Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by Arutselvan Natarajan.
Cancer immunotherapy is a treatment method in which the immune system is stimulated to communicate with the tumor at the TME to detect, target, and destroy the growth of the cancer cells or malignancy.
- immunotherapy
- immune checkpoint inhibitors
- immuno-nanomaterials
1. Introduction
The immune system defends the body against pathogens, which suggests extensive implications for its use in therapeutic interventions. For one, many characteristics of cancer cells are similar to those of healthy cells, thus making it difficult for the immune system to identify them as foreign elements. It also enables the biomolecules expressed by tumors at the tumor microenvironment (TME) to evade detection by immune cells. Cytokines may even form a tumor-supportive immune microenvironment that inhibits antitumor activity and transmits signals that directly promote tumor growth [1,2,3][1][2][3]. Therefore, new treatment strategies are required to overcome these challenges in detecting cancer cells and thereby preventing tumor development.
Cancer immunotherapy is a treatment method in which the immune system is stimulated to communicate with the tumor at the TME to detect, target, and destroy the growth of the cancer cells or malignancy [4]. Currently, several clinical studies are using immunotherapy as a first line of treatment for cancer, which has enormous potential in comparison to conventional treatments such as surgery, chemotherapy, and radiotherapy [5]. Additionally, immunotherapies are showing significant advancements in the treatment of solid tumors.
A recent report on immunologic drugs, in which monoclonal antibodies (mAbs) were used for solid tumors in poor-prognosis patients, showed increased survival by 20% when compared to chemo- and radiotherapy. Patients lived longer when monoclonal antibodies, molecular inhibitors, or chemotherapy were given directly to the tumor site [6]. Immunotherapy provides a minimally invasive, targeted cancer treatment that can work where other treatments have failed. Combining immunotherapy with other treatments can also improve the effects. However, immunotherapy is not widely accessible to patients due to the high cost—up to USD 250,000 per patient [7]. Consequently, tailored immune drug administration is an essential treatment method for solid tumor therapy and should be integrated into ongoing clinical trials.
1.1. Challenges in Immunotherapy
Cancer immunotherapy based on immune checkpoint inhibition (ICI) for patient screening remains complex in terms of clinical decision-making. ICI therapy is only effective for patients with mismatch repair deficit (dMMR) or high-microsatellite instability (MSI-H) in their metastatic colorectal cancer (mCRC) [9][8]. In addition, neurological immune-related adverse events (irAEs) are increasingly common in oncologic treatment approaches, especially in patients treated with ICIs and combination therapy [10][9]. The immune-related response criteria (irRC) clearly define an important concept necessary for the assessment of immune-related responses; however, several pitfalls and issues related to irRC remain to be solved [11][10].
Cancer treatments using ICI have short-term and long-term side effects, a wells as long-term, or chronic, side effects many of which are currently unfolding. A new study reported that ICI can cause a range of long-term side effects, but most of them are mild, with more than 40% of the patients affected [12][11]. These chronic effects were skin rashes, hypothyroidism, and joint pain. Monitoring immune checkpoint blockade (ICB) therapy can provide more information to address these issues. Measuring IC expressions in the TME over time could provide clinicians with useful information for evaluating potential outcomes. In addition, biomarkers that can predict therapy response to ICB can be utilized for further advanced-precision immunotherapy.
1.2. Need for Targeted Cancer Immunotherapy
Treatment response by immunotherapeutic drugs is characterized by immune cell activation, which can have adverse effects on normal cells. Hence, a controlled mechanistic pathway is required to understand the genomic sequence and mutations in the tumor to prescribe treatments. Effective transport of cancer-immune drugs at the molecular level can be delivered through engineering nano- and microcarriers functionalized using targeted moieties. However, the regulated release kinetics of immune drugs at the intra-tumoral region would follow burst release rather than a controlled fashion. To improve drug release patterns, externally triggered sources across electromagnetic regions, such as photothermal, sound, and radiation (X-rays and radiofrequency), are utilized for personalized medicine.
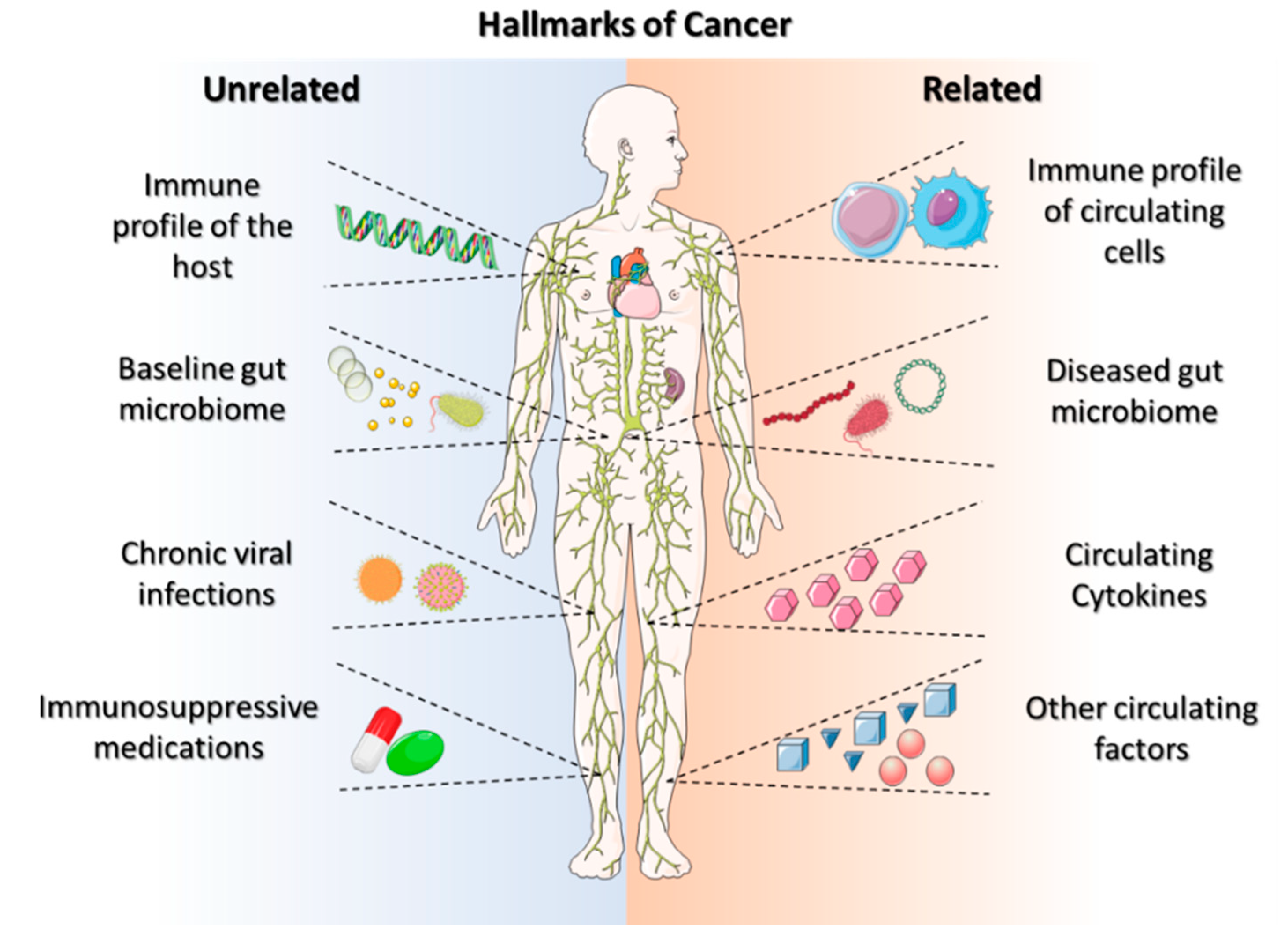
The ability of solid tumors to bypass the body’s antitumor immune response is becoming a hallmark characteristic of cancers (Figure 1). Galluzzi et al. defined the hallmarks of anticancer immunotherapy as immunogenicity, immunosuppression, susceptibility, composition, localization, and functionality [13][12]. These hallmarks of anticancer immunotherapy can lead to a successful treatment modality for personalized care. Systemic activation reduces the sensitivity of immunotherapies and potentially hinders the anticancer-immune-drug response [14][13]. The beneficial effects of anticancer immunotherapy are dependent on (i) their innate capacity to elicit a tumor-targeting immune response, (ii) their capacity to establish an immunosuppressive TME, and (iii) their sensitivity to immune-effector mechanisms [15][14]. These hallmarks aid in developing personalized immunotherapeutic regimes, requiring improved effectiveness and multiparametric evaluations. However, there is a need to search for the hallmark identifications that improve immune-based therapeutic outcomes, beginning with the identification and usage of ICIs, which provide prognostic insights into cancer immunotherapy.
