Nanoparticles纳米颗粒 (NPs) have been widely used as target delivery vehicles for therapeutic goods; however, compared with inorganic and organic nanomaterials, protein nanomaterials have better biocompatibility and can self-assemble into highly ordered cage-like structures, which are more favorable for applications in targeted drug delivery.) 已被广泛用作治疗产品的靶向运载工具;然而,与无机和有机纳米材料相比,蛋白质纳米材料具有更好的生物相容性,可以自组装成高度有序的笼状结构,更有利于在靶向药物递送方面的应用。
- protein cage
- nanoparticles
- drug delivery
1. Introduction
一、简介
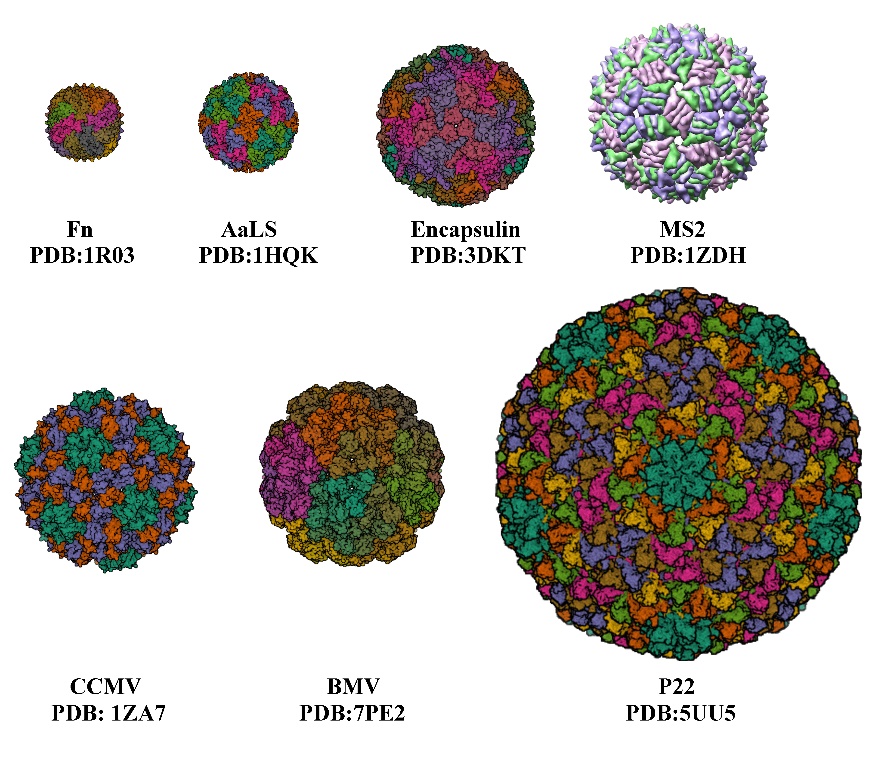
The development of nanocarriers for drug delivery has been of great interest to many researchers over recent decades, as they overcome the limitations of traditional drug delivery methods [1]. Nanoparticles (NPs) have unique properties in terms of their physical, optical, chemical, and therapeutic properties. Traditional drug delivery carriers, such as non-nanomedicines and inorganic NPs, face high toxicity and low delivery efficiency, making naturally derived nanocarriers viable alternatives. Protein nanocages [2], such as viruses, virus-like particles, and ferritin, are formed by the self-assembly of protein subunits to form cage-like structures, and they consist of varying numbers of subunits to form protein cages of varying sizes, with cage diameters ranging from a few nanometers to tens of nanometers (Figure 1, Table 1), which are suitable for the encapsulation and application of various drugs, respectively. Virus-like particles (VLPs) are a class of multimeric proteins derived from viral capsids that self-assemble into protein cage NPs with hollow interiors. The creation and structure of VLPs are identical to those of wild-type viruses, but since they lack the viral genome, they are not harmful. The virus’s shell is a symmetrical supramolecular complex composed of proteins. Each icosahedral virus contains 12 pentameric substructures that are located according to their triangulation number (T). When the subunits remain the same size, increasing the number of subunits will increase the T number and the size of the capsid [3]. Among protein NPs, VLPs are the most advantageous. In terms of biocompatibility, cellular absorption efficiency, and stability in PBS buffers, cell culture media, and mouse serum [4–6], VLPs surpass conventional organic or inorganic NPs. Targeted peptide-modified VLPs that are primarily localized in tumor tissues can prevent the dissemination of DOX to multiple organs, hence reducing therapy side effects [7]. VLPs can also reduce cytotoxicity from encapsulated toxic cargoes [8].
