Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Catherine Yang and Version 1 by Rachna Mehta.
The nervous system requires immune cells to fight foreign body invasion including the pathogenic infection. This function is accomplished by microglia, which also play many other roles in the central nervous system including elimination of apoptotic cells, synaptic pruning, supporting neuronal survival, clearing debris, etc. Under healthy conditions, cyto-morphologically, microglia possess long thin processes and a small cell body, useful for debris clearance. However, upon exposure to inflammation, it becomes “activated” to function as immune cells of the central nervous system and develop short, thick processes and a larger cell body. They phagocytose the pathogens, release inflammatory mediators, and regulate T-cell activity. A variety of neurotransmitter receptors are expressed on the microglia which facilitate bidirectional communication between neurons and microglia. It has been shown that withdrawal of noradrenaline (NA) is a necessity for generation of rapid eye movement sleep (REMS). It has been proposed that REMS has evolved to maintain NA level in the brain. Further, we argue that NA exerts neuromodulatory action on microglia and facilitates immune function of the brain. Thus, REMS modulates immune functions of the brain.
- neuroinflammation
- microglia
- noradrenaline
- sleep loss
1. Role of NA in Microglial Activation
Recent reports suggest the role of NA as a key modulator of microglial activities. Various types of adrenergic receptors (ARs) are present on microglia. However, interestingly, it has been reported that although resting microglia primarily expresses β2 ARs, under proinflammatory conditions they can express α2A ARs as well [88][1]. Activation of microglial β2 ARs by NA downregulates the expression of proinflammatory genes [89][2], whereas α2A activation upregulates proinflammatory cytokines. Therefore, depending on subtypes of ARs, NA can activate or inhibit the microglia [90][3]. Microglial activation is inhibited by pretreatment with β-AR blockers such as propranolol. Propranolol also increases microglial process surveillance activity [91][4]. Moreover, NA inhibits nitric oxide production from microglia and thus, attenuates free radical production. Thus, NA may modulate sensitivity of microglia to respond to tissue damage, which holds therapeutic potential against neurodegeneration (Figure 1).
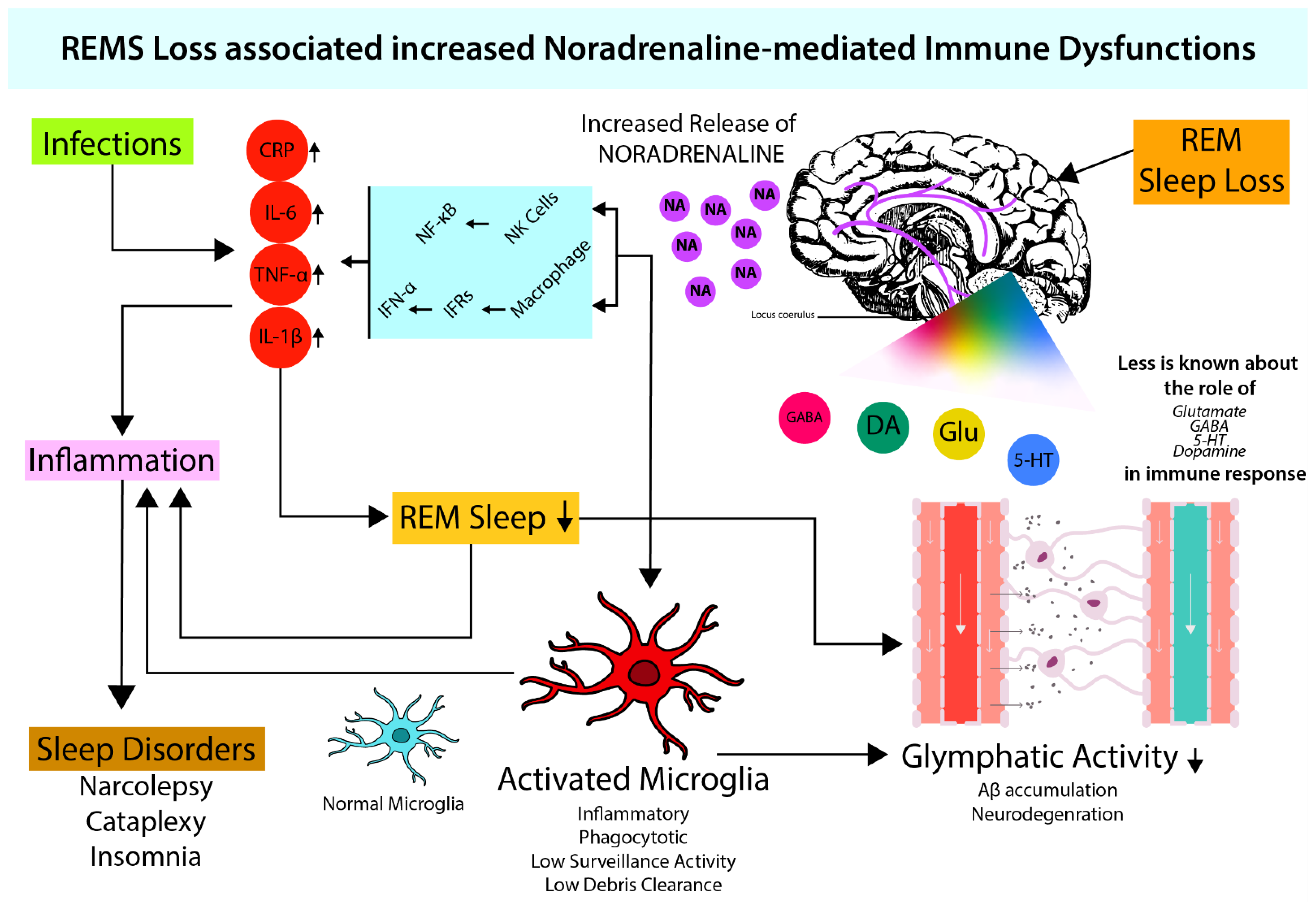
