Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Guendalina Lucarini and Version 2 by Beatrix Zheng.
Cutaneous melanoma is a severe neoplasm that shows early invasiveness of the lymph nodes draining the primary site, with increased risk of distant metastases and recurrence. The tissue biomarker identification could be a new frontier to predict the risk of early lymph node invasiveness, especially in cases considered by current guidelines to be at low risk of lymph node involvement and not requiring evaluation of the sentinel lymph node (SLN).
- melanoma
- lymph nodes
- biomarker
1. Lymphovascular Invasion (LVI)
In the literature, it is reported that lymphovascular invasion (LVI), which consists of the finding during routine histological examination of melanoma tumor cells within lymphatic or hematic vessels, correlates with a worse clinical outcome [1][17]. The use of highly specific and sensitive antibody biomarkers for melanoma (S-100, Mart-1), for cell proliferation (Ki-67, PCNA), and for lymphatic vessels (LYVE-1, D2-40) allowed a better assessment of LVI. All these biomarkers were assessed by immunohistochemistry directly on primary melanoma tissue. In addition, other parameters that are better analyzed with these biomarkers are lymphatic vessel density (LVD) and peritumoral lymphatic new vessel formation [2][3][18,19]. Specifically, LVI was estimated to be present in 50% of LVI-negative patients with hematoxylin/eosin staining after immunohistochemistry (IHC) [4][5][6][20,21,22].
Some studies suggest that intratumoral or peritumoral LVI and LVD may correlate with sentinel node loco-regional lymph node metastasis and patient survival, but the results are not unequivocal [4][7][8][9][10][20,23,24,25,26].
2. VEGF
Lymphangiogenesis is a necessary condition for tumor involvement of the lymphatic and lymph node system. Glycoproteins of the vascular endothelium growth factor (VEGF) family, produced by tumor cells themselves but also tumor-associated fibroblasts and tumor-associated macrophages, help stimulate lymphangiogenesis [11][27]. VEGF also acts both on endothelial cells and in an autocrine way on tumor cells by promoting their survival and proliferation [12][28].
The assessment through immunohistochemistry on melanoma tissue of the biomarkers VEGF-C, VEGF-D and the VEGFR receptor to predict SLN positivity has been proposed in the literature, but the results are currently inconsistent. A study by Gallego et al. [13][29] showed that VEGF-C in the peritumoral versus intratumoral site is associated with SLN positivity. The use of immunohistochemistry and polymerase chain reaction (PCR) for VEGF-C, VEGF-D, and VEGFR-3 proved to be a good predictor of SLN positivity [10][14][26,30]. However, other studies have not shown an association among VEGF-A, VEGF-D, or VEGFR receptors [15][16][31,32].
Recently, a study by Toberer et al. [17][33] showed in a statistically significant manner in a sample of 58 patients that VEGFR-3 expression is an independent predictor of SLN positivity and could be used in the future when choosing to perform SLN. VEGF-A was also statistically significantly shown to be more highly expressed in the tumor tissue of SLN-positive patients; however, on binary logistic regression, it did not prove to be an independent factor for SLN outcome.
The ambiguous results require further multicenter prospective studies to better clarify the role of VEGF and VEGFR in predicting SLN involvement.
3. Tetraspanin CD9
Tetraspanin CD9 is a transmembrane protein that plays a key role in tumor progression, as it functions as a metastasis suppressor in some neoplasms [18][34]. Specifically, its reduced expression correlates with metastatic progression of the malignancy and poor prognosis. CD9, when overexpressed, enhanced melanocyte motility, suggesting that its overexpression may partly cause the invasion activity of melanoma cells across the Matrigel [19][20][35,36].
A recent study [21][37] conducted on cutaneous melanoma evaluated CD9 expression by immunohistochemistry and immunofluorescence, showing that CD9 was not expressed in thin melanomas, whereas it reappeared in 46% of intermediate and thick melanomas at specific areas of invasion, near or within blood or lymphatic vessels. All of these CD9 stained tumors showed a positive SLN, highlighting that CD9 expression may be a strong predictor of SNB positivity and, therefore, an excellent biomarker for assessing SLN status.
CD9 is also uniformly expressed in melanocytic nevi, leading peopleus to initially consider a protective role of this protein. However, in invasive melanoma, as already seen in other tumors [22][23][24][25][26][27][38,39,40,41,42,43], an increase in CD9 correlates with lymph node metastases, distant metastases, and a worse outcome [21][37]. This can be explained by considering that tetraspanins can act as both suppressors and promoters of metastases depending on the status of the cell membrane and vesicular structures [28][44]. Some vitro studies on transendothelial migration of melanoma cells showed a significant role of CD9 in tumor–endothelial/lymphatic cell interaction and vascular dissemination of tumor cells [29][45]. Therefore, the role of CD9 in determining SLN status seems interesting, as also confirmed by Erovic et al. [30][46] showing that this tetraspanin is a valid marker for lymphatic endothelial cells and able to promote transmigration of tumor cells through the adherence to lymphatic vessels (Figure 1). Further studies are needed to evaluate its role as a biomarker of the SLN especially in patients with intermediate to thick melanoma.