Figure
Several protein cages of different sizes. Fn, Ferritin cage; AaLS, Aquifex aeolicus; encapsulin protein cage; MS2; CCMV, Cowpea chlorotic mottle virus; BMV, Bromemosaic virus; P22, Bacteriophage.
Today, researchers are paying more attention to the functionalization of protein cage NPs. It can be flexibly designed by protein engineering, and the inner and outer surfaces of protein cage NPs can be altered in a variety of ways, including affinity tags, antibodies, particular amino acids (e.g., cysteine), and targeting peptides to carry out particular functions of various purposes [9]. The main topic of this presentation will be the utilization of protein cage NPs as drug carriers to encapsulate pharmaceuticals. Additionally, it describes how protein cages are used to encapsulate tiny molecules, nucleic acids, peptides, and proteins for imaging, cancer therapy, delivering nucleic acids, and many other purposes. These methods will serve as a foundation for the later creation of novel protein cages.
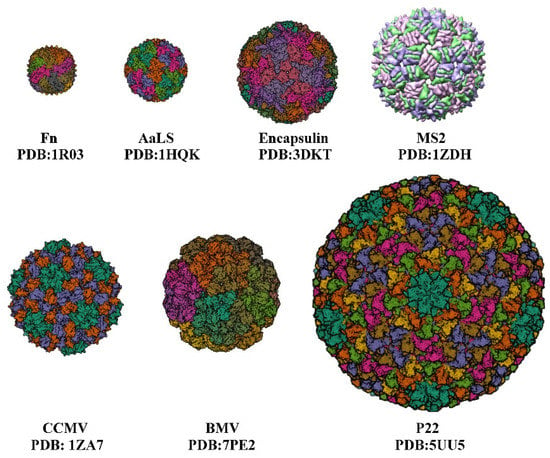
Table 1. Several triangulations and external/inner diameters of protein cage NPs.
|
Protein Cage NPs Type |
T 1 |
External/Inner Diameter |
Number of Identical Subunits |
References |
|
Fn |
- |
12 nm/8 nm |
24 |
[10] |
|
AaLS |
1 |
15.4 nm/9 nm |
60 |
[11] |
|
Encapsulin |
1 |
24 nm/20 nm |
60 |
[12] |
|
P22 |
7 |
PC 59.6 nm/44.6 nm EX/WB 64.8 nm/51.6 nm |
420 |
[13] |
|
CCMV |
3 |
28 nm/18 nm |
180 |
[14,15] |
|
MS2 |
3 |
27 nm/- |
180 |
[16] |
|
BMV |
3 |
28 nm/- |
180 |
[17] |
1 Triangulation number.
2. Strategies for Drugs Encapsulation in Various Protein Cage Nanoparticles
Due to their good biocompatibility, protein cage NPs are commonly used to encapsulate drugs and enzymes. Protein cage NP encapsulation generally uses the following methods:
(1) Diffusion. It is only possible to load a limited amount of small molecule cargoes (<5 Å) or metal ions smaller than the pores of the protein cage NPs. Additionally, some protein cage NPs allow drugs to enter the interior of the protein cage NPs by thermally responding to cargo pores. (2) Electrostatic adsorption. It facilitates the selective adsorption of oppositely charged drugs by charged protein cage NPs. (3) Covalent modification. Enhanced encapsulation efficiency of drugs can be achieved by chemical modification or specific short peptide modification. (4) Disassembly/reassembly. Reversible disassembly or reorganization of protein cage NPs by adjusting pH or salt concentration. (5) Fusion expression. The fusion and expression of encapsulated drugs on protein cages, by combining drugs with scaffold proteins and encapsulating them in cages, can increase the local concentration of the reaction.