1.3. Nanotechnology in Cancer Immunotherapy
Nanomaterials ranging from 1 to 100 nm were used for immunotherapy with combinational drugs for effective cancer treatment [16][15]. Typically, these targeted nanoparticles (NPs) were composed of a drug-concentrated core and a functionalized outside layer, or shell. These engineered nanoparticles were further optimized based on size, shape, and surface properties to increase their efficacy and reduce side effects. Nanomaterials with different compositions were used to generate an effective cancer immunotherapy based on ICIs.
Anticancer drugs currently used in clinical settings are hydrophobic, thereby making it hard for them to travel in aqueous-body environments [17][16]. Encapsulation is one strategy used for loading hydrophobic drugs in a nanoparticle carrier. This increases the concentration of hydrophobic drugs in the human system by a factor of over 5 × 104 [18][17]. Hydrophilic drugs come in the form of both macromolecules and small molecules, with some limitations. For instance, cellular uptake is poor because they struggle to cross hydrophobic membranes, have low bioavailability, and have a short half-life. However, this has been combated through the use of nanoparticles, which protect and deliver hydrophilic drugs.
“Naturally targeted” immune cells, such as macrophages, monocytes, neutrophils, and dendritic cells (DCs), phagocytose the free NP drugs to transport them to the cancer site. Targeting cancerous cells using selective binding to specific receptors is possible due to ligands such as small organic molecules, peptides, antibodies, and nucleic acids. This design allows for the transport and simultaneous delivery of multiple drugs to a target, which is useful for multimodal therapy techniques. Through the enhanced permeability and retention (EPR) effect, NPs can easily drive into the TME. NPs are a flexible platform to use in cancer immunotherapy, displaying potential in a variety of fields such as imaging and drug delivery without harming another internal system [19][18]. These factores show the cutting-edge treatment ability of cancer immunotherapy that dynamically changes the immune system’s ability to fight cancer [20][19].
2. Immune-Targeting Nanomaterials
Immunotherapy can be classified as either active or passive immunotherapy. Active immunotherapy directly stimulates the immune system to target cancer cells, whereas passive immunotherapy activates immune cells through external agents like monoclonal antibodies, drugs, and cytokines. Cancer immunotherapy remains challenging, however, as it can have life-threatening side effects due to the severity of the disease [21][20]. The lack of targeting is the fundamental cause of immunotherapy failure against cancer. Nontargeted ICIs elicit a critical immunological response in the host that affects even normal cells. Immunotherapy must be delivered accurately to get the optimal therapeutic effect in cancer patients [22][21]. Clinical trials of cancer immunotherapies have shown promising outcomes for immunological checkpoint inhibition, adoptive T cell therapy, therapeutic cancer vaccines, and ablation-enhanced immunogenic cell death (Figure 2). Coloading of drugs into a nanocarrier platform can efficiently deliver the drug to the subcellular levels of cancer residing in the TME [23][22].
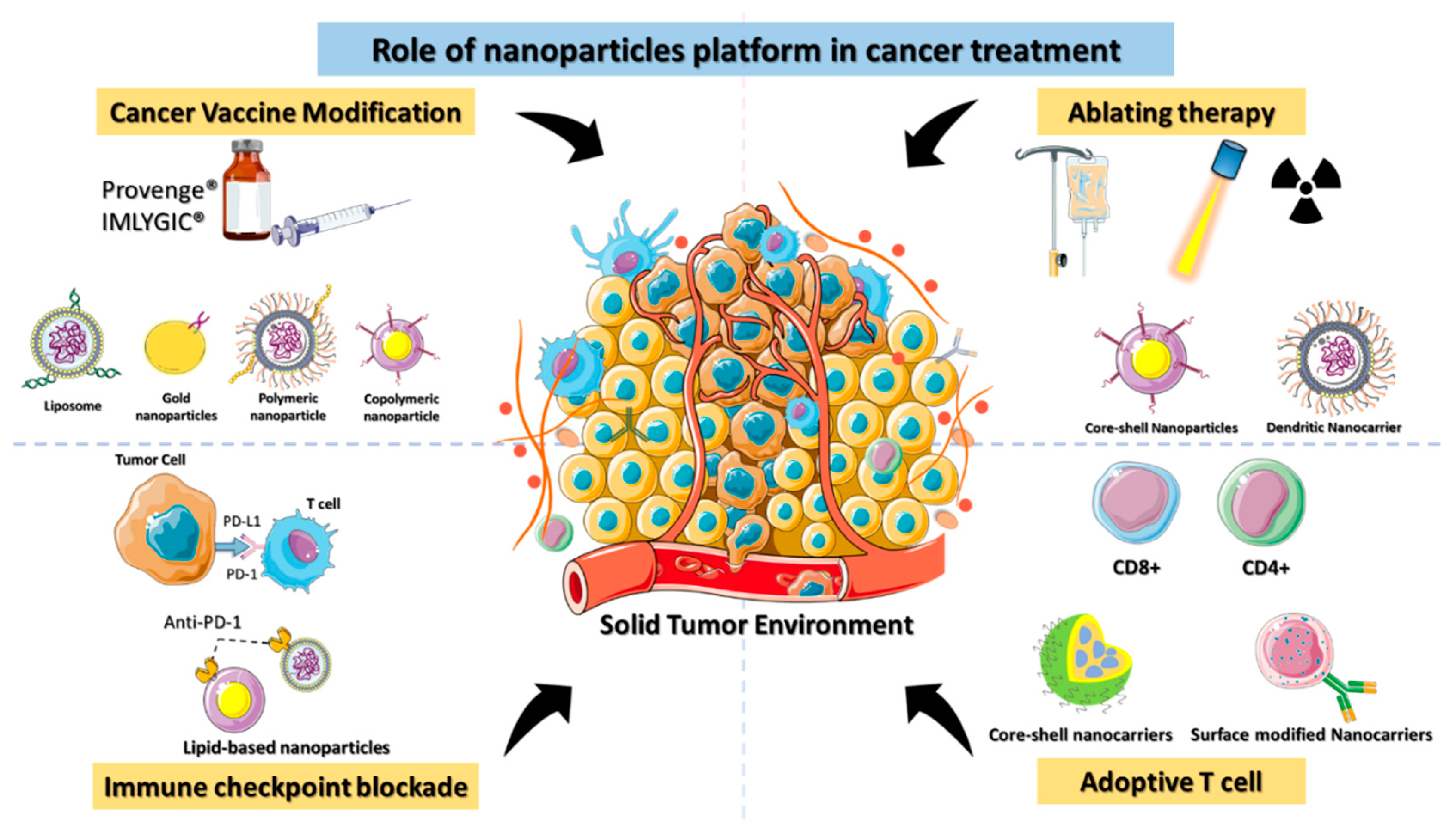
Figure 2.
Schematic depiction of the role of nanomaterials platforms in various immunotherapies for cancer.
2.1. Nanocarriers for Immune Checkpoint Inhibitor (ICI) or Blockade (ICB)
Recently, cancer immunotherapies using ICIs or ICBs have shown remarkable advancements in the treatment of advanced-stage cancers, displayed by improved patient outcomes. When these ICIs bind to the surface of partner proteins such as T cells, they turn “off” T cell signals and render them inactive. In other words, the ICIs inhibit cytokine expression, which prohibits a proper immune response from the T cell. ICIs block the checkpoint proteins of the tumor cells from binding to the ligand, resulting in a deeply improved prognosis. The ability of ICIs to regulate both innate and adaptive immune cells allows them to effectively renew the immune response and restore tumor-cell infiltration [24][23]. The US FDA-approved 16 immunomodulators, nine ICIs, and multiple cytokines and adjuvants and these are available as cancer immunotherapies [25][24].
