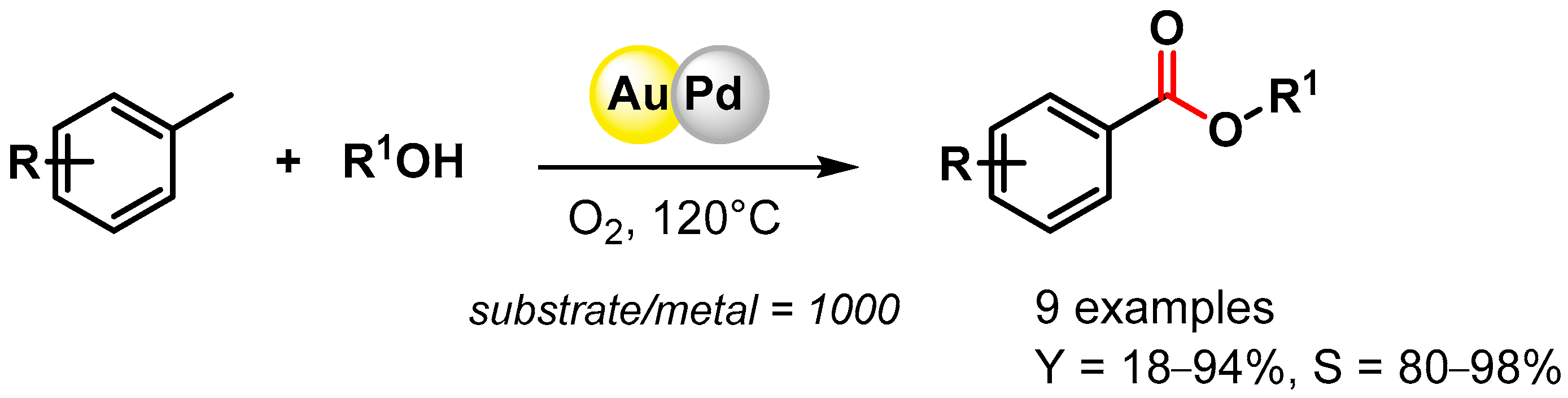
Scheme 8.
Direct oxidative esterification of methyl arenes in presence of AuPd/MIL-101.
In 2018, Varma and coworkers [75][24] synthesized graphitic-carbon-nitride-supported palladium nanoparticles (Pd@g-C3N4) for the photocatalytic C–H activation of benzyl alcohols to methyl esters with high yield and selectivity by using visible-light irradiation, under air, at room temperature (91–96% isolated yield). The photocatalyst was recycled and reused up to five times without losing its activity (from 96 to 95% yield) and with negligible Pd leaching.
An oxidative esterification of benzaldehyde via C–H activation at room temperature, using visible-light irritation and Ag/Co3O4 supported on a reduced graphene oxide (rGO) nanocomposite in the presence of alcohol as solvent was reported by Zhang in 2020 [76][25]. High conversion (72–90%) and selectivity (up to 99%) were obtained with a wide variety of alcohols (ROH, R = Me, Et, Pr, iPr, Bu).
2.2. Alkyl Arenes C(sp3)–H Activation
3. Alkyl Arenes C(sp3)–H Activation
The catalyzed oxidative C−H activation of alkyl arenes is a chemical process to obtain aromatic ketones directly. Recently, a large number of metal and metal oxide nanoparticles that efficiently promote oxidative C–H functionalization in mild conditions have been widely developed (Scheme 9) [67,71,73,77,78,79,80][16][20][22][26][27][28][29].
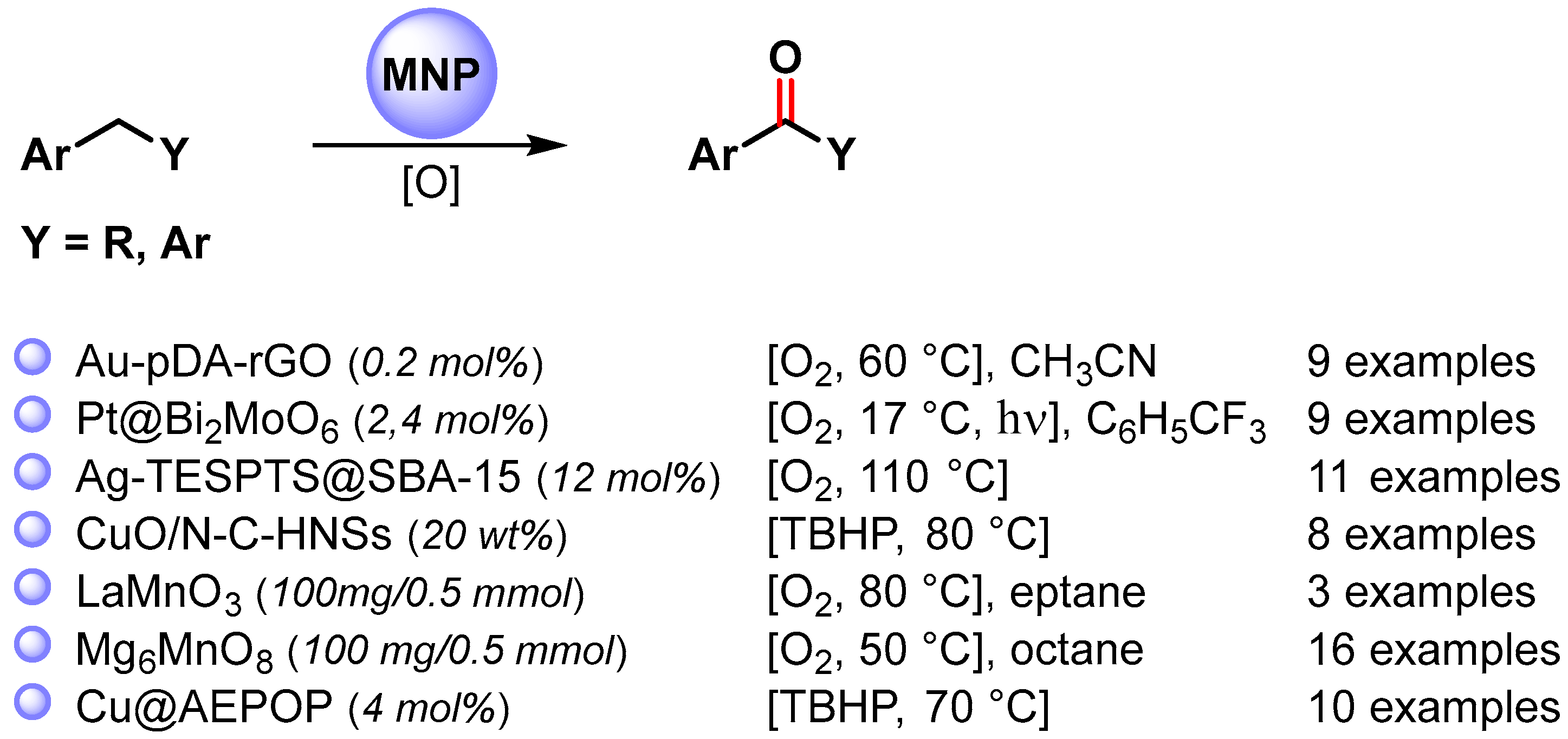
Scheme 9.
C(sp3)–H bond oxidation of alkylarenes to ketones.
The Au-NPs-based catalyst (Au-pDA-rGO) used by Sama and coworker in the selective oxidation of toluene to benzoic acid was also used for the selective benzylic C–H bond activation of alkyl arenes to the corresponding ketones [71][20]. The Au nanocomposite under an O2 atmosphere (10 bar) in the presence of N-hydroxyphthalimide (NHPI) leads to the desired oxidation products under mild and neutral reaction conditions with high efficiency and selectivity (80–99.5% conversion, up to 98.8% selectivity).
In 2018, Cheng and coworkers [77][26] reported a selective oxidation of alkyl aromatics under visible-light irradiation, at 17 °C in CF3C6H5, using O2 as an oxidant and Pt NPs supported on Bi2MoO6 semiconductor nanoplates as the photocatalyst. Generally, ketone was obtained as the main product, but for a number of substrates, alcohol was also observed. Under visible-light irradiation, the Bi2MoO6 nanoplates produce photogenerated holes and electrons: the holes oxidize the alkyl aromatic and form the radical cation, while the photogenerated electrons transfer to Pt NPs. Pt nanoparticles, as cocatalysts, suppress the recombination of a photogenerated charge, promote oxygen reduction and activate the benzylic C–H bonds. The stability of Pt/Bi2MoO6 nanoplates was investigated in a cycling experiment, and no significant activity loss was observed after six consecutive runs (from 90 to 87% conversion).
The Ag-TESPTS@SBA-15 catalyst [67][16], reported by Biswas and coworkers for the selective oxidation of toluene derivatives to the corresponding aldehyde, was also employed in the oxidative C–H bond activation of alkyl aromatics, under eco-friendly reaction conditions, to produce the corresponding ketones. The reactions exhibit good yields (59–80%) and excellent selectivities (91–94%) toward the formation of carbonyl compounds, and very good TON and TOF were also obtained.
Highly active CuO nanoparticles supported on nitrogen-doped carbon hollow nanospheres (CuO/N-CHNSs) were obtained by Kantam and coworkers in 2021 [78][27]. The catalyst was efficiently used in the selective C(sp3)–H bond oxidation of alkyl aromatics to corresponding ketones under mild conditions by using TBHP as the oxidant and water as the green solvent at 80 °C. High conversion and selectivity (up to 99%) were obtained for a wide range of aromatic hydrocarbons. Moreover, the catalyst was easily recovered and reused for up to 5 runs without a significant decrease of catalytic activity (from 98 to 92% yield). The high catalytic activity and reusability was attributed to the synergistic effect between finely dispersed CuO nanoparticles and nitrogen-doped carbon hollow nanospheres support.
LaMnO3 Perovskite nanoparticles were used by Türkmen and coworkers in 2021 [79][28] for the promotion of the oxidative C(sp3)–H activation of alkylarenes by using O2 (1 bar) as the sole oxidant and heptane as the reaction medium at 80 °C. The proposed mechanism involves the formation of surface-bound superoxide anions by the activation of O2 on the surface of LaMnO3 and the successive abstraction of the benzylic hydrogen atom producing the radical intermediate. High yields of ketones were obtained, but the used regeneration protocol did not allow the fully recovery of the initial activity of the spent catalyst.