Figure 1. REMS maintains optimum NA levels in brain. During sleep (which also includes REMS) loss, there is an increased NA release in the brain (Mehta et al., 2017) which induces macrophages and NK-cell activity. Increased macrophage activity leads to increased cytokine levels (IL-6, TNF-α, IL-1β) via NF-κB signaling resulting in inflammation (Sugama & Kakinuma, 2021; Sugama et al., 2019). Both increased cytokine levels and NA provide favorable conditions for microglial activation (Liu et al., 2019). Activated microglia leads to attenuated glymphatic activity (Benveniste et al., 2019; Leng et al., 2021), which elevates neuroinflammation and causes neurodegeneration. Neuroinflammation-induced cytokines further inhibit sleep (Okun et al., 2004; Kheirandish-Gozal & Gozal, 2019).
Microglia under resting conditions may have constructive roles in surveillance, such as debris clearance, pruning, remodeling, and functioning of synapse. However, activated microglia may disturb the homeostasis and may appear to be self-destructive. Using pharmacological and chemogenetic approaches, it has been shown that NA signaling in awake mice suppresses microglial surveillance activity [92][5]. Thus, upon sleep loss (including REMS loss), increased levels of NA can cause neuroinflammation by glial activation, leading to neurotoxicity, loss of tissue integrity, and aggravated tissue damage leading to impairment of brain functions [93][6]. The severity of loss of function depends on quantity and quality of sleep loss, chronicity of the condition(s), and effects of recovery.
The role of microglia in neurodegenerative diseases viz. Alzheimer’s disease (AD) and Parkinson’s disease is of late gaining global attention. Microglia are activated by amyloid beta (Aβ) accumulated in AD, and that produces IL-1 and TNF-α. Normally, this mechanism helps clear the Aβ and τ-protein aggregates by microglial phagocytosis and cytokine activity which maintain homeostasis. However, if the formation of plaques and tangles increases, significantly overwhelming the microglial response, AD sets in. Furthermore, if there is overproduction of cytokines, it leads to neurotoxicity and (neuronal and glial) cell death [94][7]. Thus, NA plays a key role on one hand by facilitating microglial activation and clearance of Aβ peptide by phagocytosis, and on the other hand, it prevents Aβ-induced increase in proinflammatory cytokine release [95][8]. As the degeneration causes loss of NA-ergic neurons, the protective care of NA is withdrawn, resulting in the loss of neuron–neuron and neuron–glia communication, microgliosis, and defective Aβ clearance leading to neurodegenerative diseases, e.g., AD [96][9]. This view may be supported by the fact that a low dose of NA favors neuronal survival and growth, while its high dose facilitates neuronal degeneration [23][10].