Throughout the decade, many mAb-based ICI drugs have shown remarkable results towards several IC targets, e.g., cytotoxic T lymphocyte-associated protein 4 (CTLA-4), and programmed cell death 1 or ligand 1 and 2 (PD-1 or PD-L1 or PD-L2), and show successful results in the clinic [26][25]. These induce remarkable tumor regression and long-lasting recovery in certain cancer patients. For example, acute lymphoblastic leukemia (ALL) is one of the highly resistant cancer to cure, but T cells expressing chimeric antigen receptors (CARs) have shown improved prognosis [27][26]. In clinical trials, a variety of immunostimulatory mAbs, small molecules that reverse cancer-associated immunosuppression, and tumor-targeting therapeutic vaccines are used for treatments. Several immunotherapeutic cancer treatments are becoming more widely used and some ICIs have even reached late-stage clinical testing.
When utilizing nontargeted drug delivery, patients may be at risk for developing autoimmune reactions and related adverse effects [28][27]. Critical challenges in ICI-based cancer immunotherapies include targeted localization, protein infusion stability, and regulated ICI release. ICI blockage of the receptor on T cells results in more peripheral T cell recruitment, leading to an autoimmune reaction [29][28]. Nanotechnology-assisted administration of ICIs has benefits, including enhanced drug accumulation in tumors, simultaneous delivery of multiple ICIs, real-time delivery monitoring, and even facilitating advanced delivery techniques. The engineered nanoparticles with ICIs are better able to reach the tumor via enhanced permeability and retention (EPR) effects. The nanomedicine-based ICIs can penetrate complicated tumor environments by overcoming physiological barriers such as the blood–brain barrier and the host immune response (Figure 3).
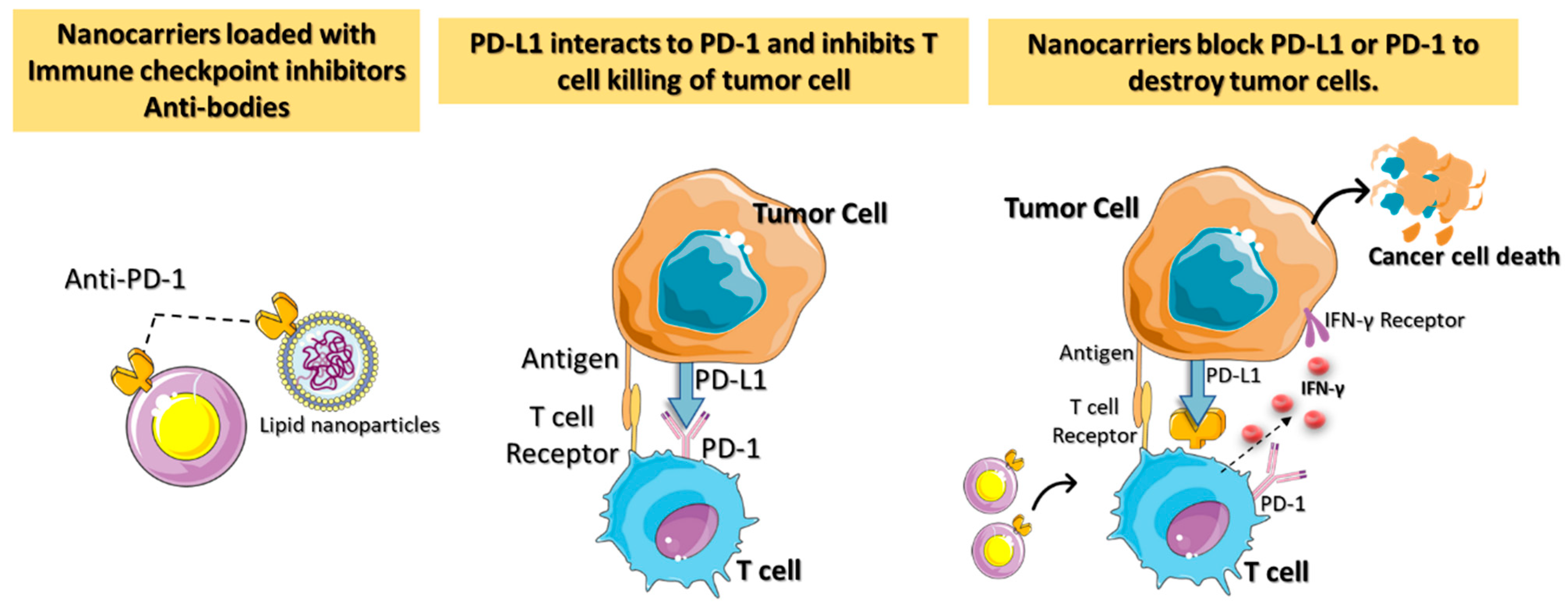
Figure 3. A diagrammatic representation of the immune checkpoint’s blockage using nanocarriers loaded with checkpoint inhibitors and antibodies for targeted cancer immunotherapy. Antitumor immune responses are regulated by proteins called checkpoints, such as PD-L1 on tumor cells and PD-1 on T cells. When PD-L1 binds to PD-1, it inhibits the activity of T cells and prevents them from killing tumor cells. Targeted nanocarriers functionalized with an immune checkpoint inhibitor (anti-PD-L1 or anti-PD-1) allow T cells to destroy tumor cells by preventing PD-L1 from attaching to PD-1.
Polo-like kinase 1 (PLK1) enzymes are key mitotic kinases that are overexpressed in a variety of cancers and exhibit oncogenic properties. PLK1 inhibition kills cancer cells and upregulates PD-L1 expression in the TME through the mitogen-activated protein kinase (MAPK) pathway. Yantesee et al. developed a nanoplatform strategy to express the relationship between PD-L1 antibody and PLK-1 inhibitor to target ICIs to improve the survival of non-small cell lung cancer (NSCLC). The nanoplatform consists of mesoporous silica nanoparticles (MSNs) loaded with the immunotherapy drug Volasertib (PLK-1 inhibitor) and functionalized with PD-L1 on the surface of nanostructures to target cancer cells. This combination of strategies is defined as the ARAC platform (antigen-release agents and a checkpoint). The increase in the uptake of MSNs in NSCLC via PD-L1, thereby delivering PLK-1 in the cytosol, leads to cellular apoptosis. In vivo evaluation of ARAC has shown an improved survival rate (up to 30 days) and a threefold reduction in the tumor volume compared to Volasertib. Inhibiting PLK1 results in an increase in PD-L1 expression in the remaining surviving cancer cells. The PD-L1 antibody-targeted ARAC significantly enhances adaptive antitumor immunity and T cell activity by elevating the CD8+ ratio and tumor infiltration. The subsequent dosage of ARAC results in immunosuppression in the TME [30][29].
A decline in the efficacy of ICBs in cancer immunotherapy is often attributed to tumor suppressors, which act as the driving force behind tumorigenesis. Despite the cell-autonomous tumor-suppressive effects, evidence indicates that the p53 protein modulates the TME by altering the contact of tumor cells with immune cells. When p53 is restored genetically, myeloid cell activation is upregulated, and tumor antigen-specific adaptive immunity is enhanced. Shi et al. developed specific hybrid nanoparticles with a lipid-based poly (lactic-co-glycolic acid) (PLGA) polymer core to transport p53 mRNA transfection complexes [31][30]. Targeted p53 mRNA nanoparticles suppressed the pro-tumorigenic M2-type tumor-associated macrophage (TAM) and enhanced the MHC-1 expression with antitumor immunity. Glycogen synthase kinase 3 (GSK-3) works as an unanticipated tumor suppressor in certain cancers [32][31], as it is responsible for phosphorylating the oncoprotein known as mouse double minute 2 homologs (MDM2). The MDM2 protein is the most important regulator of the p53 protein [33][32]. Thus, instead of restoring the p53, direct inhibition of GSK3 effectively reduces PD-1 expression and promotes the proliferation of cytotoxic T cells, resulting in long-term T cell memory. However, delivery of the GSK-3 small molecule is challenging due to its relatively short half-life and high availability in off-targeted organs. Thierry et al. reported a nano formulation containing a GSK-3 inhibitor loaded with PEG-PLGA nanoparticles to block the PD-L1 ICP [34][33]. Results indicate that the nanoplatform enhances the efficacy of the ICB therapy, thereby improving cancer treatment.