In 2022, Mg6MnO8 murdochite nanoparticles were used by Hara and coworkers [80][29] in the C–H oxidation of a wide variety of alkylarenes to ketones at 50 °C in the presence of O2 (1 atm) as the sole oxidant and n-octane as the reaction medium. On the basis of kinetic and mechanistic studies, it was hypothesized that the oxidation proceeds via a basicity-controlled C–H activation mechanism involving O2 activation. The murdochite was recovered and reused for 6 runs without changing its high catalytic activity (from 96 to 99% yield).
In the same year, Wang and coworkers [73][22] designed copper nanoparticles supported on porous amide and ether functionalized organic polymer (Cu@AEPOP) as a heterogeneous catalyst for the efficient oxidative C–H bond activation of alkyl arenes to ketones (93–99% yield). The copper nano-catalyst showed remarkable activity at 70 °C, in the presence of TBHP, 70 wt% in water, which was used as the oxidant and the solvent. Hot filtration experiments showed that no Cu ion was leached into the solution and that heterogeneous catalysis was operative: Cu+2 nanoparticles promote the decomposition of TBHP with the formation of surface radical species responsible for C–H benzylic activation by the chain-propagating steps.
In 2015, Ellis and coworkers [81][30] reported the preparation of Pd NPs supported on multiwalled carbon nanotubes (Pd(II)/MWCNT) for the oxidative C(sp3)–H activation of 8-methylquinoline in the presence of PhI(OAc)2 as the oxidant (Scheme 10). When the reaction was performed in MeOH as a solvent, a near-quantitative yield (99%) of 8-(methoxymethyl)quinoline was obtained in 10 min at 100 °C, while in acetic acid at 120 °C for 10 min, the desired 8-(acetoxymethyl)quinoline was obtained in 90% yield. The authors showed that the reactions were faster with Pd(II)/MWCNT than with homogeneous Pd(OAc)2 (Scheme 10). The catalyst was recycled 16 times with minimal reduction of catalytic activity (from 98 to 90% conversion) and very little metal leaching.
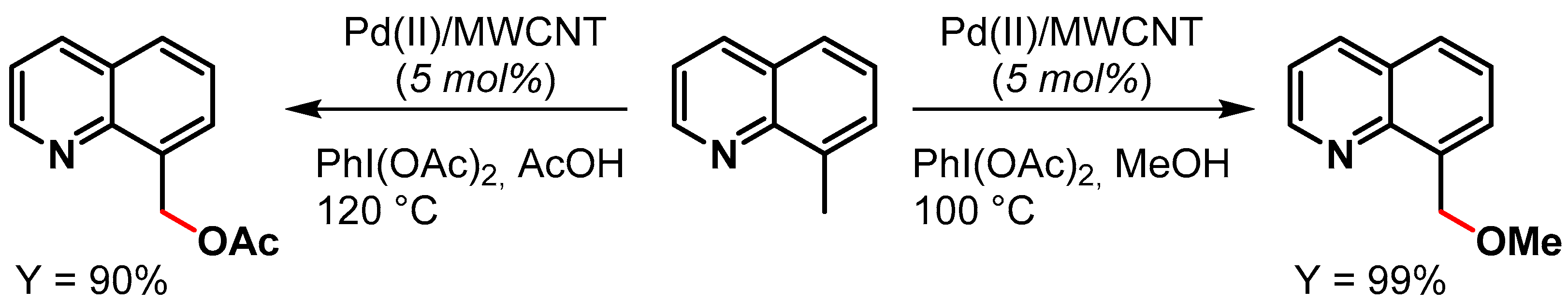
Scheme 10.
Oxidative C(sp3)–H activation of 8-methylquinoline.
More recently, Zhang and coworkers [82][31] prepared monodisperse CuPd alloy nanoparticles supported on reduced graphene oxide (CuPd/rGO) as efficient catalyst for the directed C(sp3)–H activation of 8-methylquinolines in the presence of PhI(OAc)2 as the oxidant and carboxylic acids to form the corresponding 8-(acyloxymethyl)quinoline.
In both papers [81[30][31],82], a N-directing group assisting the Pd(II)/Pd(IV) mechanism via a cyclopalladated intermediate was reported (Scheme 11).
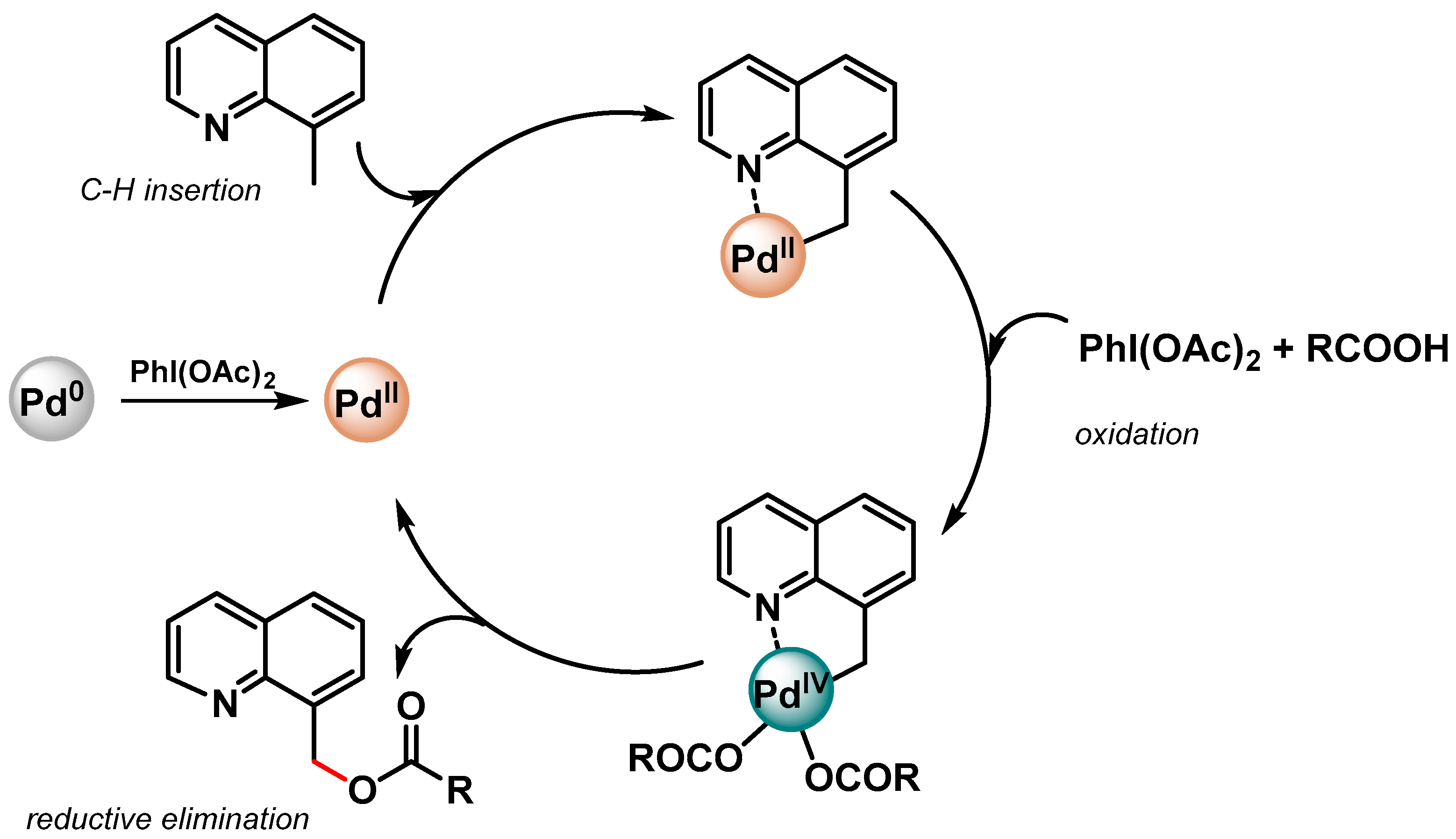
Scheme 11.
Proposed mechanism for N-chelation-directed Pd(II)/Pd(IV) C–H activation.
2.3. Cycloalkane C(sp3)–H Activation
4. Cycloalkane C(sp3)–H Activation
The catalytic oxidative C–H activation of cycloalkanes under mild conditions is one of the principal objectives of catalysis chemistry. Indeed, cycloalkanols and cycloalkanones are involved in the production of commodity chemicals, such as polymers, paints, plastics, etc. For example, the oxidative C−H activation of cyclohexane to cyclohexanone and cyclohexanol is the key step of the commercial production of nylon-6 and nylon-6,6 polymers.
Among the metal nanoparticles, Au NPs have been widely used in the oxidative C–H activation of less reactive cycloalkanes (Scheme 12) [67,83,84,85,86][16][32][33][34][35].
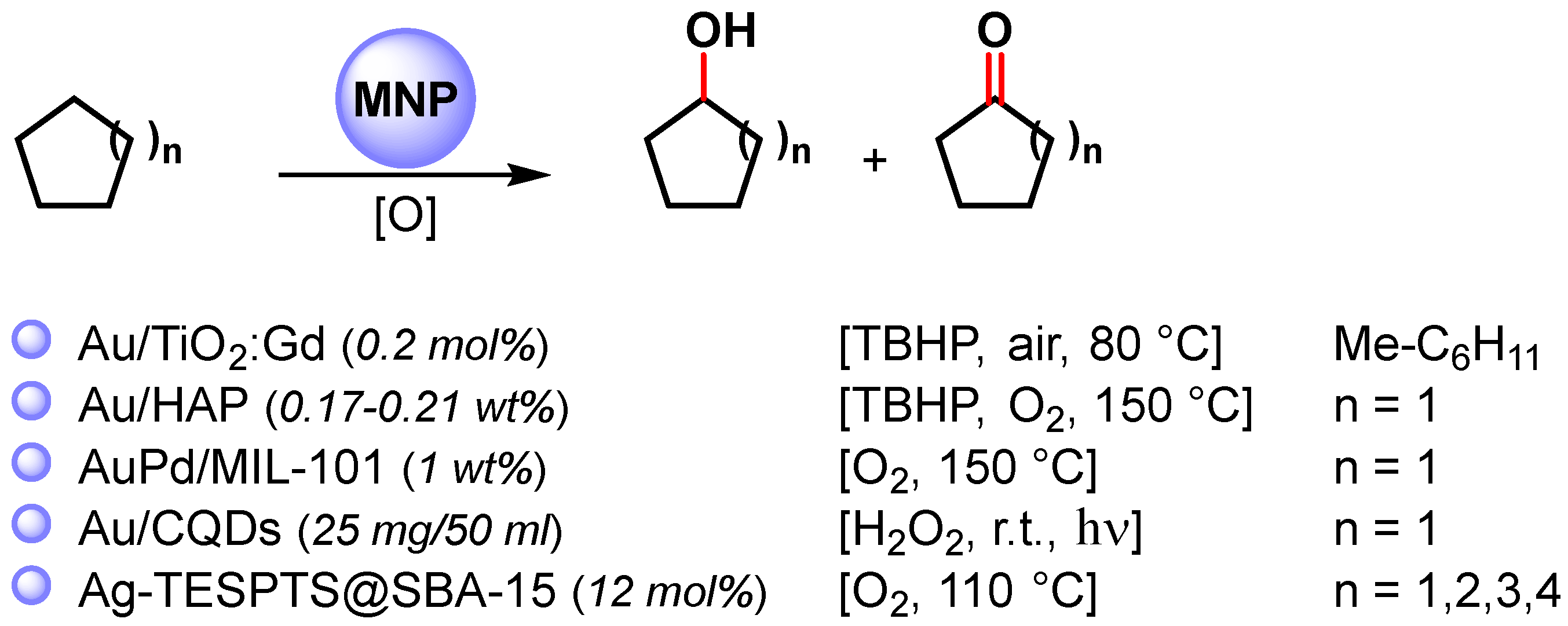
Scheme 12.