2.1. Diffusion
Passive diffusion allows drugs to be loaded into Human H ferritin (HFn) through protein pores. The drugs are packaged into the cage-like structure only after passing through the pores. Although there are fewer drugs loaded into the HFn nanocages, the packaging efficiency is low, and they are limited to some small molecule drugs (with size <5 Å) or some metal ions. Thus, a natural temperature-sensitive thermal-response channel on HFn shell has been discovered, facilitating drug entry into protein cages [6]. A ferritin cage with a 4-fold channel was formed by artificially removing the E-helix formed by the C-terminal channel. To ensure the stability of the protein cage NPs, Nicked-HF partially forms a quadruple channel, and the drug is actively encapsulated into the cage-like structure via this channel [18]. Similarly, the natural encapsulins have pores of 3–4 Å in diameter, which are only suitable for some metal ions to pass through. With the pores modified to 11 Å, Tb
2. Strategies for Drugs Encapsulation in Various Protein Cage Nanoparticles
2.1. Diffusion
Passive diffusion allows drugs to be loaded into Human H ferritin (HFn) through protein pores. The drugs are packaged into the cage-like structure only after passing through the pores. Although there are fewer drugs loaded into the HFn nanocages, the packaging efficiency is low, and they are limited to some small molecule drugs (with size <5 Å) or some metal ions. Thus, a natural temperature-sensitive thermal-response channel on HFn shell has been discovered, facilitating drug entry into protein cages [6]. A ferritin cage with a 4-fold channel was formed by artificially removing the E-helix formed by the C-terminal channel. To ensure the stability of the protein cage NPs, Nicked-HF partially forms a quadruple channel, and the drug is actively encapsulated into the cage-like structure via this channel [18]. Similarly, the natural encapsulins have pores of 3–4 Å in diameter, which are only suitable for some metal ions to pass through. With the pores modified to 11 Å, Tb3+ is able to diffuse into the cage and the transport rate is increased 7-fold, making it easier for encapsulins to transport drugs [19] (Figure 2a). The passive diffusion of drugs has certain limitations, and drugs can be lost during transport.
2.2. Electrostatic Adsorption
The use of electrostatic charge-mediated interactions facilitated the selective encapsulation of negatively/positively charged drugs by positively/negatively charged protein cage NPs. Escherichia coli alkaline phosphatase (PhoA) can be encapsulated by the positively charged protein capsid MS2, and the negatively charged acidic peptide can be fused to PhoA, which can further improve the encapsulation efficiency [20]. Negatively charged siRNA can interact electrostatically with the arginine-binding domain of the CCMV [14]. Archaeoglobus fulgidus ferritin (AfFtn) has negatively charged proteins inside, which can encapsulate positively charged gold nanoparticles [21] (Figure 2b). In addition, positively charged green fluorescent protein GFP (+36) was used to confirm that positively charged particles could be encapsulated into AfFtn [22]. Layer-by-layer, the functional protein cage NPs formed by adding oppositely charged macromolecules to the surface will have greater application opportunities [23].
2.3. Covalent Modification
The two main types of covalent modifications used to encapsulate drugs are covalent modifications using natural and non-natural amino acid residues [24], and protein-mediated covalent modifications [25].
2.3.1. Covalent Modification of Natural and Non-Natural Amino Acid Residues
The side chains of canonical amino acids are reactive, meaning they have the ability to form biocompatible covalent bonds [24]. These amino acids include cysteine, lysine, glutamic acid, aspartic acid, and tyrosine. Cysteine covalent crosslinking relies on the free sulfhydryl group on the cysteine, is chemically loaded onto protein cage NPs by thiol-maleimide Michael-type addition, and can occur at any desired location on the protein surface, e.g., inside or outside the protein cage NPs. There are more established synthetic protocols for maleimide-linked drugs, which are relatively easy to obtain, so cysteine covalent links are commonly used. Aldoxorubicin (AlDox) is a commonly used anticancer prodrug that encapsulates the drug through cysteine residues-maleimide Michael-type addition to 123-site Cys located on the outer surface of encapsulin protein cage NPs, and the activity of AlDox is not reduced by a covalent modification to the protein cage [12].