2.2. Nanocarrier for Adoptive Cell Transfer (ACT)
Adoptive cell therapy (ACT) involving T cells derived from tumor-infiltrating lymphocytes (TIL) has the potential for both immunostimulatory and immunosuppressive cancer therapies [34]. However, ACT is still a barrier to a robust activation of the host immune system, the regulation of signaling pathways, and the maintenance of a progression-free state. The use of the nanoplatform can improve the production, application, and efficacy of T cell immunotherapy for a variety of cancer conditions [35].
 Tumor resistance can be developed through the stimulation of the innate immune system. The development of active nanocarriers facilitates binding with the innate immune system to regulate “danger signals”. The activation of danger signals at the tumor site by Toll-like receptors such as retinoic-acid-inducible gene I (RIG-I), nucleotide-binding oligomerization domain (NOD), and stimulator of interferon genes (STING) improves T cell response [40][39]. Current clinical research indicates that natural ligands for STING and cyclic dinucleotides (CDNs) are effective agonists for inducing potent antitumor immunity. CDNs are susceptible to nucleases and impermeable to cell membranes, which hinders their systemic delivery. Irvine et al. formulated PEGylated lipids conjugated with CDNs that self-assemble in lipid nanodiscs (LNDs) [41][40]. Discoid-shaped nanoparticles (LNDs-CDNs) are optimal for intravenous delivery and show effective penetration within the tumor model that leads to tumor necrosis.
Effective cancer cytosol penetration of CDN-loaded lipid nanomaterials triggers antitumor immunity through CD8+ T cell and natural killer (NK) cell activation [42][41]. STING activation pathways trigger NK cells to produce interferon γ (IFN-γ), promoting PD-1 expression in tumor cells [43][42]. However, the data from studies implies that a single dose is insufficient to activate NK cells [44][43]. Therefore, a combination of PD-1 antibody with the CDN agonists loaded in lipid nanoparticles has an antitumor impact in lung metastasis under multiple-dose cycles. Ferroptosis is an iron-dependent form of apoptosis for tumor suppression. Cancer cells undergoing ferroptosis also trigger IFN-γ, which promotes the CD8+ T lymphocytes in the TME. The expression profile analysis identified miR-21-3p as the highly upregulated microRNAs that regulate ferroptosis. Chunying Li’s group examined the anti-PD-1 immunotherapy impact of miR-21-3p-loaded gold nanoparticles in a preclinical tumor model [45][44]. T-cell-adoptive cancer immunotherapies can be improved by integrating the nanosystem loaded with the immune drugs.
Tumor resistance can be developed through the stimulation of the innate immune system. The development of active nanocarriers facilitates binding with the innate immune system to regulate “danger signals”. The activation of danger signals at the tumor site by Toll-like receptors such as retinoic-acid-inducible gene I (RIG-I), nucleotide-binding oligomerization domain (NOD), and stimulator of interferon genes (STING) improves T cell response [40][39]. Current clinical research indicates that natural ligands for STING and cyclic dinucleotides (CDNs) are effective agonists for inducing potent antitumor immunity. CDNs are susceptible to nucleases and impermeable to cell membranes, which hinders their systemic delivery. Irvine et al. formulated PEGylated lipids conjugated with CDNs that self-assemble in lipid nanodiscs (LNDs) [41][40]. Discoid-shaped nanoparticles (LNDs-CDNs) are optimal for intravenous delivery and show effective penetration within the tumor model that leads to tumor necrosis.
Effective cancer cytosol penetration of CDN-loaded lipid nanomaterials triggers antitumor immunity through CD8+ T cell and natural killer (NK) cell activation [42][41]. STING activation pathways trigger NK cells to produce interferon γ (IFN-γ), promoting PD-1 expression in tumor cells [43][42]. However, the data from studies implies that a single dose is insufficient to activate NK cells [44][43]. Therefore, a combination of PD-1 antibody with the CDN agonists loaded in lipid nanoparticles has an antitumor impact in lung metastasis under multiple-dose cycles. Ferroptosis is an iron-dependent form of apoptosis for tumor suppression. Cancer cells undergoing ferroptosis also trigger IFN-γ, which promotes the CD8+ T lymphocytes in the TME. The expression profile analysis identified miR-21-3p as the highly upregulated microRNAs that regulate ferroptosis. Chunying Li’s group examined the anti-PD-1 immunotherapy impact of miR-21-3p-loaded gold nanoparticles in a preclinical tumor model [45][44]. T-cell-adoptive cancer immunotherapies can be improved by integrating the nanosystem loaded with the immune drugs.
2.2. Nanocarrier for Adoptive Cell Transfer (ACT)
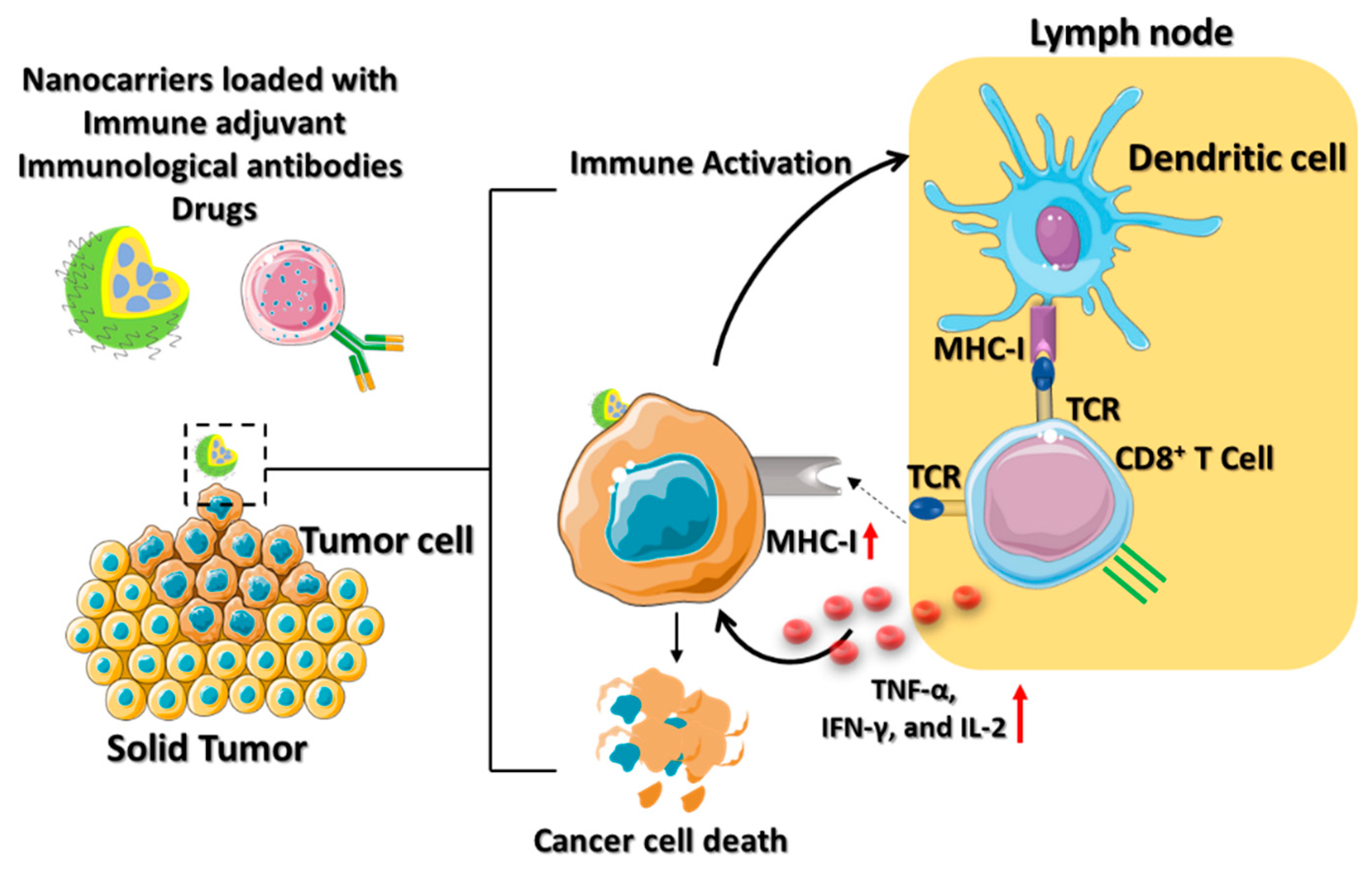
Adoptive cell therapy (ACT) involving T cells derived from tumor-infiltrating lymphocytes (TIL) has the potential for both immunostimulatory and immunosuppressive cancer therapies [35]. Howeversplatin, ACT is still a barrier to a robust activation of the host immune system, the regulation of signaling pathways, and the maintenance of a progression-free state. The use of the nanoplatform can improve the production, application, and efficacy of T cell immunotherapy for a variety of cancer conditions [36]. Cisplatin, also lso known as cis-diamminedichloroplatinum (II) (CDDP), is an effective cancer drug in clinical trials [37][36]. The effectiveness of cisplatin in inhibiting tumor growth has been established; however, its organ toxicity presents a significant challenge. Yao and his colleagues utilized the CDDP drug-loaded polyethylene glycol (PEG)–polyglutamate block copolymer micelle nanoparticles (CDDP-NPs) for antitumor immune response [38][37]. The longer retention time of the CDDP-NPs in the TME upregulates the major histocompatibility complex class I (MHC-I), leading to the activation of the amyloid precursor protein (APP) pathway. The DCs are attributed to the interaction between the T cell receptor (TCR) and the peptide it recognizes by the APP (Figure 4). Thus, the CDDP-NPs indirectly activate the tumor antigen-presenting CD8+ T cell through the TCR signaling pathways. The OX40 is a tumor necrosis factor receptor superfamily (TNFRSF) domain that acts as a costimulatory antigen molecule expressed in the CD8+ T cells [39][38]. Therefore, the use of CDDP-NPs along with the OX40 agonists (aOX40) had a remarkable therapeutic effect in inducing T cell infiltration in the tumor environment. The promotion of aOX40 would activate the proinflammatory cytokines and secrete TNF-α, IFN-γ, and IL-2 for killing the tumor. Immunotherapies utilizing CDDP-NPs remain in the preclinical phase of development. It is crucial to analyze the efficacy of nanomedicine in the clinic to determine the possible role of T cell infiltration in the tumor.
