Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Eliza Gruczyńska-Sękowska and Version 2 by Catherine Yang.
Polystyrene (PS) is a thermoplastic polymer made of aromatic hydrocarbon monomer styrene that is derived from fossil-fuels. The synthesis of PS is based on the free radical polymerization of styrene using free-radical initiators. It is mostly used in solid (high impact and general purpose PS), foam and expanded PS forms. The main advantages of PS are low-cost, easy processing ability, and resistance to ethylene oxide, as well as radiation sterilization. Polylactide (PLA)—biodegradable and compostable aliphatic polyester—is one of the key biopolymers with the largest market significance.
- crude oil-based
- bio-based polymers
- chemical structure
- properties
- application
1. Introduction
Polystyrene (PS) is a thermoplastic polymer (Figure 13) made of aromatic hydrocarbon monomer styrene that is derived from fossil-fuels [1][53].
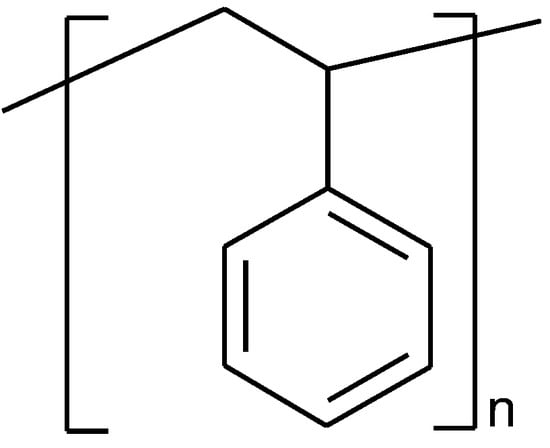
Figure 13.
The fragment of polystyrene structure.
The synthesis of PS is based on the free radical polymerization of styrene using free-radical initiators. It is mostly used in solid (high impact and general purpose PS), foam and expanded PS forms. The main advantages of PS are low-cost, easy processing ability, and resistance to ethylene oxide, as well as radiation sterilization. It is, however, not resistant to organic solvents such as cyclic ethers, ketones, acids, and bases. The most popular general purpose PS (GPPS or unmodified PS) is transparent, brittle, and rigid, which makes this kind of material suitable for laboratory purposes, such as diagnostic and analytical, and medical packaging (e.g., Petri dishes, tissue culture trays, pipettes, test tubes). For high-strength products, high-impact PS (HIPS) is competitive with polypropylene and PVC [2][54]. It is typically used in thermoformed products, such as catheters, heart pumps, and epidural trays, and toys, packaging, and electronic appliances. Owing to its high dimensional stability and easy processing, it is often chosen for the preproduction prototypes in 3D-printing technique [3][55]. As a result of strong C-C and C-H bonds present in the structure, PS is resistant to biodegradation without special treatment such as copolymerization and fictionalization. However, it was proved that some bacterial species are able to form biofilm on the PS surface, which leads to its partial degradation [4][56]. PS can be recycled using several methods. Mechanical recycling is the one with lowest cost, but it has many limitations. The main obstacle is efficient separation of PS from the plastic waste stream. Currently, PS is sorted using near-infrared technologies and complementary sorting methods, including density, electrostatics, selective dissolution, and flotation [5][57]. The latter—froth flotation—is the most common due to its low cost and the possibility to separate polymers with similar density. To increase the flotation effectiveness, surface modification can be performed, e.g., in the presence of KMnO4 [6][58] or K2FeO4 [7][59]. Recycled PS exhibits worse mechanical properties than neat polymer, and reduction in molecular mass is also observed. Nevertheless, many products made of recycled PS can be found on the market, e.g., pencils, doors, window frames, cups, plates, and bottles; some of them even approved for food contact [8][60]. Chemical recycling of PS is less common due to the high cost. It leads to the production of styrene, and other useful chemicals such as benzene, toluene, indan, ethylbenzene, and benzoic acid, via pyrolysis and oxidation. Recently, a novel simple and low-cost method has been reported that enables the oxidative cleavage of PS to benzoic acid, formic acid, and acetophenone by singlet oxygen at ambient temperature and pressure [9][61]. PS waste can also be converted to biodegradable PHAs [10][62]. To summarize, PS is one of the most important polymers present in our daily life. However, once it has fulfilled its designed purpose, it is not easily degradable. Chemical recycling is not economically convenient since the feedstocks are cheaper than the process itself; additionally, mechanical recycling is limited due to the low separation efficiency of PS from the plastic waste stream. That is why there is an urgent need to find sustainable alternatives that can at least partially replace petroleum-based PS in use. The most popular green substitutes for PS are cellulose and thermoplastic starch used as thermal insulation materials (foams) [11][12][63,64], and poly(vinyl alcohol) for bead-foaming process [13][65] and polylactide [14][66].