As NA levels tend to increase, REMS appears to bring the level of NA to protective levels and homeostasis is maintained [20][11]. In case of loss of sleep (including REMS loss), an initial small increase in NA activates microglia to exert protection. However, extended loss of REMS causes significant increase in NA, due to non-cessation of REM OFF neurons in LC, which is likely to affect many other systems causing damage to the brain. The Weresearchers propose that such significant increase in NA level in the brain might be responsible for behavioral changes particularly under chronic sleep disturbed conditions (Figure 2). Thus, dose dependent effect of NA on neuronal survivability supports ourthe contention; the molecular action of NA on microglia in evolution needs further study. Based on such study, it has been proposed that REMS has evolved to maintain the brain level of NA [21][12].
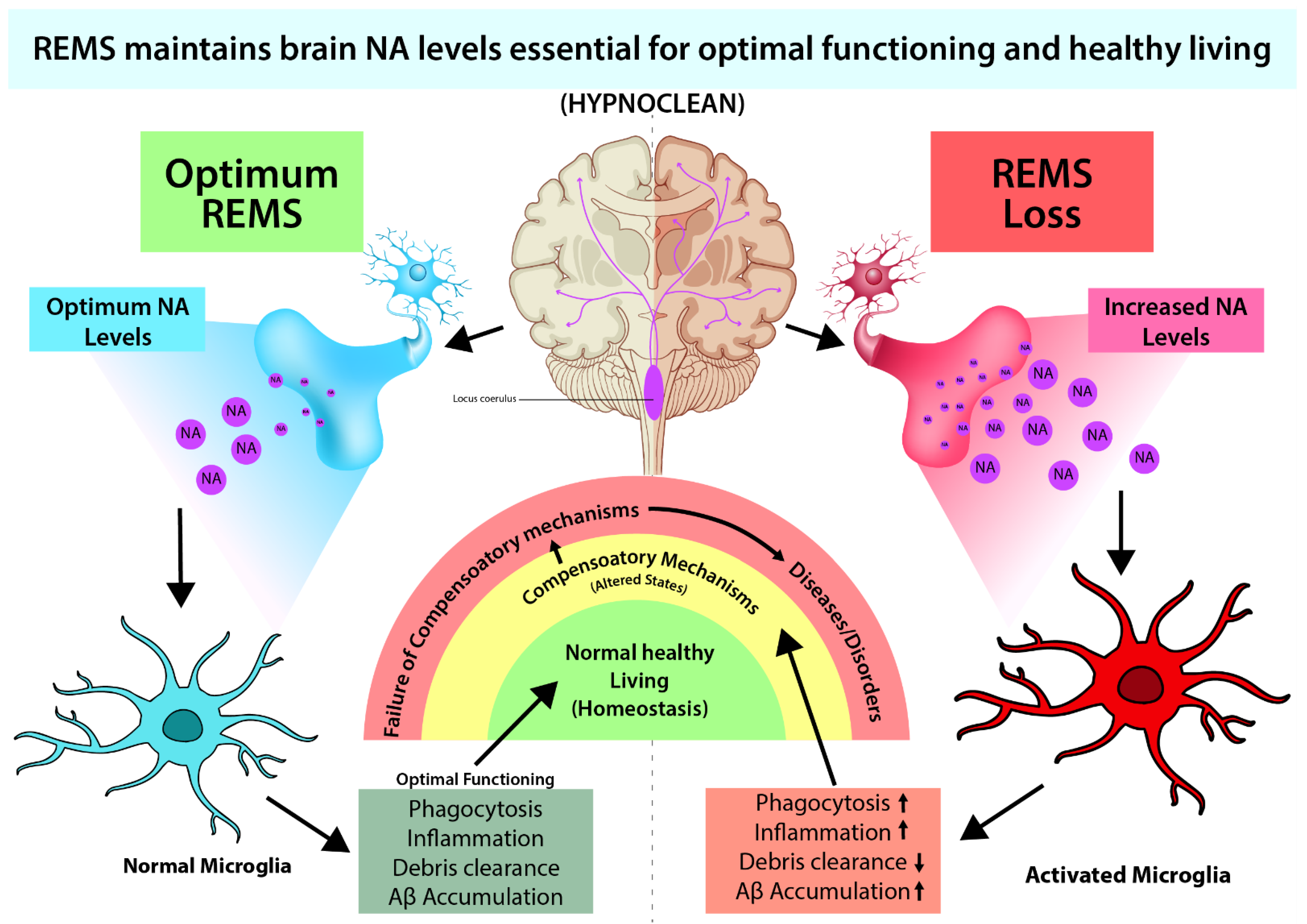
Figure 2. REMS loss-induced elevated brain NA causes many associated symptoms, such as immune dysfunctions, reduced waste clearance, etc., which can be ameliorated by preventing excessive NA action (Sugama & Kakinuma, 2021; Sugama et al., 2019; Liu et al., 2019).