Figure 4. Schematic illustration which depicts the mechanism of ACT using various nanocarriers. The nanocarriers, such as lipid-based nanoparticles, nanodiscs, or core–shell nanoparticles are coloaded with immune drugs, antibodies, and adjuvants for active cancer immunotherapy. The immune activation of the dendritic cells contributes to immunological responses to the TME by secreting IFN-γ, which is promoted by the effector activities of CD8+ T cells to cause tumor death.
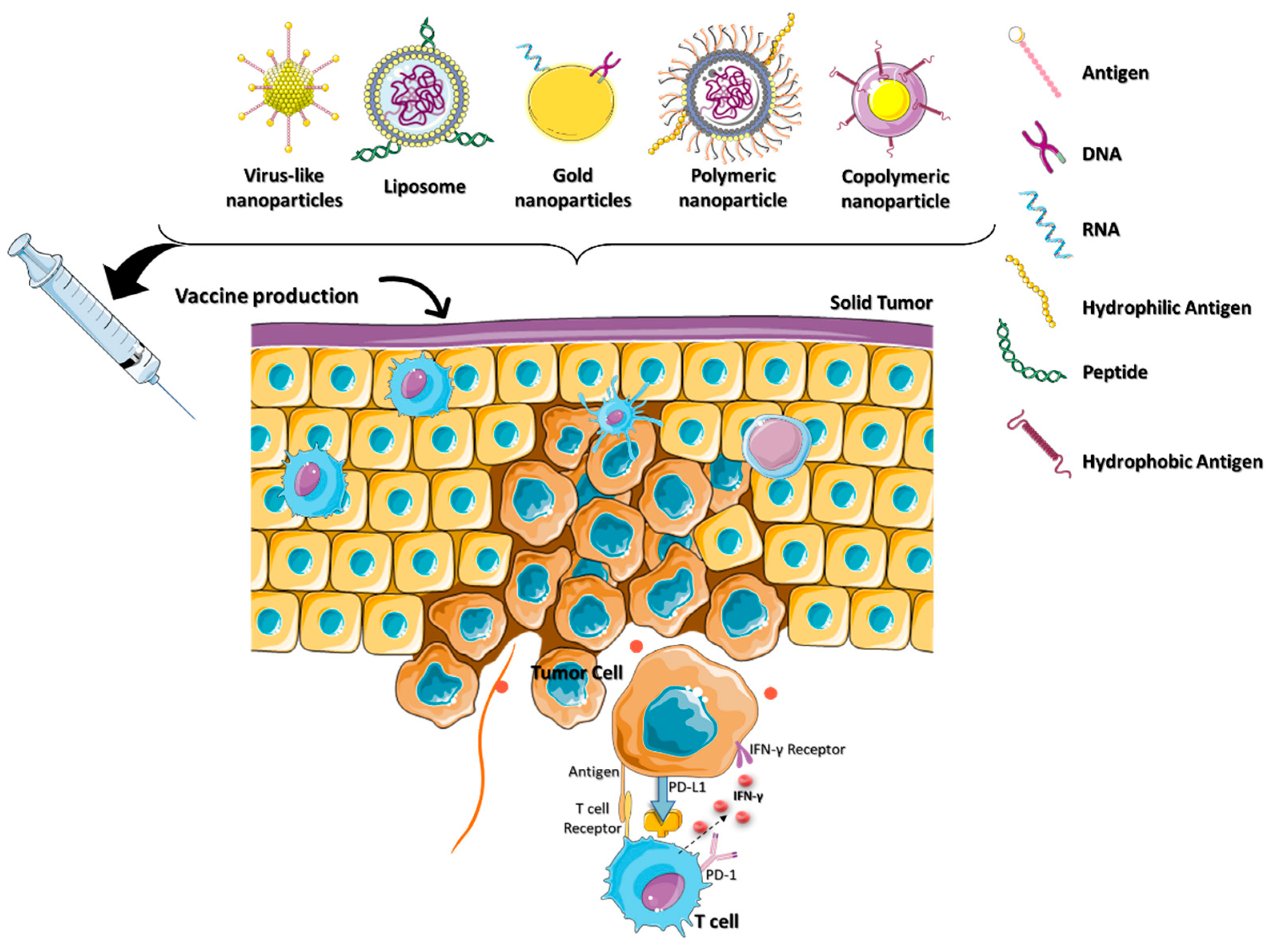
2.3. Nanocarriers for Cancer Vaccine
Cancer vaccines generally use tumor-associated antigens (TAAs) and tumor-specific antigens (TSAs) to boost the patient’s immune system. Therapies involving TSAs or neoantigens selectively expressed in the tumor have been at the forefront of cancer immunotherapy. Vaccines based on neoantigens have the potential to circumvent central immune tolerance and activate tumor-specific T cells [46][45]. Sipuleucel-T (Provenge®) and talimogene laherparepvec (IMLYGIC®) are some of the DCs-based cancer vaccines currently in phase I clinical trials [47,48][46][47]. However, poor targeting of the neoantigen-based cancer vaccines results in a low affinity to the TCR, making it incapable of mediating an effective antitumor response. Combining the NPs platform with the neoantigen-targeted vaccine delivery has therapeutic potential in cancer immunotherapy. Jadidi and colleagues were the first to report that the combined suppression of lymphocyte-associated gene 3 (LAG3) and PD-1 by DC vaccines improves anticancer efficacy in the TME. LAG3 is an inhibitory receptor generated by immune cells to trigger T cell dysfunction [49][48]. Initiating immunosuppressive responses and promoting the proliferation of cancer cells, LAG3 promotes the development of cancer [50][49]. Specific siRNAs and LAG3/PD-1 antibodies were loaded onto chitosan–dextran sulfate–lactate (TMC-DS-L) nanoparticles, which led to increased IFN-γ production at the tumor site [51][50]. The primary obstacles in cancer immunotherapy are multiple drug resistance (MDR) and the inadequate accumulation of cancer vaccines in the TME [52][51]. Yao et al. developed a zwitterionic polymer employing poly (carboxy betaine methacrylate) (PBCMA) as a polymer shell over mesoporous organo silica nanoparticles (MONs) to overcome MDR in the TME [53][52]. PCBMA is loaded with redox-responsive sulfur dioxide (SO2), prodrug molecules (DN 2,4 dinitrobenzene-sulfonyl chloride), and chemotherapeutic drugs (DOX, doxorubicin) to treat MDR cancer effectively. The downregulation of P-glycoprotein in the presence of SO2 makes cancer cells more susceptible to chemotherapy-induced apoptosis. PCBMA increases the nanomedicine’s intratumor accumulation, resulting in a 94.8% reduction in tumor growth. The redox-responsive SO2 sensitizes cells, which aids DOX in prolonging the apoptosis in the TME. Preclinical immuno compatibility development requires the controlled activation of innate immunity [54][53]. Biopolymers (proteins, nucleic acids, collagen, chitosan, etc.), synthetic polymers (polystyrene, poly (lactic-co-glycolic acid) (PLGA), and poly (amino ester) (PBAEs)), and stimuli-responsive polymers loaded with immunoadjuvants have MDR capabilities that promote immunological activity. These polymers enable multi adjuvant synergy in cancer immunotherapy by stabilizing drug–cargo transport and activating proinflammatory cytokines. Thus, the camouflage of polymeric nanoparticles for the delivery of cancer vaccines provides an efficient platform for cancer immunotherapy (Figure 5).