Oxidative C–H activation of cycloalkanes.
In 2010, Caps and coworkers [83][32] prepared Au NPs supported on gadolinium-doped titania (Au/TiO2:Gd) with < 3 nm overage size. The catalyst efficiently catalyzed, at 80 °C, in the presence of TBHP and aerobic conditions the methylcyclohexane/stilbene co-oxidation reaction, which led to the formation of 1-metylcicloexanol. The oxidation proceeds via a complex radical chain mechanism in which Au NPs activate TBHP and produce an alkylperoxy radical through a reaction of a tertiary alkyl radical with atmospheric O2.
In 2011, Tsukuda and coworkers [84][33] synthesized ultrasmall Au NPs (<2 nm) with atomically controlled sizes (Aun, with n = 10, 18, 25, 39) immobilized on hydroxyapatite (Au/HAP) for the selective C–H oxidation of cyclohexane to cyclohexanol and cyclohexanone, at 150 °C, in the presence of TBHP and O2 as oxidants. The turnover frequency increased with an increase in the size, reaching values as high as 18 500 h−1 Au atom−1 at n = 3, and thereafter decreased with a further increase in size.
The effect of Au nanoparticle size on catalytic activity was also observed by Golovko and coworkers [87][36] in the aerobic oxidation of the allylic C–H bond of cyclohexene under mild conditions (65 °C). The authors showed that catalytic activity was observed only when >2 nm Au nanoparticles size had formed, while <2 nm particles were inactive in cyclohexene oxidation. The size dependency observed suggested that Au NPs promote the abstraction of allylic hydrogen with the formation of a cyclohexenyl radical that then reacts with dissolved O2.
In 2013, bimetallic AuPd NPs supported on zeolite-type metal-organic frameworks (AuPd/MIL-101) were obtained by Li and coworkers [85][34]. The AuPd alloy catalyst was used in the selective C–H oxidation of cyclohexane at 150 °C in the presence of O2 as the sole oxidant with high TOF (19 000 h−1) and >80% selectivity to cyclohexanone and cyclohexanol. The Au−Pd bimetallic catalysts showed a higher catalytic activity than pure metal or Au+Pd physical mixture in terms of cyclohexane conversion, suggesting that the enhanced surface electron density of the bimetallic Au−Pd catalyst, compared with monometallic Au, favors O2 adsorption and activation to form a surface superoxo species.
In 2014, Zhang and coworkers [86][35] reported the preparation of Au NPs supported on carbon quantum dots (Au/CQDs) and their use in the photocatalytic selective C–H oxidation of cyclohexane to cyclohexanone at room temperature, under visible-light irradiation and in the presence of H2O2 as an oxidant. The best conversion efficiency (63.8%) and selectivity (99.9%) was obtained under green-light irradiation, which matches with the surface plasma resonance zone of AuNPs. Light absorption enhanced the catalytic activity of Au/CQDs toward H2O2 decomposition to hydroxy radicals, suggesting that HO. is the active oxygen species for this photocatalytic C–H activation.
More recently, highly dispersed Ag NPs supported over 2D hexagonally ordered functionalized mesoporous silica material (Ag-TESPTS@SBA-15, AgMS) was reported by Biswas and coworkers [67][16]. Alongside the selective benzylic C(sp3)–H bond oxidation of toluenes and alkyl arenes to the corresponding carbonyl compounds described above, the authors showed that the catalyst was also active in the oxidative C–H activation of less reactive cyclic alkanes to ketones with good yield (61–69%) and selectivity (73–81%).
The copper-catalyzed oxidative esterification of carboxylic acids with common alkanes via C(sp3)–H bond dehydrogenation was reported by Li and coworker in 2015 [62][11]. The preparation of allylic esters was catalyzed by CuO in the presence of di-tert-butylperoxide (DTBP) as oxidant and a catalytic amount of K2CO3 as promoter (Scheme 13). Radical inhibitors, such TEMPO, completely suppressed the reaction, indicating a radical pathway for the C–H oxidative dehydrogenation and esterification cascade.
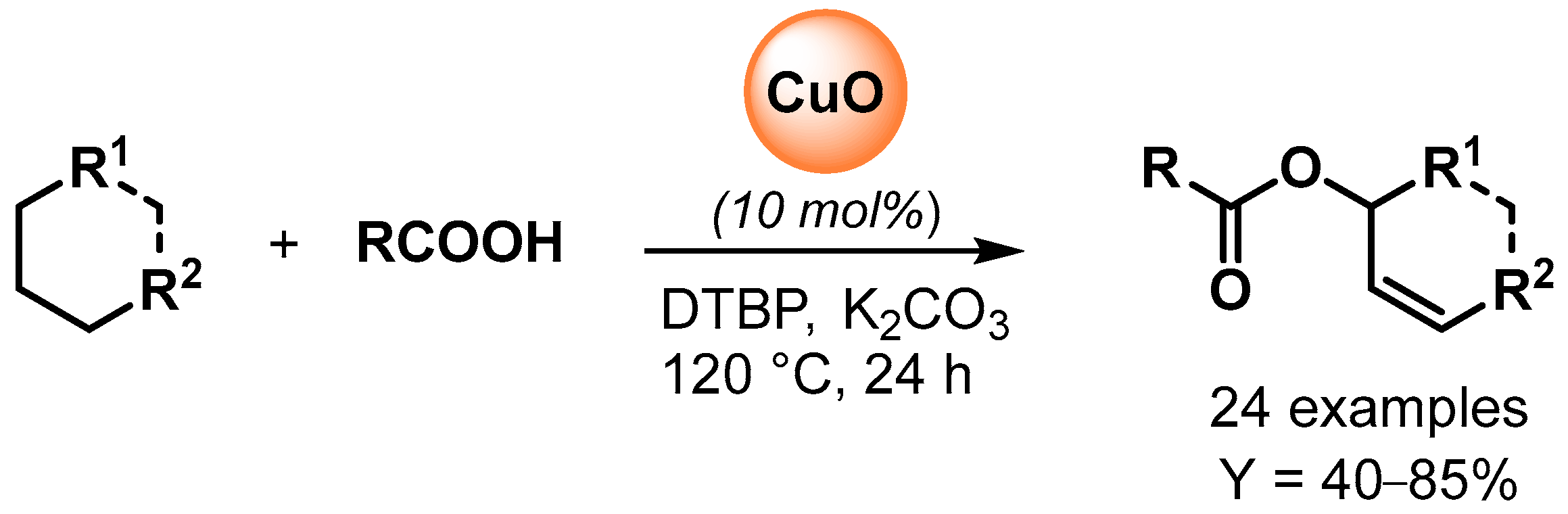
Scheme 13.
Copper-catalyzed oxidative coupling of carboxylic acids with alkanes.
The direct esterification of benzoic acid with cyclic ether via C(sp3)–H bond activation by using non-noble metal nanoparticles was firstly reported by Kantam and coworkers in 2015 [88][37] and then by Jia and coworkers in 2021 [89][38] (Scheme 14) [88,89][37][38].
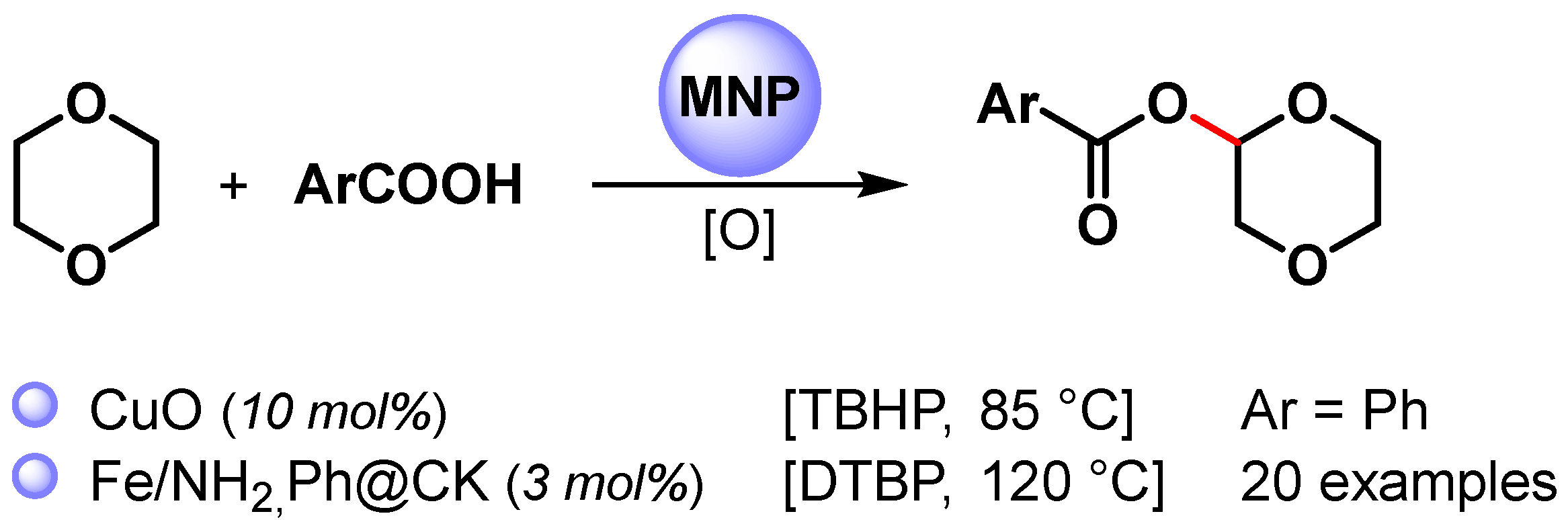
Scheme 14.