Polylactide (PLA)—biodegradable and compostable aliphatic polyester (Figure 24)—is one of the key biopolymers with the largest market significance. The global volume of PLA production was around 457,000 metric tons in 2021, which accounted for 29% of the total biodegradable bioplastics production worldwide [15][67]. The PLA production on industrial scale is either based on the ring-opening polymerization (ROP) of lactide, (method applied by NatureWorks LLC, Plymouth, MN, United States, and Corbion N.V., Amsterdam, the Netherlands) or direct polycondensation of lactic acid in an azeotropic solution (applied by Mitsui Toatsu Chemicals, Inc., Tokyo, Japan) [16][68]. In both cases, high molecular mass PLA is obtained; however, solvent-free ROP is preferable for production in large scale. In this case, optically pure L-lactic or D-lactic acid is produced as a monomer of PLA by microbial fermentation from renewable resources such as molasses, whey, sugar cane, and plants with high starch content [17][69]. Next, LA is condensed to form low molecular mass prepolymer PLA, which undergoes a controlled depolymerization to a cyclic dimer of lactate–lactide. The polymerization of lactide is generally catalyzed by tin octanoate and requires short reaction time at a temperature of about 440–460 K [18][70].
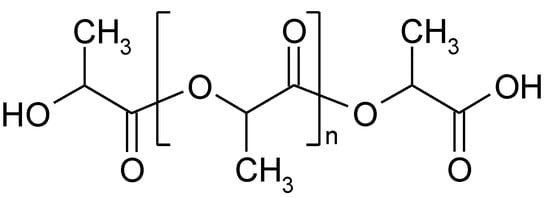
Figure 24.
The fragment of polylactide structure.
The mechanical properties of PLA are similar to those of PS and polyethylene terephthalate (PET) [19][71]. It can also be a sustainable alternative to polypropylene (PP) and PVC. PLA is as rigid and brittle as PS, and its resistance to fats and oils resembles PET [19][71]. Although CO2, O2, N2, and H2O permeabilities for PLA are higher than for PET, but lower than for PS [20][72], therefore many attempts to improve the PLA barrier properties have been reported, e.g., by introducing nanofillers with a lamellar structure [21][73]. In addition, it is characterized by a high tensile modulus and resistance to UV radiation. Good mechanical and optical properties allow PLA to compete with the existing crude oil-based thermoplastics. PLA containing approx. 5% of D-repeating units is a transparent, colorless, and relatively rigid material resembling PS [22][74]. An extra advantage of PLA is its easy processing ability through conventional melt processes such as extrusion, injection molding, compression molding, or blow molding, which are also used for other commercial polymers, namely PS and PET [23][75].
The properties of PLA depend on the polymer molecular mass and the degree of crystallinity [24][76]. Stereochemistry also plays an important role. The stereochemical composition and distribution of monomer units along the polyester chain affect the properties of PLA [22][74]. L-PLA (PLLA) and D-PLA (PDLA) are composed of lactic acid units of the same chirality [25][77]. They are isotactic, stereoregular, and partially crystalline polymers (degree of crystallinity up to 60%), the glass-transition temperature (Tg) is approx. 320–330 K, and the melting point is 440–470 K [22][26][27][29,74,78]. On the other hand, D,L-PLA is an amorphous polymer with a Tg of about 330 K. It shows worse mechanical properties and degrades faster than PLLA and PDLA. The highest melting point, about 500 K, shows a racemic mixture of PLLA and PDLA, in which chains of different chirality form a densely packed network. Compared to the parent polymers, the resulting racemic PLA (PDLLA) has enhanced functional properties, such as mechanical strength, durability, and thermal and hydrolytic stability [28][79].