Figure 5. The cancer vaccination nanoparticles were camouflaged for specific functions in the TME. Nanovesicles containing a cancer vaccine comprised of a variety of antigens and biomolecules are being developed for use in cancer immunotherapy.
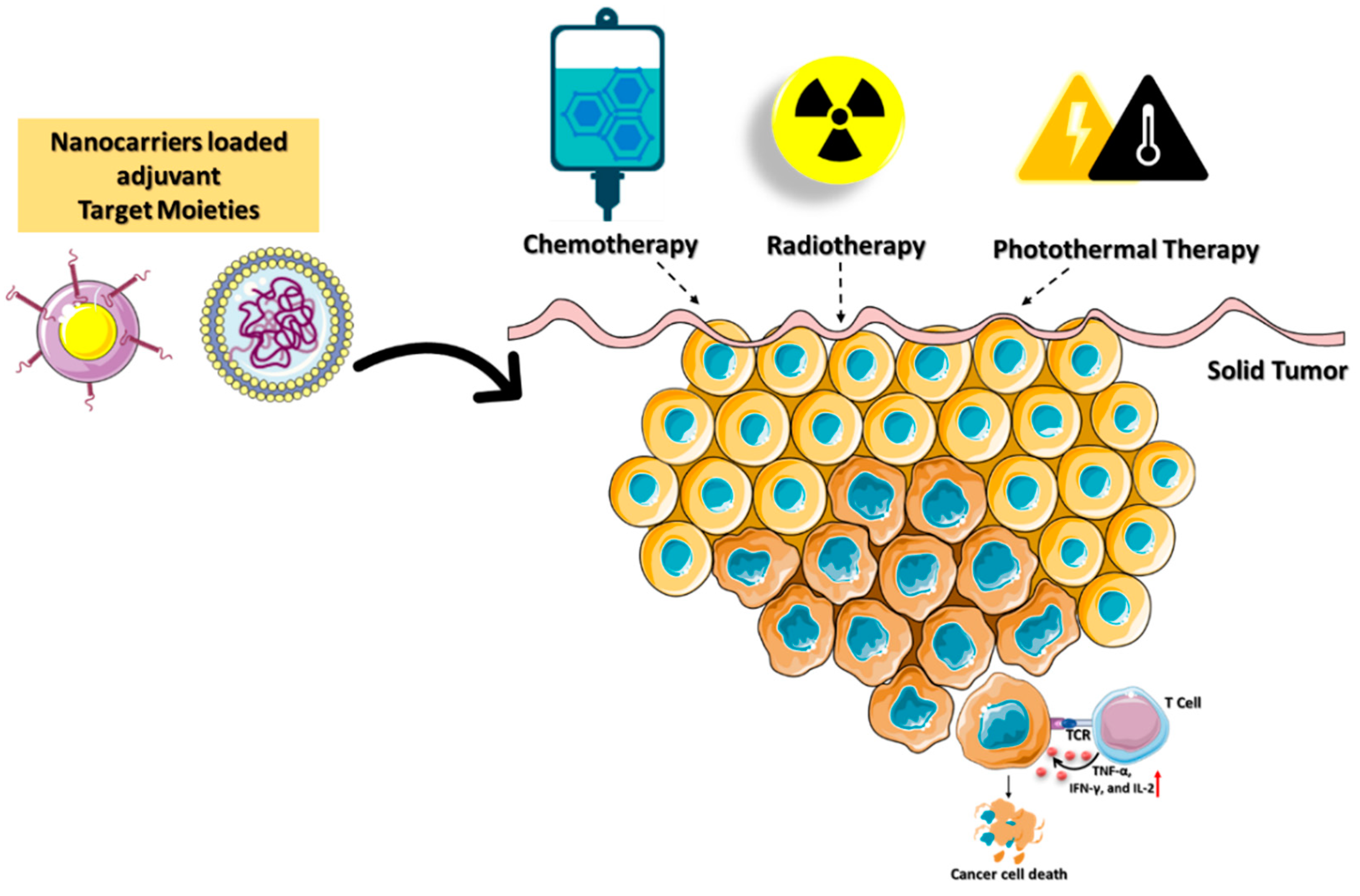
2.4. Nanocarriers for Immunogenic Cell Death (ICD)
Immunotherapy is a treatment method to eradicate cancer cells with a high degree of specificity and minimal side effects while preventing their recurrence. Chemotherapy, photothermal therapy (PTT), radiotherapy, and reactive-oxygen-species (ROS)-mediated therapies can induce immunogenic cell death (ICD), which is used to improve cytotoxic-based antitumor immunity. Cancer immunotherapies have had limited success in ablation treatment due to the development of local and systemic immunosuppression and immune evasion. Local radiation therapy in the TME destroys cancer cells by activating the immune system [55][54]. When radiation and immunotherapy are combined on a nanoplatform, they have the potential to enhance the overall therapeutic efficacy [56][55]. These methods have the potential to improve patient outcomes, but they encounter challenges in optimizing the radiation dose, toxicity, and timing of combined therapies [57][56]. Chemotherapeutics can induce ICD and release tumor-specific antigens that are effective against numerous types of cancer [58][57]. Combining chemotherapeutics and immune adjuvants is a viable method for achieving synergistic therapeutic benefits. Deng et al. reported a nanocarrier composed of liposomal spherical nucleic acids (SNAs) and FDA-approved 1,2-dioleoyl-sn-glycerol-3-phosphoethanolamine (DOPE) for enhanced cellular absorption and stability against nucleases [59][58]. Conjugating chemotherapeutics such as doxorubicin (DOX) with DOPE triggered the ICD (DOPE-DOX) to secrete tumor-specific antigens. CpG oligodeoxynucleotides (CpGs) and matrix metalloproteinases-9 (MMP-9) are immunostimulatory reagents (DOPE-MMP-CpG) that amplify the immune response and promote immune cell penetration into the TME, respectively. Tumor cell MMP-9 and glutathione stimulated lipid-encapsulated SNAs to release DOX and CpGs into the TME. However, immunosuppression and cytotoxic side effects continue to restrict the use of these nanocarriers. Yibru et al. developed a pH-responsive polyamidoamine (PAMAM) dendritic nanoparticles into the copolymer hydrogel (PCLA-PEG-PCLA) for the codelivery of DOX (PAMAM-DOX). PCLA-PEG-PCLA (poly(caprolactone-co-lactide)-poly (ethylene glycol)-b-poly(caprolactone-co-lactide) is a thermo reversible polymer ability to integrate with PAMAM-DOX nanocarriers for targeted drug delivery. At body temperature, PAMAM-DOX dendritic nanocarriers form a gel and facilitate a sustained DOX release in the TME. The combination of the immune activation with various cancer ablation techniques enhances the death of tumors. IND coupled with PAMAM-DOX also promotes the activate NK cells to attack tumor cells. However, immunological adjuvants in combination with chemotherapy remain resistant in treating some forms of cancer, especially triple-negative breast cancer (TNBC). The photothermal immunotherapy (PTT) technique has also been demonstrated to reduce tumor development and enhance immune response. However, PTT efficiency in clinical applications is hindered by its poor stability, low therapeutic potency, and loss of affinity in the multistep delivery carrier synthesis [60][59]. A research team led by Zou developed a mesoporous carbon nanocomposite (MCN) loaded with IR792 a near-infrared (NIR) laser dye (IR792-MCN) [61][60]. Photothermal immunotherapy using NIR laser irradiation is improved by combining the PD-L1 antibody with the nanocarrier (IR792-MCN@ICB). The delivered IR792-MCN@ICB irradiated at 808 nm efficiently kills tumor cells by inducing DC maturation and secreting cytokines. PD-L1 gene silencing in conjunction with photothermal immunotherapy promotes tumor-infiltrating CD8+ and CD4+ T cells, which reduces tumor growth and prevents cancer metastasis. Cancer immunotherapy based on ICDs in combination with inhibitors as adjuvants plays a significant role in determining the effectiveness of personalized therapy (Figure 6).