Direct esterification of benzoic acids with cyclic ether via C(sp3)–H bond activation.
When CuO nanoparticles were used in the presence of TBHP as an oxidant at 85 °C, 1,4-dioxane underwent a cross-dehydrogenative coupling reaction with benzoic acid, producing the corresponding benzoate in 91% yield [88][37].
A wide variety of α-acyloxy ethers were obtained in moderate to good yields (up to 75%) via the direct cross-dehydrogenative-coupling reaction of arylcarboxylic acids with cyclic ethers by using Fe NPs supported on organo-modified coal-bearing kaolin (NH2,Ph@CK) as a heterogeneous catalyst and DTBP as an oxidant at 120 °C [89][38]. The competing kinetic isotope effect (1,4-dionane/1,4-dioxane-d8, kH/kD = 2.5) and control experiments with TEMPO suggested a radical pathway in which the C(sp3)–H bond cleavage of ether was the rate-determining step.
2.4. Aromatic C(sp2)–H Activation
5. Aromatic C(sp2)–H Activation
The direct C(sp2)–H activation of aromatics by using metal and metal–oxide nanoparticles has been widely explored, but the preparation and the use of cost-effective nano-catalysts under milder reaction conditions remain a challenge.
In particular, the synthesis of phenols is a very important process in the fine-chemical industry and their preparation by direct C–H hydroxylation is highly desirable. Recent examples of the direct C–H functionalization of benzene to phenol, catalyzed by metal nanoparticles, are shown in Scheme 15 [76,90,91,92][25][39][40][41].
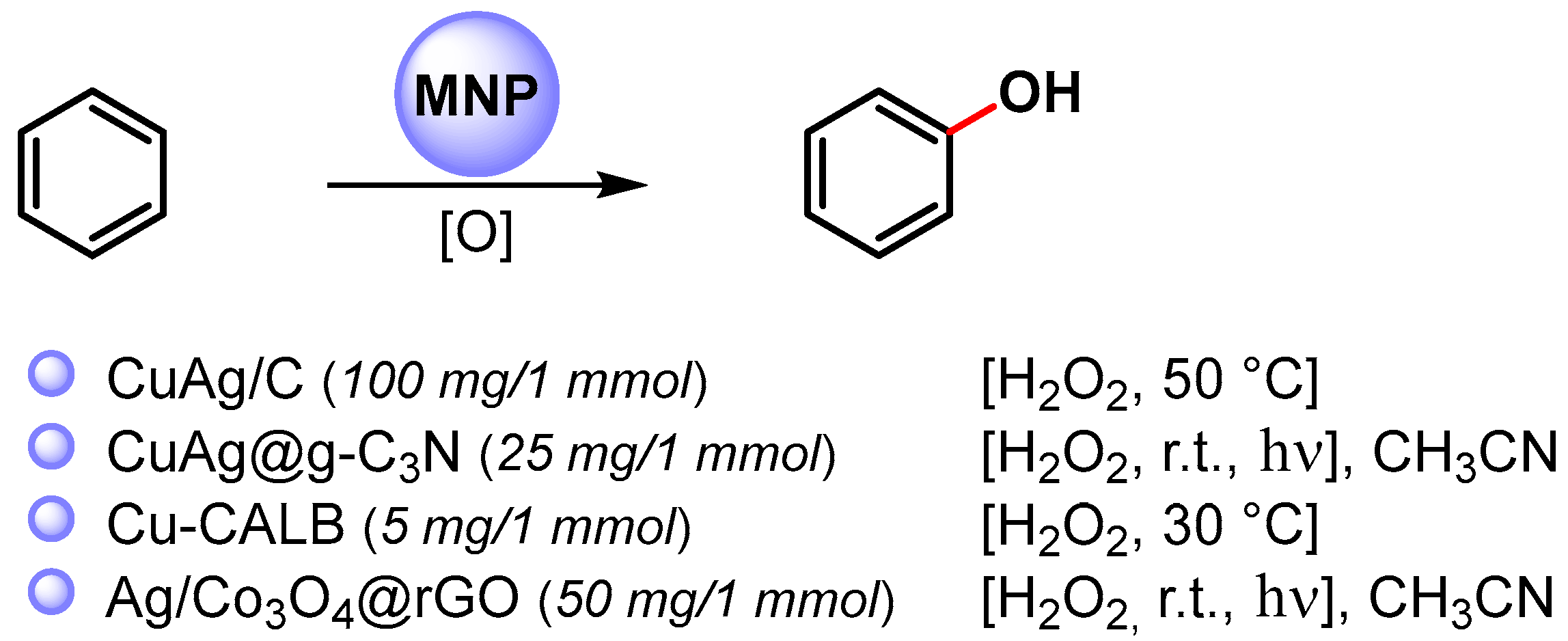
Scheme 15.
Direct hydroxylation of benzene via C–H activation.
In 2016, Jiang and coworkers [90][39] reported the preparation of bimetallic Cu-Ag NPs supported on biochar (Cu-Ag/C) obtained by the one-pot fast pyrolysis of Cu-Ag preloaded onto biomass waste and its use in the direct hydroxylation of benzene to phenol by using H2O2 as an oxidant at 50 °C. The best reaction yield (35%) and selectivity (96%) was obtained with acetic acid as the reaction medium. The catalyst showed only a slightly decrease of performance over three consecutive runs, indicating that Cu-Ag/C has good reusability.
A year later, Varma and coworkers [91][40] used photoactive bimetallic Cu-Ag NPs immobilized onto a graphitic carbon nitride surface (CuAg@g-C3N) for the C–H activation and hydroxylation of benzene. Quantitative conversion was obtained in 30 min, in CH3CN, by using H2O2 as an oxidant, at room temperature under visible-light irradiation (20 W domestic bulb). In dark conditions, the reaction did not proceed. Graphitic carbon nitride support absorbs the visible light promoting the degradation of hydrogen peroxide into hydroxyl radicals.
In both papers [90,91][39][40], it was shown that bimetallic Cu-Ag NPs were more active than monometallic Cu or Ag NPS, suggesting a synergistic effect between Ag and Cu in the formation of the hydroperoxide radical and the activation of the C–H bond on the benzene ring. Moreover, it was hypothesized that the carbonaceous supports grip benzene molecules on its surface through non-covalent interactions, thus facilitating the C–H bond breaking and the attack of HO..
In 2020, Palomo and coworkers [92][41] synthesized nanobiohybrids containing copper nanoparticles by using the enzyme Lipase B from Candida antarctica (CAL-B) in combination with copper sulfate. Depending on the experimental conditions, enzymes facilitate the formation of a particular Cu species, such as Cu(0)NPs, Cu(0)/Cu2ONPs, Cu2ONPs or Cu3(PO4)2NPs. The catalytic activity of heterogeneous enzyme–copper nanobiohybrids was evaluated with the direct C–H activation hydroxylation of benzene using H2O2 as an oxidant, in aqueous medium at 30 °C. The enzyme-copper nanobiohybrid obtained in phosphate buffer without treatment with a reducing agent (Cu-CALB-PHOS-NR) showed the highest activity, and, when the hydroxylation was carried out in the presence of 20% acetonitrile as co-solvent, phenol was obtained with 24% yield and 83% selectivity. A radical mechanism that involves the degradation of H2O2 via a Fenton-like reaction to produce a hydroxyl radical and Cu(III) was hypothesized.
In same year, Zhang and Liu [76][25] reported the preparation of small-sized Ag NPs (2–10 nm) supported on Co3O4 nanoparticles embedded on the surface of reduced graphene oxide. The photocatalyst efficiently catalyzed the hydroxylation of benzene to phenol via C–H activation in acetonitrile, under visible-light irradiation. A conversion of 99% and 96% phenol selectivity was obtained in 30 min. The high catalytic activity was ascribable both to the high dispersion of the nanocomposite over the support and to the high surface area. The nanocomposite was conveniently recovered and reused for seven runs without the loss of its catalytic activity (≥98% conversion, ≥95% selectivity). The formation of phenol takes place via a radical pathway on the surface of the photocatalyst where the photo-active bimetallic NPs promote the degradation of hydrogen peroxide into hydroxyl radical and the noncovalent interactions of benzene with graphene surface activate the substrate toward C–H hydroxylation.
High selective C–O bond formation via the C(sp2)−H bond activation of aromatic compounds can be achieved by using palladium catalysis combined with an intramolecular directing group, such as a pyridinyl or other nitrogen-functional groups. In most cases, homogeneous Pd(II) catalysts are used, and only a few examples have been reported in the use of heterogeneous Pd NPs (Scheme 16) [81,93,94][30][42][43]. Generally, these reactions utilize a Pd(II)/Pd(IV) catalytic cycle (see Scheme 11) [81,82][30][31].