2. Possible Effect of NA on the Glymphatic System through Microglia (HYPNOCLEAN)
The glymphatic system is a recently identified glia-dependent waste clearance pathway in the brain and constitutes the brain’s “front end” waste drainage system [97][13]. It is a transportation system that plays a significant role in the clearance of debris produced in the brain, including Aβ. Impaired glymphatic clearance has been linked to neurodegenerative diseases (Figure 1). The process of debris clearance occurs when the rapidly entering cerebrospinal fluid in the peri-arterial space exchanges with the interstitial fluid in the surrounding parenchyma. This interstitial fluid flows towards the peri-venous space, and the final exchange of debris-filled fluid occurs before drainage into the lymphatic vessels [90][3]. The glymphatic system has been closely linked to the sleep–wake cycle. As waste generation is a continuous process, its disposal is executed by a normal physiological process. Normal sleep is one such process that helps maintain the brain waste drainage across the lifespan of an individual [98][14]. As such, lifestyle and factors affecting sleep cycle such as diet, alcohol intake, exercise, meditation, temperature, light, sleep posture, intermittent fasting, and chronic stress modulate glymphatic clearance. Its functioning has been found to differ not just in sleep and wakefulness, but also during specific stages of sleep [97][13]. During sleep, the interstitial fluid volume fraction increases to 23% as compared to 14% during wakefulness [99][15]. This was closely related to the faster glymphatic transport and waste clearance during sleep in rodents [100][16]. It has also been observed that glymphatic activity varies with body positions. A supine or lateral decubitus position has positive effects on debris clearance by higher glymphatic activity, whereas a prone position seems to be negatively associated with debris clearance [98][14].
Recent studies suggest that during sleep, the glymphatic system operates through microglial activation. Aquaporin-4 (AQP-4) is a major water channel in the central nervous system and AQP-4 is essential in exchanging cerebrospinal fluid and interstitial fluids in the perivascular space. AQP-4 is significantly expressed in astrocytes and microglia; hence, their actions may be instrumental for adequate functioning of the glymphatic system during sleep. During dementia-associated sleep loss, an age-related decline in AQP-4 polarization has been shown. Mice with a dysfunctional AQP-4 channel were not able to clear Aβ efficiently [90,97][3][13]. The basis for sleep-induced enhancement of glymphatic transport appears to be closely twined with NA-ergic [101][17] neuronal activity. NA release during wakefulness suppresses glymphatic clearance by decreasing the amount of interstitial space. Blocking NA release expands the interstitial volume, enhances glymphatic clearance that boosts removal of metabolic waste products from the brain, and protects the brain. Another study has shown that mice treated with a combination of dexmedetomidine (NA antagonist) and isoflurane is more effective in increasing glymphatic activity than treating them with only isoflurane. Under normal condition, as NA level is lowest in the brain during REMS, it is likely that glymphatic system would be maximally effective during this stage. Such correlations underline the importance of REMS in maintaining normal brain functioning, primarily by clearing debris (waste). Because of this, wthe researchers introduce a new term for function of sleep as “Hypnoclean”, i.e., to clean the brain fluid during sleep.
References
- Gyoneva, S.; Traynelis, S.F. Norepinephrine modulates the motility of resting and activated microglia via different adrenergic receptors. J. Biol. Chem. 2013, 288, 15291–15302.