Figure 6. Schematic illustration of immunogenic cell death mediated by targeted nanocarriers conjugated with stimulating molecules such as immunological adjuvants and agonistic antibodies. The combination of immune activation with various cancer-ablation techniques enhances the death of tumors.
References
- Burkholder, B.; Huang, R.Y.; Burgess, R.; Luo, S.; Jones, V.S.; Zhang, W.; Lv, Z.Q.; Gao, C.Y.; Wang, B.L.; Zhang, Y.M.; et al. Tumor-induced perturbations of cytokines and immune cell networks. Biochim. Biophys. Acta 2014, 1845, 182–201.
- Gonzalez, H.; Hagerling, C.; Werb, Z. Roles of the immune system in cancer: From tumor initiation to metastatic progression. Genes Dev. 2018, 32, 1267–1284.
- Kartikasari, A.E.R.; Huertas, C.S.; Mitchell, A.; Plebanski, M. Tumor-Induced Inflammatory Cytokines and the Emerging Diagnostic Devices for Cancer Detection and Prognosis. Front. Oncol. 2021, 11, 692142.
- Vesely, M.D.; Schreiber, R.D. Cancer immunoediting: Antigens, mechanisms, and implications to cancer immunotherapy. Ann. N. Y. Acad. Sci. 2013, 1284, 1–5.
- Pucci, C.; Martinelli, C.; Ciofani, G. Innovative approaches for cancer treatment: Current perspectives and new challenges. Ecancermedicalscience 2019, 13, 961.
- Zahavi, D.; Weiner, L. Monoclonal Antibodies in Cancer Therapy. Antibodies 2020, 9, 34.
- Nixon, N.A.; Blais, N.; Ernst, S.; Kollmannsberger, C.; Bebb, G.; Butler, M.; Smylie, M.; Verma, S. Current landscape of immunotherapy in the treatment of solid tumors, with future opportunities and challenges. Curr. Oncol. 2018, 25, e373–e384.
- Li, K.; Luo, H.; Huang, L.; Luo, H.; Zhu, X. Microsatellite instability: A review of what the oncologist should know. Cancer Cell Int. 2020, 20, 16.
- Fan, S.; Ren, H.; Zhao, L.; Yin, J.; Feng, G.; Wang, J.; Guan, H. Neurological immune-related adverse events associated with immune checkpoint inhibitors: A review of the literature. Asia Pac. J. Clin. Oncol. 2020, 16, 291–298.
- Nishino, M.; Ramaiya, N.H.; Hatabu, H.; Hodi, F.S. Monitoring immune-checkpoint blockade: Response evaluation and biomarker development. Nat. Rev. Clin. Oncol. 2017, 14, 655–668.
- Patrinely, J.R., Jr.; Johnson, R.; Lawless, A.R.; Bhave, P.; Sawyers, A.; Dimitrova, M.; Yeoh, H.L.; Palmeri, M.; Ye, F.; Fan, R.; et al. Chronic Immune-Related Adverse Events Following Adjuvant Anti-PD-1 Therapy for High-risk Resected Melanoma. JAMA Oncol. 2021, 7, 744–748.
- Galluzzi, L.; Chan, T.A.; Kroemer, G.; Wolchok, J.D.; Lopez-Soto, A. The hallmarks of successful anticancer immunotherapy. Sci. Transl. Med. 2018, 10, eaat7807.
- Bai, R.; Chen, N.; Li, L.; Du, N.; Bai, L.; Lv, Z.; Tian, H.; Cui, J. Mechanisms of Cancer Resistance to Immunotherapy. Front. Oncol. 2020, 10, 1290.
- Rameshbabu, S.; Labadie, B.W.; Argulian, A.; Patnaik, A. Targeting Innate Immunity in Cancer Therapy. Vaccines 2021, 9, 138.
- Gavas, S.; Quazi, S.; Karpinski, T.M. Nanoparticles for Cancer Therapy: Current Progress and Challenges. Nanoscale Res. Lett. 2021, 16, 173.
- Anselmi, M.; Borbely, A.; Figueras, E.; Michalek, C.; Kemker, I.; Gentilucci, L.; Sewald, N. Linker Hydrophilicity Modulates the Anticancer Activity of RGD-Cryptophycin Conjugates. Chemistry 2021, 27, 1015–1022.
- Li, Q.; Li, X.; Zhao, C. Strategies to Obtain Encapsulation and Controlled Release of Small Hydrophilic Molecules. Front. Bioeng. Biotechnol. 2020, 8, 437.
- Subhan, M.A.; Yalamarty, S.S.K.; Filipczak, N.; Parveen, F.; Torchilin, V.P. Recent Advances in Tumor Targeting via EPR Effect for Cancer Treatment. J. Pers. Med. 2021, 11, 571.
- Tan, S.; Li, D.; Zhu, X. Cancer immunotherapy: Pros, cons and beyond. Biomed. Pharmacother. 2020, 124, 109821.
- Teixidor, E.; Bosch-Barrera, J. the dark side of immunotherapy: Challenges facing the new hope in cancer treatment. Ann. Transl. Med. 2019, 7, S183.
- Chevolet, I.; Speeckaert, R.; Schreuer, M.; Neyns, B.; Krysko, O.; Bachert, C.; Hennart, B.; Allorge, D.; van Geel, N.; van Gele, M.; et al. Characterization of the in vivo immune network of IDO, tryptophan metabolism, PD-L1, and CTLA-4 in circulating immune cells in melanoma. Oncoimmunology 2015, 4, e982382.
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751–760.
- He, X.; Xu, C. Immune checkpoint signaling and cancer immunotherapy. Cell Res. 2020, 30, 660–669.
- Mokhtari, R.B.; Sambi, M.; Qorri, B.; Baluch, N.; Ashayeri, N.; Kumar, S.; Cheng, H.M.; Yeger, H.; Das, B.; Szewczuk, M.R. The Next-Generation of Combination Cancer Immunotherapy: Epigenetic Immunomodulators Transmogrify Immune Training to Enhance Immunotherapy. Cancers 2021, 13, 3596.
- Qin, S.; Xu, L.; Yi, M.; Yu, S.; Wu, K.; Luo, S. Novel immune checkpoint targets: Moving beyond PD-1 and CTLA-4. Mol. Cancer 2019, 18, 155.
- Greenbaum, U.; Mahadeo, K.M.; Kebriaei, P.; Shpall, E.J.; Saini, N.Y. Chimeric Antigen Receptor T-Cells in B-Acute Lymphoblastic Leukemia: State of the Art and Future Directions. Front. Oncol. 2020, 10, 1594.
- Yetisgin, A.A.; Cetinel, S.; Zuvin, M.; Kosar, A.; Kutlu, O. Therapeutic Nanoparticles and Their Targeted Delivery Applications. Molecules 2020, 25, 2193.
- Yu, X.; Fang, C.; Zhang, K.; Su, C. Recent Advances in Nanoparticles-Based Platforms Targeting the PD-1/PD-L1 Pathway for Cancer Treatment. Pharmaceutics 2022, 14, 1581.
- Reda, M.; Ngamcherdtrakul, W.; Nelson, M.A.; Siriwon, N.; Wang, R.; Zaidan, H.Y.; Bejan, D.S.; Reda, S.; Hoang, N.H.; Crumrine, N.A.; et al. Development of nanoparticle-based immunotherapy targeting PD-L1 and PLK1 for lung cancer treatment. Nat. Commun. 2022, 13, 4261.
- Xiao, Y.; Chen, J.; Zhou, H.; Zeng, X.; Ruan, Z.; Pu, Z.; Jiang, X.; Matsui, A.; Zhu, L.; Amoozgar, Z.; et al. Combining p53 mRNA nanotherapy with immune checkpoint blockade reprograms the immune microenvironment for effective cancer therapy. Nat. Commun. 2022, 13, 758.