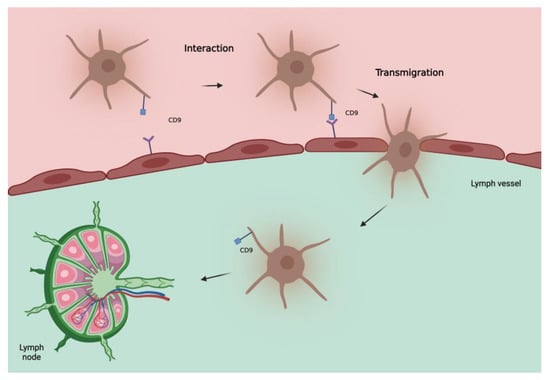
Figure 1. Hypothetical model of involvement of tetraspanin CD9 in metastatic spread of melanoma cells to lymph nodes. CD9, localized at the melanoma cell-endothelial/lymphatic cell contact area during active transmigration of tumor cells across endothelial/lymphatic vessels, facilitates lymph node invasion.
4. LYVE-1 and D2-40
Lymphatic vessel endothelial hyaluronan receptor-1 (LYVE-1), a selective marker of lymphatic vessels, and D2-40, an endothelial marker known as podoplanin, were used to assess LVI in cutaneous melanomas. Their distribution was assessed by immunohistochemistry directly on primary melanoma tissue. Using both of these biomarkers, no correlation was found between LVI and SLN positivity [31][47]. However, if LVI was associated with the presence of intratumoral lymphatic vessels, the possibility of predicting SLN status increased, with a positive predictive value of 80% and a negative predictive value of 72%.
D2-40 was used alone to assess LVI in cutaneous melanoma <2 mm, showing good predictive ability for positive SLN [32][48]. These data suggest that LVI assessment implemented using LYVE-1 and D2-40 may be an indicator for SLN status.
5. Gene Expression Profile Test (31-GEP)
31-GEP is a test used to stratify the risk of SLN positivity, identifying high-risk (>5%) patients who are candidates for SLN biopsy. 31-GEP allows evaluation of molecular expression signatures to guide staging in patients with melanoma [33][34][35][36][37][38][49,50,51,52,53,54].
31-GEP is performed on a sample of primary melanoma tissue normally fixed in formalin and embedded in paraffin. Real-time polymerase chain reaction (RT-PCR) is used for the evaluation of expressed genes. From a clinical point of view, this method can be used at the same time as the histological examination. 31-GEP is based on evaluating, directly on the melanoma tissue sample, the expression of gene levels of a panel containing several genes. Specifically, markwers can be found can find markers for cell migration/chemotaxis/metastasis, secretory molecules, adhesion, lymphocyte invasion, transcription factors, differentiation/proliferation structural proteins, and surface receptors (CXCL14, SPP1, CLCA2, S100A9, S100A8, BAP-1, MGP, GJA1, DSC1, PPL, LTA4H, TRIM29, KRT6B, KRT14, CRABP2, SPRRIB, TACSTD2, CLCA2, ROBO1, CST6, SAP130, ID2, EIF1B, ARG1, AQP1, RBM23, and TYRP1) [39][55].
Genetic tests can identify a class 1 low risk of SLN positivity or a class 2 high risk. Class 1 is considered to have a very low risk of both metastasis and mortality, also providing greater tranquillity for patients and improving their quality of life [37][53].
DecisionDx-Melanoma has recently been proposed as an additional risk assessment parameter for SLN positivity, integrating the 31-GEP test with other clinical/pathological aspects for the evaluation of SLN in patients with thin melanoma [40][41][56,57].
This could, in the near future, introduce a new variable to be considered, highlighting population groups with a >5% risk of SLN positivity, optimizing the treatment.
All the melanoma tissue biomarkers are summarized in Table 12.
Table 12.
summary of reviewed biomarkers for SLN positivity.
| Biomarker | Rationale | Sample | Results | |||||
|---|---|---|---|---|---|---|---|---|
| VEGFR-3 Toberer et al. [17] | VEGFR-3 Toberer et al. [33] |
Involved in the stimulation of lymphangiogenesis | 58 patients |
| ||||
| Tetraspanin CD9 Lucarini et al. [21] | Tetraspanin CD9 Lucarini et al. [37] |
Transmembrane protein, key role in tumor progression Both suppressor and promoter of metastases, depending on the status of the cell membrane and vesicular structures |
140 patients Melanocytic nevus 20 Primary melanoma 120 |
|
| |||
| LYVE-1 Doeden et al. [31] | LYVE-1 Doeden et al. [47] |
LYVE-1 selective marker of lymphatic vessels | 94 patients |
| ||||
| D2-40 Fohn et al. [32] | D2-40 Fohn et al. [48] |
D2-40 endothelial marker (podoplanin) In combination, better histological definition of LVI |
158 patients Primary melanomas ≤ 2.0 mm |
| ||||
| 31-GEP Vetto et al. [39] | 31-GEP Vetto et al. [55] |
Gene expression profile test, (markers for cell migration/chemotaxis/metastasis, secretory molecules, adhesion, lymphocyte invasion, transcription factors, differentiation/proliferation structural proteins and surface receptors) Identifies high-risk (>5%) patients, candidates for SLN |
690 patients (total validation cohort, retrospective) staged I–III follow-up median 7 years 1421 patients (prospectively-tested) |
|
Vascular endothelium growth factor receptor-3 (VEGF), statistically significant (ss), lymphatic vessel endothelial hyaluronan receptor-1 (LYVE-1), lymphovascular invasion (LVI), sentinel lymph node (SLN), and gene expression profile test (31-GEP).