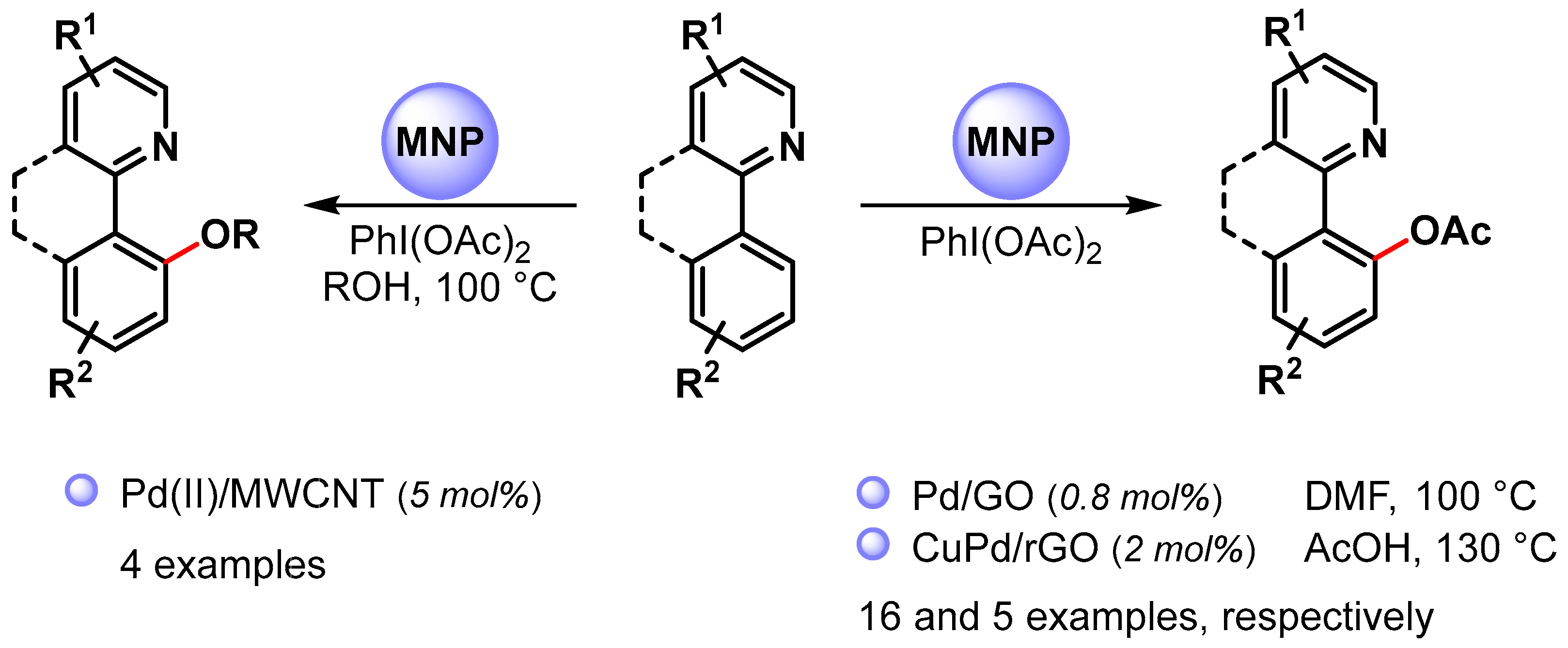
Scheme 16.
N-Chelation-directed C(sp2)–H activation for C–O bond formation.
In 2015, Ellis and coworkers [81][30] used Pd(II) NPs supported on multiwalled carbon nanotubes (Pd(II)/MWCNT) in the selective N-chelation-directed C−H activation reactions of benzo[h]quinoline in the presence of PhI(OAc)2 as an oxidant and alcohols as a solvent at 100 °C. When methanol was used as a solvent, 9-metoxy-derivatives were obtained in high yield (90%). However, as the size of the alcohol increased (ethanol, isopropanol), the product yield decreased (73% and 33%, respectively), and, in the case of tert-butyl alcohol, no product was observed.
In 2017, Junmin Zhang and coworker [93][42] reported the use of Pd NPs supported on graphene oxide (Pd/GO) for the selective acetoxylation of 2-aryl pyridines by using PhI(OAc)2 as an oxidant in DMF at 100 °C. High yields were obtained for a wide variety of substrates (up to 98%), and the authors showed that the catalytic efficiency of PdNPs/GO was superior to that of free Pd(OAc)2. A hot filtration test and a Hg poisoning test suggested a heterogeneous catalysis but a recycling test showed a drastic decrease in yield on the third run. This was mainly attributed to palladium leaching in the reaction medium (>40% in the first cycle) and to Pd NPs aggregation.
More recently, Wu Zhang and coworkers [94][43] prepared CuPd alloy nanoparticles immobilized on reduced graphene oxide (CuPd@rGO) for the N-chelation assisted C(sp2)–H bond acetoxylation using different directing group, including pyridine, pyrazole, benzothiazole and pyrimidine. The reactions were performed in chlorobenzene in the presence of PhI(OAc)2 and acetic acid at 130 °C. Acetoxy derivatives were obtained in moderate to good yield, and the best result was obtained with pyridine as the directing group (90% yield). The catalyst was recovered and reused for five cycles with only a slight decrease in catalytic activity (from 94 to 85% yield).
In 2022, high-performance Pd NPs supported on nitrogen-doped reduced graphene oxide (Pd/N-RGO) were developed by Movahed and coworker [95][44]. The nanohybrid catalyst was used as a heterogeneous catalyst for the ortho C–H acyloxylation of N-arylcarbamates with carboxylic acid in the presence of K2S2O8 as an oxidant in dichloroethane (DCE) at 120 °C (Scheme 17). Good yields were obtained with both aliphatic and aromatic carboxylic acid (up to 95%), but bulky alkyl side-chain carboxylic acid (t-Bu and i-Pr) showed no reactivity. Moreover, the presence of an electron-donating group on phenyl-carbamate led to a higher yield compared to the electron-withdrawing group. These effects, together with the experiment on the radical scavenger TEMPO that showed no effect on the reaction yield, were consistent with an electrophilic palladation pathway with the formation of a cyclopalladated intermediate in which K2S2O8 plays an important role in maintaining the Pd(II)/Pd(0) and Pd(II) Pd(IV) catalytic cycle. The heterogeneous catalyst was reused ten times (from 93 to 75% yield), and additionally, when the Pd(OAc)2 homogeneous catalyst was used, only a moderate yield was obtained, indicating that the nitrogen-doped graphene support could improve the activity and the stability of the Pd catalyst.
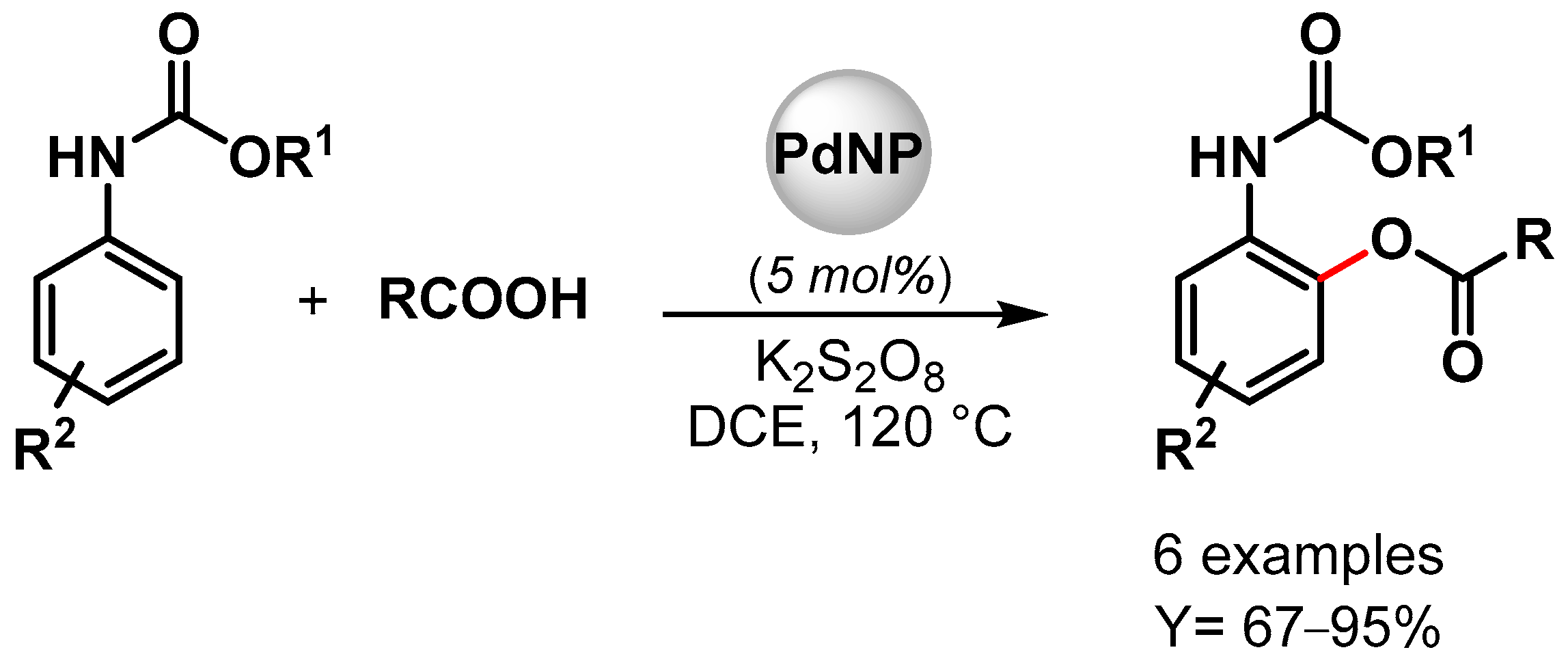
Scheme 17.
Pd/N-RGO-catalyzed C–H acyloxylation of N-arylcarbamates.
2.5. Olefinic C(sp2)–H Activation
6. Olefinic C(sp2)–H Activation
In 2016, Banerjee and coworkers [96][45] reported the synthesis of poly-substituted furans via the oxidative C–H/C–H functionalization of 1,3-dicarbonyls and α,β-unsaturated carbonyl compounds catalyzed by CuO nanoparticles in the presence of TBHP as an oxidant in aqueous ethanol as a green solvent (Scheme 18). A control experiment with TEMPO suppressed the reaction, indicating a radical pathway with CuO nanoparticles promoting the decomposition of TBHP with the formation of surface radical species that activated the C(α)–H abstraction of 1,3-dicarbonyl and the successive conjugate addition/domino reactions. High yields of substituted furans were obtained for a large number of substrates, and good recyclability was obtained for up to six cycles, but a slight drop in the yield was observed thereafter (from 81 to 70% yield). The XRD pattern of recycled CuO NPs after the fourth run showed that the morphology of the particles was unchanged but that the size increased from 10 to 15 nm.