- Mills, C.N.; Nowsheen, S.; Bonner, J.A.; Yang, E.S. Emerging roles of glycogen synthase kinase 3 in the treatment of brain tumors. Front. Mol. Neurosci. 2011, 4, 47.
- Kulikov, R.; Boehme, K.A.; Blattner, C. Glycogen synthase kinase 3-dependent phosphorylation of Mdm2 regulates p53 abundance. Mol. Cell Biol. 2005, 25, 7170–7180.
- Badiee, P.; Maritz, M.F.; Thierry, B. Glycogen kinase 3 inhibitor nanoformulation as an alternative strategy to inhibit PD-1 immune checkpoint. Int. J. Pharm. 2022, 622, 121845.
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat. Rev. Immunol. 2020, 20, 651–668.
- Yang, L.; Shi, P.; Zhao, G.; Xu, J.; Peng, W.; Zhang, J.; Zhang, G.; Wang, X.; Dong, Z.; Chen, F.; et al. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct. Target Ther. 2020, 5, 8.
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378.
- Yao, H.; Shen, N.; Ji, G.; Huang, J.; Sun, J.; Wang, G.; Tang, Z.; Chen, X. Cisplatin Nanoparticles Promote Intratumoral CD8(+) T Cell Priming via Antigen Presentation and T Cell Receptor Crosstalk. Nano Lett. 2022, 22, 3328–3339.
- Bansal-Pakala, P.; Halteman, B.S.; Cheng, M.H.; Croft, M. Costimulation of CD8 T cell responses by OX40. J. Immunol. 2004, 172, 4821–4825.
- Liu, Z.; Han, C.; Fu, Y.X. Targeting innate sensing in the tumor microenvironment to improve immunotherapy. Cell Mol. Immunol. 2020, 17, 13–26.
- Dane, E.L.; Belessiotis-Richards, A.; Backlund, C.; Wang, J.; Hidaka, K.; Milling, L.E.; Bhagchandani, S.; Melo, M.B.; Wu, S.; Li, N.; et al. STING agonist delivery by tumor-penetrating PEG-lipid nanodiscs primes robust anticancer immunity. Nat. Mater. 2022, 21, 710–720.
- Nakamura, T.; Yamada, K.; Sato, Y.; Harashima, H. Lipid nanoparticles fuse with cell membranes of immune cells at low temperatures leading to the loss of transfection activity. Int. J. Pharm. 2020, 587, 119652.
- Khalifa, A.M.; Nakamura, T.; Sato, Y.; Sato, T.; Hyodo, M.; Hayakawa, Y.; Harashima, H. Interval- and cycle-dependent combined effect of STING agonist loaded lipid nanoparticles and a PD-1 antibody. Int. J. Pharm. 2022, 624, 122034.
- Messenheimer, D.J.; Jensen, S.M.; Afentoulis, M.E.; Wegmann, K.W.; Feng, Z.; Friedman, D.J.; Gough, M.J.; Urba, W.J.; Fox, B.A. Timing of PD-1 Blockade Is Critical to Effective Combination Immunotherapy with Anti-OX40. Clin. Cancer Res. 2017, 23, 6165–6177.
- Guo, W.; Wu, Z.; Chen, J.; Guo, S.; You, W.; Wang, S.; Ma, J.; Wang, H.; Wang, X.; Wang, H.; et al. Nanoparticle delivery of miR-21-3p sensitizes melanoma to anti-PD-1 immunotherapy by promoting ferroptosis. J. Immunother. Cancer 2022, 10, e004381.
- Liu, J.; Fu, M.; Wang, M.; Wan, D.; Wei, Y.; Wei, X. Cancer vaccines as promising immuno-therapeutics: Platforms and current progress. J. Hematol. Oncol. 2022, 15, 28.
- Pallerla, S.; Abdul, A.; Comeau, J.; Jois, S. Cancer Vaccines, Treatment of the Future: With Emphasis on HER2-Positive Breast Cancer. Int. J. Mol. Sci. 2021, 22, 779.
- Chen, B.Q.; Zhao, Y.; Zhang, Y.; Pan, Y.J.; Xia, H.Y.; Kankala, R.K.; Wang, S.B.; Liu, G.; Chen, A.Z. Immune-regulating camouflaged nanoplatforms: A promising strategy to improve cancer nano-immunotherapy. Bioact. Mater. 2023, 21, 1–19.
- Hassannia, H.; Chaleshtari, M.G.; Atyabi, F.; Nosouhian, M.; Masjedi, A.; Hojjat-Farsangi, M.; Namdar, A.; Azizi, G.; Mohammadi, H.; Ghalamfarsa, G.; et al. Blockage of immune checkpoint molecules increases T-cell priming potential of dendritic cell vaccine. Immunology 2020, 159, 75–87.
- Barshidi, A.; Karpisheh, V.; Noukabadi, F.K.; Kiani, F.K.; Mohammadi, M.; Afsharimanesh, N.; Ebrahimi, F.; Kiaie, S.H.; Navashenaq, J.G.; Hojjat-Farsangi, M.; et al. Dual Blockade of PD-1 and LAG3 Immune Checkpoints Increases Dendritic Cell Vaccine Mediated T Cell Responses in Breast Cancer Model. Pharm. Res. 2022, 39, 1851–1866.
- Jorgovanovic, D.; Song, M.; Wang, L.; Zhang, Y. Roles of IFN-gamma in tumor progression and regression: A review. Biomark Res. 2020, 8, 49.
- Shofolawe-Bakare, O.T.; Stokes, L.D.; Hossain, M.; Smith, A.E.; Werfel, T.A. Immunostimulatory biomaterials to boost tumor immunogenicity. Biomater. Sci. 2020, 8, 5516–5537.
- Yao, X.; Ma, S.; Peng, S.; Zhou, G.; Xie, R.; Jiang, Q.; Guo, S.; He, Q.; Yang, W. Zwitterionic Polymer Coating of Sulfur Dioxide-Releasing Nanosystem Augments Tumor Accumulation and Treatment Efficacy. Adv. Healthc. Mater. 2020, 9, e1901582.
- Weiss, A.M.; Hossainy, S.; Rowan, S.J.; Hubbell, J.A.; Esser-Kahn, A.P. Immunostimulatory Polymers as Adjuvants, Immunotherapies, and Delivery Systems. Macromolecules 2022, 55, 6913–6937.
- Formenti, S.C.; Demaria, S. Systemic effects of local radiotherapy. Lancet Oncol. 2009, 10, 718–726.
- Wang, Y.; Deng, W.; Li, N.; Neri, S.; Sharma, A.; Jiang, W.; Lin, S.H. Combining Immunotherapy and Radiotherapy for Cancer Treatment: Current Challenges and Future Directions. Front. Pharmacol. 2018, 9, 185.
- Hagan, C.T.t.; Mi, Y.; Knape, N.M.; Wang, A.Z. Enhancing Combined Immunotherapy and Radiotherapy through Nanomedicine. Bioconjug. Chem. 2020, 31, 2668–2678.
- Lerner, E.C.; Edwards, R.M.; Wilkinson, D.S.; Fecci, P.E. Laser ablation: Heating up the anti-tumor response in the intracranial compartment. Adv. Drug Deliv. Rev. 2022, 185, 114311.
- Deng, B.; Ma, B.; Ma, Y.; Cao, P.; Leng, X.; Huang, P.; Zhao, Y.; Ji, T.; Lu, X.; Liu, L. Doxorubicin and CpG loaded liposomal spherical nucleic acid for enhanced Cancer treatment. J. Nanobiotechnol. 2022, 20, 140.
- Zhou, B.; Wu, Q.; Wang, M.; Hoover, A.; Wang, X.; Zhou, F.; Towner, R.A.; Smith, N.; Saunders, D.; Song, J.; et al. Immunologically modified MnFe2O4 nanoparticles to synergize photothermal therapy and immunotherapy for cancer treatment. Chem. Eng. J. 2020, 396, 125239.
- Wang, Y.; Wang, H.; Song, Y.; Lv, M.; Mao, Y.; Song, H.; Wang, Y.; Nie, G.; Liu, X.; Cui, J.; et al. siRNA drug delivery system enhances photothermal immunotherapy for triple-negative breast cancer under near-infrared laser irradiation. J. Nanobiotechnol. 2022, 20, 96.
More