2. Packaging Application
Low toxicity, strong flavor and aroma barrier, and high transparency make PLA an ideal material for fresh food packaging, especially fruit and vegetables [29][80]. Auras et al. [30][81] tested and compared oriented PLA (OPLA) with PET and oriented PS (OPS) films intended for production of fresh fruit and vegetables storage containers. According to these results, mechanical, physical, and barrier properties of OPLA were comparable and, in some cases, better than standard OPS and PET containers. Similar studies were performed for the shelf life of blackberries [31][82] and blueberries [32][83] under retail conditions closed in the OPS and OPLA containers. In both cases the shelf life was extended, proving that PLA can be a good replacement for PS. PLA can be used also as trays for storage of mangoes, melons, and other tropical fruit. The shelf life of the fruit packed in such a way was the same as of the fruit packed in PET trays [33][84]. However, the PLA packaging is more susceptible to cracking and breaking during transport when compared with OPS or PET. Neither the sheet nor the finished product can be stored at temperatures above 313 K or relative humidity greater than 50% [34][85].
3. Three-Dimensional Printing
The filaments used in 3D printing are primarily thermoplastics. The most popular are PLA, acrylonitrile butadiene styrene (ABS) and HIPS [35][86]. In all three cases, filament can also be produced from recycled plastic, which can significantly reduce its price. It is worth mentioning that commercial filaments for 3D printing are 20 to 200 times more expensive than those of raw plastics [36][87]. The source for PLA waste is food containers and bottles, ABS filaments originating from car dashboards, and HIPS derived from refrigerators or automotive parts [37][88]. The advantages of PLA as filament for 3D printing are ease of printing, glossiness, and multicolor appearance. The dimensional accuracy of the parts printed from PLA is high since it poses less warp behavior than the other filaments. Compared to HIPS, PLA filament does not require a heated bed, it is odorless, and what is more important, it releases many fewer volatile organic compounds and exhibits lower particle emission during printing [38][89]. PLA prints have wider application than HIPS due to biocompatibility and susceptibility to biodegradation, which are important in biomedical application and tissue engineering [39][90]. Moreover, the price of 1 kg of PLA filament is comparable to that for HIPS. This is why PLA can be a good alternative to HIPS in rapid manufacturing of packaging prototypes using 3D printing technology [40][91].
4. Medical Application/Drug Delivery
Medical plastic has to be biocompatible, stable under different sterilization conditions, and robust to surface modification. While PLA fulfils all these requirements, PS is not applicable because of the cancerogenic properties of styrene and its very moderate biocompatibility [2][54]. However, there are several studies on improving the biocompatibility of PS, e.g., by nonequilibrium gaseous plasma treatment [41][92]. Both polymers can be sterilized by ethylene oxide, gamma radiation, and electron-beam radiation, however, due to the presence of a benzene ring in its structure, PS is more resistant to high radiation doses than PLA. PLA exhibits strong resistance to sterilization processes with use of an autoclave or dry heat [42][93]; standard PS is not autoclavable, but syndiotactic PS is excellent [43][94]. The main application of PS in the laboratory field is the production of different containers for a variety of liquids, cells, and bacteria, together with microspheres used as drug carriers and magnetic particles. The biocompatibility of PLA makes this material an excellent application as scaffolds for bone regeneration, implants, stents, along with bioresorbable surgical and orthopedic threads and dental implants. Owing to the good mechanical properties of PLA, it can be used in catheters, heart pumps, and epidural trays to replace PS [2][54]. PLA and PS are also used as a surface for adhesion and proliferation of fibroblast and osteoblast cell lines [44][95].
PLA is a promising bioplastic with mechanical properties comparable to those of PS. In addition to its established position as a material for biomedical applications, it can replace mass production plastics from petroleum. However, there are still challenges that need to be addressed, e.g., improvement of barrier properties, which play a very important role in maintaining food quality and safety [45][96]. Moreover, the cost of PLA manufacturing is still too high to compete with PS. That is why there is a need to find low-cost substrates and high-performance microorganisms to increase the efficiency of LA production and obtain low-cost, high-quality PLA. Another concern is the recycling of PLA. PLA can be easily degraded in the natural environment or in compost; however, the idea of introducing a large amount of waste for biodegradation is unreasonable and its transformation into chemical products more valuable than simply carbon dioxide and water should be considered. Currently, several attempts of PLA recycling have been made but an industrially feasible chemical recycling concept, in adherence to the fundamental principles of closed-loop recycling within a Circular Economy, has not yet been developed [46][97]. Other than PLA, products made from PS can be recycled, but the high cost of the recycling process and the segregation problem make the technology inefficient. Moreover, the production of biopolymers is considered more sustainable than petroleum-based materials due to the reduced net carbon footprint [47][98].