- Mori, K.; Ozaki, E.; Zhang, B.; Yang, L.; Yokoyama, A.; Takeda, I.; Maeda, N.; Sakanaka, M.; Tanaka, J. Effects of norepinephrine on rat cultured microglial cells that express alpha1, alpha2, beta1 and beta2 adrenergic receptors. Neuropharmacology 2002, 43, 1026–1034.
- Sugama, S.; Kakinuma, Y. Noradrenaline as a key neurotransmitter in modulating microglial activation in stress response. Neurochem. Int. 2021, 143, 104943.
- Sugama, S.; Takenouchi, T.; Hashimoto, M.; Ohata, H.; Takenaka, Y.; Kakinuma, Y. Stress-induced microglial activation occurs through beta-adrenergic receptor: Noradrenaline as a key neurotransmitter in microglial activation. J. Neuroinflamm. 2019, 16, 266.
- Liu, Y.U.; Ying, Y.; Li, Y.; Eyo, U.B.; Chen, T.; Zheng, J.; Umpierre, A.D.; Zhu, J.; Bosco, D.B.; Dong, H.; et al. Neuronal network activity controls microglial process surveillance in awake mice via norepinephrine signaling. Nat. Neurosci. 2019, 22, 1771–1781.
- Garbarino, S.; Lanteri, P.; Bragazzi, N.L.; Magnavita, N.; Scoditti, E. Role of sleep deprivation in immune-related disease risk and outcomes. Commun. Biol. 2021, 4, 1304.
- Lee, C.Y.; Landreth, G.E. The role of microglia in amyloid clearance from the AD brain. J. Neural. Transm. 2010, 117, 949–960.
- Jardanhazi-Kurutz, D.; Kummer, M.P.; Terwel, D.; Vogel, K.; Thiele, A.; Heneka, M.T. Distinct adrenergic system changes and neuroinflammation in response to induced locus ceruleus degeneration in APP/PS1 transgenic mice. Neuroscience 2011, 176, 396–407.
- Leng, F.; Edison, P. Neuroinflammation and microglial activation in Alzheimer disease: Where do we go from here? Nat. Rev. Neurol. 2021, 17, 157–172.
- Somarajan, B.I.; Khanday, M.A.; Mallick, B.N. Rapid Eye Movement Sleep Deprivation Induces Neuronal Apoptosis by Noradrenaline Acting on Alpha1 Adrenoceptor and by Triggering Mitochondrial Intrinsic Pathway. Front. Neurol. 2016, 7, 25.
- Mehta, R.; Giri, S.; Mallick, B.N. REM sleep loss-induced elevated noradrenaline could predispose an individual to psychosomatic disorders: A review focused on proposal for prediction, prevention, and personalized treatment. EPMA J. 2020, 11, 529–549.
- Singh, A.; Das, G.; Kaur, M.; Mallick, B.N. Noradrenaline Acting on Alpha1 Adrenoceptor as well as by Chelating Iron Reduces Oxidative Burden on the Brain: Implications with Rapid Eye Movement Sleep. Front. Mol. Neurosci. 2019, 12, 7.
- Reddy, O.C.; van der Werf, Y.D. The Sleeping Brain: Harnessing the Power of the Glymphatic System through Lifestyle Choices. Brain. Sci. 2020, 10, 868.
- Xie, L.; Kang, H.; Xu, Q.; Chen, M.J.; Liao, Y.; Thiyagarajan, M.; O’Donnell, J.; Christensen, D.J.; Nicholson, C.; Iliff, J.J.; et al. Sleep drives metabolite clearance from the adult brain. Science 2013, 342, 373–377.
- Iliff, J.J.; Wang, M.; Liao, Y.; Plogg, B.A.; Peng, W.; Gundersen, G.A.; Benveniste, H.; Vates, G.E.; Deane, R.; Goldman, S.A.; et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci. Transl. Med. 2012, 4, 147ra111.
- Benveniste, H.; Liu, X.; Koundal, S.; Sanggaard, S.; Lee, H.; Wardlaw, J. The Glymphatic System and Waste Clearance with Brain Aging: A Review. Gerontology 2019, 65, 106–119.
- Jessen, N.A.; Munk, A.S.; Lundgaard, I.; Nedergaard, M. The Glymphatic System: A Beginner’s Guide. Neurochem. Res. 2015, 40, 2583–2599.
More