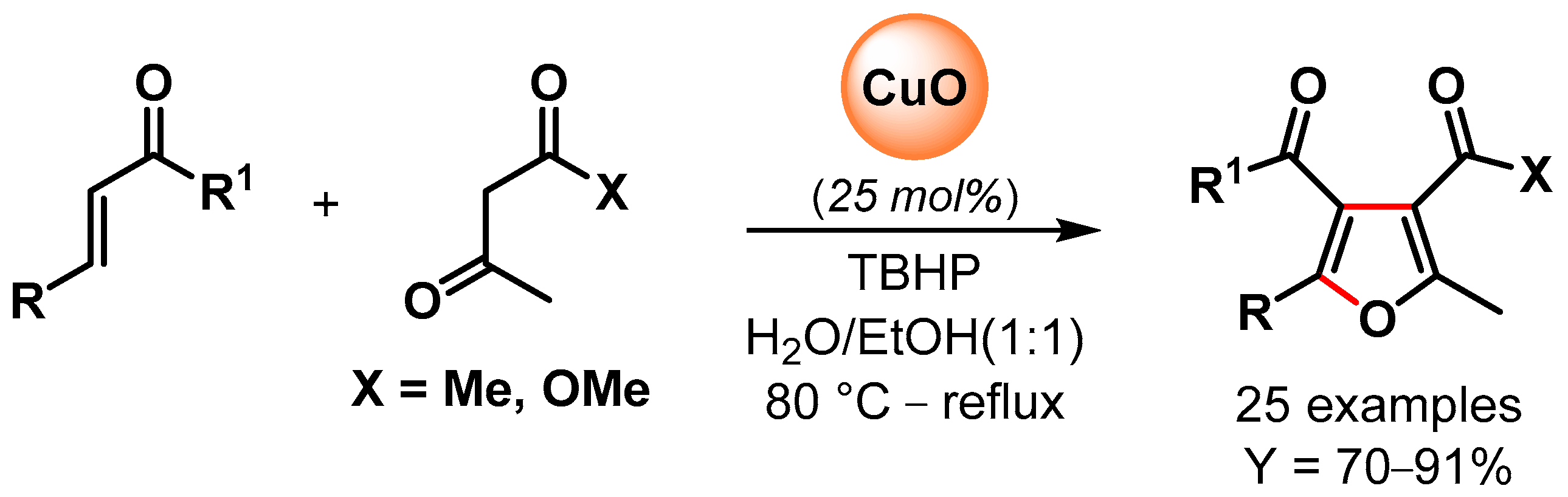
Scheme 18. CuO NPs catalyzed synthesis of substituted furans via oxidative C–H/C–H functionalization in aqueous medium.
In 2018, Yamaguchi and coworkers [97][46] reported the preparation of aurones, unusual five-membered ring flavonoids, via the direct α-olefinic C–H activation of chalcones by using Pd-on-Au bimetallic NPs supported by a CeO2 catalyst (Pd/Au@CeO2, 5 mol%) under air at 100 °C (Scheme 19). Moderate to good yields (16–72%) with high Z-selectivity (93–99%) in the aurone products were obtained. The monometallic Pd@CeO2 showed very low conversion, while Au@CeO2 was highly active, but the formation of a six-membered flavone was observed. The authors hypothesized that the role of Au was to stabilize and improve the catalytic activity of Pd(0) NPs. The control experiment showed that the reaction rate was not affected by adding the radical scavenger TEMPO, suggesting Pd(0)/Pd(II) α-olefinic C–H bond activation with the formation of a six-membered palladacycle intermediate.
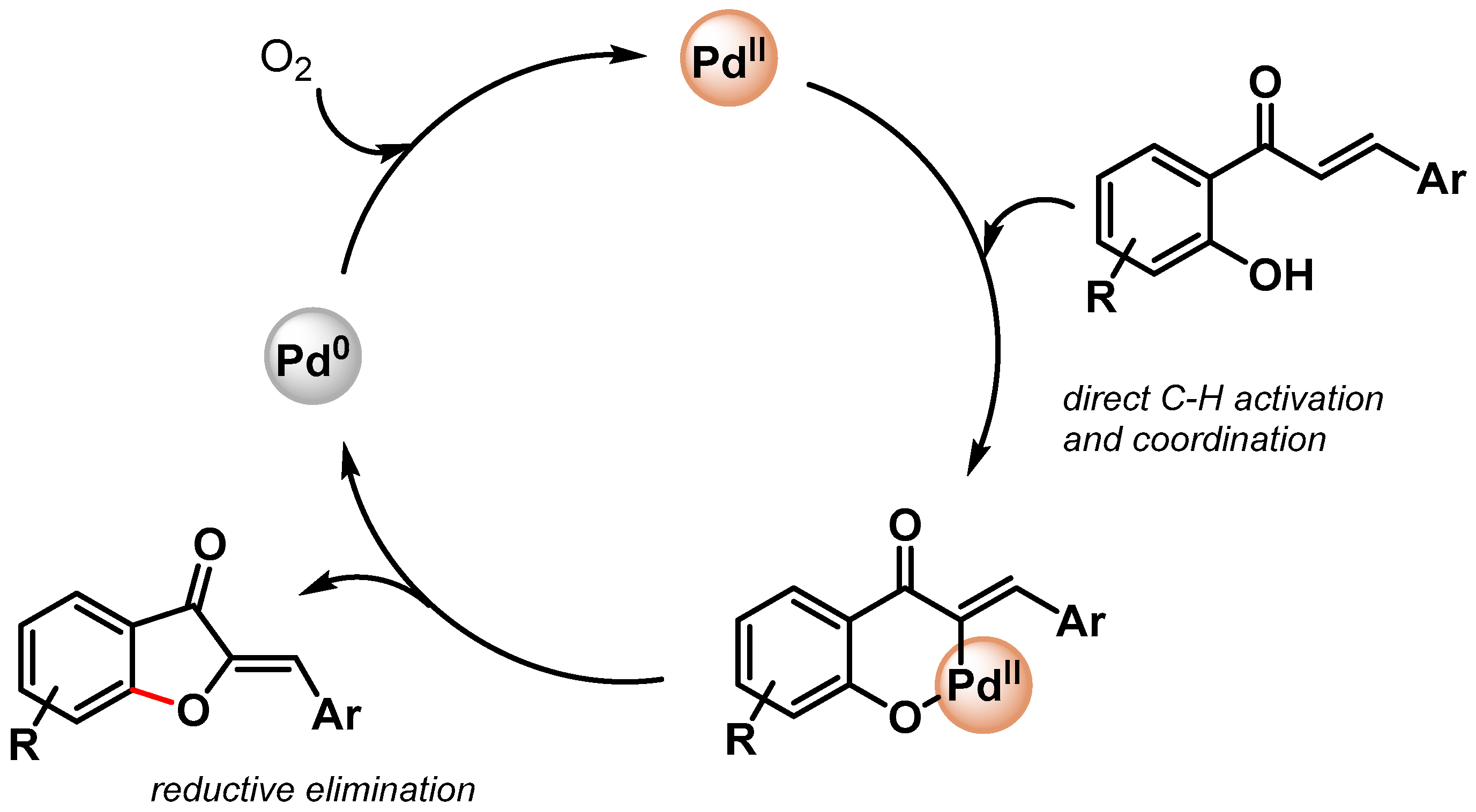
Scheme 19. Preparation of aurones via direct Pd/Au@CeO2 catalyzed α-olefinic C–H activation of chalcones.
In 2019, Maji and coworkers [98][47] prepared Pd nanoparticles deposited on amyloid fibril (α-Syn-PdNPs), a sustainable catalyst for the preparation of benzofuran involving C–C and C–O bond formation via the triple C–H bond activation of phenols and alkenes (Scheme 20). Among various oxidants, the best results were obtained with V2O5, and good to excellent yields (up to 91%) were obtained for a wide variety of substrates. The heterogeneous Pd nanocomposite was reused for up to four runs with a negligible loss of catalytic activity (from 95 to 90% yield). A plausible mechanism for the formation of benzofuran via the insertion of Pd(0) at the ortho position of phenol and the successive coordination and migratory insertion of the olefin at the Pd(II) center was hypothesized.
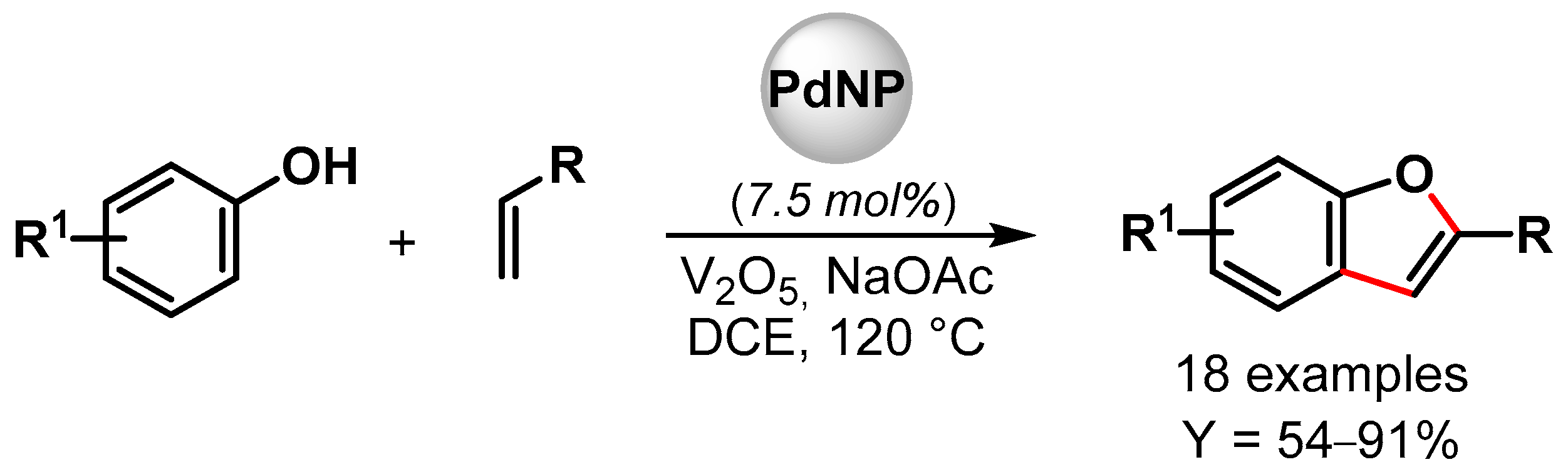
Scheme 20.
Preparation of benzofuran via C–H activation using amyloid fibril-supported PdNPs.
2.6. Formyl C(sp2)–H Activation
7. Formyl C(sp2)–H Activation
In 2014, 2,5-disubstituted 1,3,4-oxadiazoles have been synthesized by Bojja and coworkers [99][48] through oxidative C–O coupling via the direct C–H bond activation of N-aroyl-N-arylidinehydrazines using CuO nanoparticles as a catalyst (Scheme 21). Performing the reactions in the presence of Cs2CO3 as a base, in DMSO, under air at 80 °C, excellent to good yields were obtained for a large number of substrates. Moreover, an electron-withdrawing substituent on the arylidinehydrazine moiety improved the yields, probably through an increment of the acidity of the imine proton and thus facilitating the metalation step. The catalyst has been reused for three times with a negligible decrease in catalytic activity (from 90 to 85% yield).
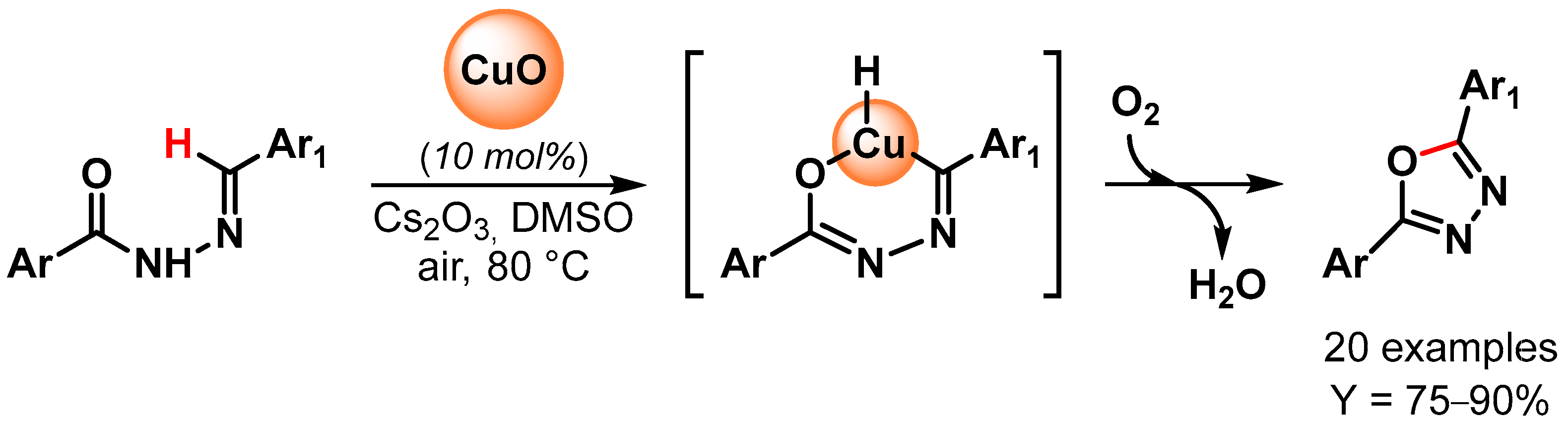
Scheme 21.
Synthesis of 2,5-disubstituted 1,3,4-oxadiazoles via direct oxidative C–H bond activation.
Recently, copper nanoparticles were investigated as environmentally friendly heterogeneous catalysts for the synthesis of enol carbamates via the oxidative coupling of N,N-dialkyl formamides with β-ketoesters [100,101][49][50] (Scheme 22).
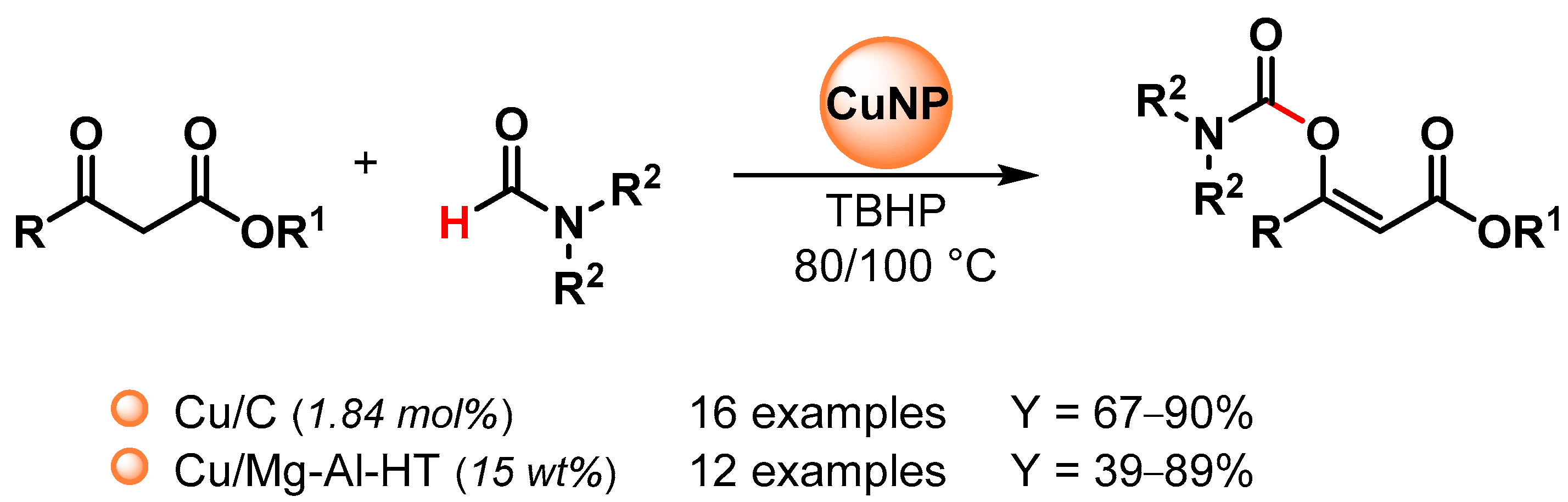
Scheme 22.
Copper catalyzed enol carbamates synthesis via direct C–H activation of dialkylformamide.
In 2016, Niknam and coworkers [100][49] reported the use of copper NPs on charcoal (Cu/C) as an effective nano-catalyst for enol carbamates synthesis via the C–H bond activation of dialkylformamides by using TBHP as an oxidant at 80 °C (Scheme 22). The catalyst was reused at least five times with no appreciable loss in catalytic activity (from 85 to 75% yield).
In 2020, Mannepalli and coworkers [101][50] described the preparation of a copper-supported Mg-Al hydrotalcite-derived oxide catalyst (Cu/Mg-Al-HT) by a co-precipitation method, followed by wet impregnation and calcination and its use in enol carbamates synthesis via the oxidative C–H bond activation of dimethylformamide by using TBHP as an oxidant at 100 °C (Scheme 22). The catalyst was recovered and reused for up to four runs without a loss in catalytic activity (from 89 to 87% conversion). Mechanistically, β-ketoester forms a coordination complex with copper, and the complex decomposes TBHP to generate the tert-butyloxy radical that can abstract the formamide hydrogen on the copper surface.
References
- Fessner, N.D. P450 Monooxygenases Enable Rapid Late-Stage Diversification of Natural Products via C−H Bond Activation. ChemCatChem 2019, 11, 2226–2242.
- Borpatra, P.J.; Deka, B.; Deb, M.L.; Baruah, P.K. Recent advances in intramolecular C–O/C–N/C–S bond formation: Via C–H functionalization. Org. Chem. Front. 2019, 6, 3445–3489.
- Newhouse, T.; Baran, P.S. If C–H Bonds Could Talk: Selective C–H Bond Oxidation. Angew. Chem. Int. Ed. 2011, 50, 3362–3374.
- Schuchardt, U.; Cardoso, D.; Sercheli, R.; Pereira, R.; da Cruz, R.S.; Guerreiro, M.C.; Mandelli, D.; Spinace, E.V.; Pires, E.L. Cyclohexane oxidation continues to be a challenge. Appl. Catal. A Gen. 2001, 211, 1–17.
- Pla, D.; Gómez, M. Metal and Metal Oxide Nanoparticles: A Lever for C–H Functionalization. ACS Catal. 2016, 6, 3537–3552.
- Dimitratos, N.; Lopez-Sanchez, J.A.; Hutchings, G.J. Selective liquid phase oxidation with supported metal nanoparticles. Chem. Sci. 2012, 3, 20–44.
- Wang, V.C.-C.; Maji, S.; Chen, P.P.-Y.; Lee, H.K.; Yu, S.S.-F.; Chan, S.I. Alkane Oxidation: Methane Monooxygenases, Related Enzymes, and Their Biomimetics. Chem. Rev. 2017, 117, 8574–8621.
- Latimer, A.A.; Kulkarni, A.R.; Aljama, H.; Montoya, J.H.; Yoo, J.S.; Tsai, C.; Abild-Pedersen, F.; Studt, F.; Noerskov, J.K. Understanding trends in C–H bond activation in heterogeneous catalysis. Nat. Mater. 2017, 16, 225–229.
- Freakley, S.J.; Dimitratos, N.; Willock, D.J.; Taylor, S.H.; Kiely, C.J.; Hutchings, G.J. Methane Oxidation to Methanol in Water. Acc. Chem. Res. 2021, 54, 2614–2623.
- Sundberg, M.R.; Zborowski, K.K.; Alkorta, I. Multiple 3cA2e bonding of methane with metal cations. Chem. Phys. Lett. 2011, 515, 210–213.
- Voutchkova, A.M.; Crabtree, R.H. Iridium-catalyzed benzylic C–H activation and functionalization of alkyl arenes. J. Mol. Catal. A Chem. 2009, 312, 1–6.
- Kesavan, L.; Tiruvalam, R.; Ab Rahim, M.H.; bin Saiman, M.I.; Enache, D.I.; Jenkins, R.L.; Dimitratos, N.; Lopez-Sanchez, J.A.; Taylor, S.H.; Knight, D.W.; et al. Solvent-Free Oxidation of Primary Carbon-Hydrogen Bonds in Toluene Using Au-Pd Alloy Nanoparticles. Science 2011, 331, 195–199.
- Binsaiman, M.I.; Brett, G.L.; Tiruvalam, R.; Forde, M.M.; Sharples, K.; Thetford, A.; Jenkins, R.L.; Dimitratos, N.; Lopez-Sanchez, J.A.; Murphy, D.M.; et al. Involvement of surface-bound radicals in the oxidation of toluene using supported Au-Pd nanoparticles. Angew. Chem. Int. Ed. 2012, 51, 5981–5985.
- Lingampalli, S.R.; Gupta, U.; Gautam, U.K.; Rao, C.N.R. Oxidation of toluene and other examples of C–H bond activation by CdO2 and ZnO2 nanoparticles. ChemPlusChem 2013, 78, 837–842.
- Das, P.P.; Chowdhury, B. Indium oxide nanoparticles embedded in TUD-1 as a highly selective catalyst for toluene to benzaldehyde oxidation using TBHP as oxidant. Chem. Pap. 2020, 74, 2091–2100.
- Biswas, R.; Das, S.K.; Bhaduri, S.N.; Bhaumik, A.; Biswas, P. AgNPs Immobilized over Functionalized 2D Hexagonal SBA-15 for Catalytic C–H Oxidation of Hydrocarbons with Molecular Oxygen under Solvent-Free Conditions. ACS Sust. Chem. Eng. 2020, 8, 5856–5867.
- Dai, Y.; Poidevin, C.; Ochoa-Hernández, C.; Auer, A.A.; Tüysüz, H. A Supported Bismuth Halide Perovskite Photocatalyst for Selective Aliphatic and Aromatic C–H Bond Activation. Angew. Chem. Int. Ed. 2020, 59, 5788–5796.
- Zhang, Z.; Yang, Y.; Wang, Y.; Yang, L.; Li, Q.; Chen, L.; Xu, D. Revealing the A-Site Effect of Lead-Free A3Sb2Br9 Perovskite in Photocatalytic C(sp3)−H Bond Activation. Angew. Chem. Int. Ed. 2020, 59, 18136–18139.
- Yan, Y.; Ye, B.; Chen, M.; Lu, L.; Yu, J.; Zhou, Y.; Wang, Y.; Liu, J.; Xiao, L.; Zou, S.; et al. Site-specific deposition creates electron-rich Pd atoms for unprecedented C−H activation in aerobic alcohol oxidation. Chin. J. Catal. 2020, 41, 1240–1247.
- Majumdar, B.; Bhattacharya, T.; Sarma, T.K. Gold Nanoparticle-Polydopamine-Reduced Graphene Oxide Ternary Nanocomposite as an Efficient Catalyst for Selective Oxidation of Benzylic C(sp3)–H Bonds under Mild Conditions. ChemCatChem 2016, 8, 1825–1835.
- Huang, C.; Su, X.; Gu, X.; Liu, R.; Zhu, H. Bimetallic oxide nanoparticles confined in ZIF-67-derived carbon for highly selective oxidation of saturated C–H bond in alkyl arenes. Appl. Organomet. Chem. 2021, 35, e6047.
- Dong, Z.; Pan, H.; Yang, L.; Fan, L.; Xiao, Y.; Chen, J.; Wang, W. Porous organic polymer immobilized copper nanoparticles as heterogeneous catalyst for efficient benzylic C–H bond oxidation. J. Saudi Chem. Soc. 2022, 26, 101397.
- Liu, H.; Chen, G.; Jiang, H.; Li, Y.; Luque, R. From alkyl aromatics to aromatic esters: Efficient and selective C–H activation promoted by a bimetallic heterogeneous catalyst. ChemSusChem 2012, 5, 1892–1896.
- Verma, S.; Nasir Baig, R.B.; Nadagouda, M.N.; Varma, R.S. Photocatalytic C–H activation and oxidative esterification using Catal. Today 2018, 309, 248–252.
- Zhang, Y.; Liu, G. Development of highly efficient and durable reduced graphene oxide decorated with Ag/Co3O4 nanocomposite towards photocatalytic C–H activation. J. Photochem. Photobiol. A Chem 2020, 394, 112494.
- Zhang, B.; Yang, X.; Li, J.; Cheng, G. Selective aerobic oxidation of alkyl aromatics on Bi2MoO6 nanoplates decorated with Pt nanoparticles under visible light irradiation. Chem. Commun. 2018, 54, 12194–12197.
- Gupta, S.S.R.; Lakshmi Kantam, M. Finely dispersed CuO on nitrogen-doped carbon hollow nanospheres for selective oxidation of sp3 C–H bonds. New J. Chem. 2021, 45, 16179–16186.
- Sahin, Y.; Sika-Nartey, A.T.; Ercan, K.E.; Kocak, Y.; Senol, S.; Ozensoy, E.; Türkmen, Y.E. Precious Metal-Free LaMnO3 Perovskite Catalyst with an Optimized Nanostructure for Aerobic C–H Bond Activation Reactions: Alkylarene Oxidation and Naphthol Dimerization. ACS Appl. Mater. Interfaces 2021, 13, 5099–5110.
- Hayashi, E.; Tamura, T.; Aihara, T.; Kamata, K.; Hara, M. Base-Assisted Aerobic C–H Oxidation of Alkylarenes with a Murdochite-Type Oxide Mg6MnO8 Nanoparticle Catalyst. ACS Appl. Mater. Interfaces 2022, 14, 6528–6537.
- Korwar, S.; Brinkley, K.; Siamaki, A.R.; Gupton, B.F.; Ellis, K.C. Selective N -Chelation-directed C–H activation reactions catalyzed by pd(II) nanoparticles supported on multiwalled carbon nanotubes. Org. Lett. 2015, 17, 1782–1785.
- Wang, F.; Huang, F.; Yu, Y.; Zhou, S.; Wang, Z.; Zhang, W. Monodisperse CuPd alloy nanoparticles supported on reduced graphene oxide as efficient catalyst for directed C−H activation. Catal. Commun. 2021, 153, 106296.
- Mendez, V.; Guillois, K.; Daniele, S.; Tuel, A.; Caps, V. Aerobic methylcyclohexane-promoted epoxidation of stilbene over gold nanoparticles supported on Gd-doped titania. Dalton Trans. 2010, 39, 8457–8463.
- Liu, Y.; Tsunoyama, H.; Akita, T.; Xie, S.; Tsukuda, T. Aerobic oxidation of cyclohexane catalyzed by size-controlled au clusters on hydroxyapatite: Size effect in the sub-2 nm regime. ACS Catal. 2011, 1, 2–6.
- Long, J.; Liu, H.; Wu, S.; Liao, S.; Li, Y. Selective oxidation of saturated hydrocarbons using Au-Pd alloy nanoparticles supported on metal-organic frameworks. ACS Catal. 2013, 3, 647–654.
- Liu, R.; Huang, H.; Li, H.; Liu, Y.; Zhong, J.; Li, Y.; Zhang, S.; Kang, Z. Metal nanoparticle/carbon quantum dot composite as a photocatalyst for high-efficiency cyclohexane oxidation. ACS Catal. 2014, 4, 328–336.
- Donoeva, B.G.; Ovoshchnikov, D.S.; Golovko, V.B. Establishing a Au nanoparticle size effect in the oxidation of cyclohexene using gradually changing Au catalysts. ACS Catal. 2013, 3, 2986–2991.
- Priyadarshini, S.; Amal Joseph, P.J.; Lakshmi Kantam, M. Copper catalyzed cross-coupling reactions of carboxylic acids: An expedient route to amides, 5-substituted γ-lactams and α-acyloxy esters. RSC Adv. 2013, 3, 18283–18287.
- Bao, U.; Muschin, T.; Bao, A.; Bao, Y.S.; Jia, M. FeNP-loaded coal-bearing kaolin catalysts for the direct esterification of benzoic acid with cyclic ether via C(sp3)–H bond activation. Green Chem. Lett. Rev. 2021, 14, 565–577.
- Tian, K.; Liu, W.J.; Zhang, S.; Jiang, H. One-pot synthesis of a carbon supported bimetallic Cu-Ag NPs catalyst for robust catalytic hydroxylation of benzene to phenol by fast pyrolysis of biomass waste. Green Chem. 2016, 18, 5643–5650.
- Verma, S.; Nasir Baig, R.B.; Nadagouda, M.N.; Varma, R.S. Hydroxylation of Benzene via C–H Activation Using Bimetallic 3N4. ACS Sust. Chem. Eng. 2017, 5, 3637–3640.
- Losada-García, N.; Rodríguez-Otero, A.; Palomo, J.M. Tailorable synthesis of heterogeneous enzyme-copper nanobiohybrids and their application in the selective oxidation of benzene to phenol. Catal. Sci. Technol. 2020, 10, 196–206.
- Zhang, Y.; Zhao, Y.; Luo, Y.; Xiao, L.; Huang, Y.; Li, X.; Peng, Q.; Liu, Y.; Yang, B.; Zhu, C.; et al. Directed Aromatic C–H Activation/Acetoxylation Catalyzed by Pd Nanoparticles Supported on Graphene Oxide. Org. Lett. 2017, 19, 6470–6473.
- Mondal, J.; Borah, P.; Sreejith, S.; Nguyen, K.T.; Han, X.; Ma, X.; Zhao, Y. Morphology-tuned exceptional catalytic activity of porous-polymer-supported Mn3O4 in aerobic sp3 C–H bond oxidation of aromatic hydrocarbons and alcohols. ChemCatChem 2014, 6, 3518–3529.
- Salarinejad, N.; Dabiri, M.; Movahed, S.K. Directed aromatic C–H functionalization of N-arylcarbamates and quinazolinones catalyzed by palladium nanoparticles supported on nitrogen-doped graphene. Colloids Interface Sci. Commun. 2022, 47, 100606.
- Payra, S.; Saha, A.; Guchhait, S.; Banerjee, S. Direct CuO nanoparticle-catalyzed synthesis of poly-substituted furans: Via oxidative C–H/C–H functionalization in aqueous medium. RSC Adv. 2016, 6, 33462–33467.
- Yatabe, T.; Jin, X.; Mizuno, N.; Yamaguchi, K. Unusual Olefinic C–H Functionalization of Simple Chalcones toward Aurones Enabled by the Rational Design of a Function-Integrated Heterogeneous Catalyst. ACS Catal. 2018, 8, 4969–4978.
- Jayarajan, R.; Kumar, R.; Gupta, J.; Dev, G.; Kadu, P.; Chatterjee, D.; Bahadur, D.; Maiti, D.; Maji, S.K. Fabrication of an amyloid fibril-palladium nanocomposite: A sustainable catalyst for C–H activation and the electrooxidation of ethanol. J. Mater. Chem. A 2019, 7, 4486–4493.
- Murty, M.S.R.; Penthala, R.; Buddana, S.K.; Prakasham, R.S.; Das, P.; Polepalli, S.; Jain, N.; Bojja, S. Recyclable CuO nanoparticles-catalyzed synthesis of novel-2,5-disubstituted 1,3,4-oxadiazoles as antiproliferative, antibacterial, and antifungal agents. Med. Chem. Res. 2014, 23, 4579–4594.
- Saberi, D.; Mansoori, S.; Ghaderi, E.; Niknam, K. Copper nanoparticles on charcoal: An effective nanocatalyst for the synthesis of enol carbamates and amides via an oxidative coupling route. Tetrahedron. Lett. 2016, 57, 95–99.
- Vishwakarma, R.; Gadipelly, C.; Nakhate, A.; Deshmukh, G.; Mannepalli, L.K. Copper supported Mg–Al hydrotalcite derived oxide catalyst for enol carbamates synthesis via C–H bond activation of formamides. Catal. Commun. 2020, 147, 106150.
More