AaLS isolated from the hyperthermophile Aquifex aeolicus has been genetically modified to contain cysteine at position 108, which has been used to encapsulate drugs such as AlDox and bortezomib [11]. The Gd (III)-DOTA-Mal complexes were also successfully linked to the cysteine residue at position 108 of the AaLS protein cage [26]. Selenocysteine (Sec) is oxidized to selenocysteine dimer (Sec2) in air. Using AaLS mutants with one cysteine, Sec is encapsulated at sequence position 122 of each subunit of AaLS, and Sec2 effectively forms a Sec sulfide linked coupling with AaLS without altering the capsid’s original structure [27]. Using reducing agents such as glutathione or dithiothreitol, the encapsulated Sec can be quantitatively released from AaLS–ICSec [27]. Ferritin-based nanocarriers with 20 nm diameter utilize internally mutated cysteine residues to encapsulate SOD [28]. In addition, Salmonella typhimurium bacteriophage P22 VLPs cysteine mutants (S133C or K118C) were also used to encapsulate AlDox [29].
Lysine is mostly exposed on the surface of protein cage NPs and can be used to link small molecule drugs, and the primary amine (R-NH2) on lysine reacts with N-hydroxysuccinimidyl esters (NHS-esters). The carboxyl groups of aspartic acid and glutamic acid can react with primary amines in the presence of carbodiimide. The phenol group of tyrosine can be coupled and the tyrosine can be interlinked with other tyrosine [30]. This method of mutating specific amino acids on the protein cage NPs also has limitations, potentially leading to changes in the original structure of the protein cage NPs or a reduction in the structural stability of the protein cage NPs.
2.3.2. Protein-Mediated Encapsulation Strategies
Spytag (ST)/Spycatcher (SC) covalent attachment strategy: The protein cage NP’s structure is unaffected by ST fusion expression outside of the protein cage NPs, and SC can target to identify ST and create covalent isopeptide bonds [31]. After modifying SC on the drug, the drug can be encapsulated onto protein cage NPs; protein cage NPs have the same formation process and structure as wild viruses, but they are not infectious and can be used for vaccine production. Seasonal influenza A virus (IAV) often undergoes gene mutations or gene recombination, resulting in the need for frequent updates of IAV and the use of P22 VLPs as a vector for constructing vaccines, which have different globular head structural domains of hemagglutinin (HA) proteins, allowing for rapid updates of IAV. Expression of SP at the C-terminus of the CP of P22 VLPs allows ST to be expressed outside of VLPs and ST/SC to bind covalently, resulting in a P22-HA
2.2. Electrostatic Adsorption
The use of electrostatic charge-mediated interactions facilitated the selective encapsulation of negatively/positively charged drugs by positively/negatively charged protein cage NPs. Escherichia coli alkaline phosphatase (PhoA) can be encapsulated by the positively charged protein capsid MS2, and the negatively charged acidic peptide can be fused to PhoA, which can further improve the encapsulation efficiency [20]. Negatively charged siRNA can interact electrostatically with the arginine-binding domain of the CCMV [14]. Archaeoglobus fulgidus ferritin (AfFtn) has negatively charged proteins inside, which can encapsulate positively charged gold nanoparticles [21].2.3. Covalent Modification
用于封装药物的两种主要共价修饰类型是使用天然和非天然氨基酸残基的共价修饰 [ 24 ],以及蛋白质介导的共价修饰 [ 25 ]。2.3.1. 天然和非天然氨基酸残基的共价修饰
典型氨基酸的侧链具有反应性,这意味着它们能够形成生物相容的共价键 [ 24 ]]. These amino acids include cysteine, lysine, glutamic acid, aspartic acid, and tyrosine. Cysteine covalent crosslinking relies on the free sulfhydryl group on the cysteine, is chemically loaded onto protein cage NPs by thiol-maleimide Michael-type addition, and can occur at any desired location on the protein surface, e.g., inside or outside the protein cage NPs. There are more established synthetic protocols for maleimide-linked drugs, which are relatively easy to obtain, so cysteine covalent links are commonly used. Aldoxorubicin (AlDox) is a commonly used anticancer prodrug that encapsulates the drug through cysteine residues-maleimide Michael-type addition to 123-site Cys located on the outer surface of encapsulin protein cage NPs, and the activity of AlDox is not reduced by a covalent modification to the protein cage [12 ]。 从超嗜热菌 Aquifex aeolicus 中分离出的AaLS经过基因改造,在 108 位含有半胱氨酸,该半胱氨酸已用于封装药物,例如 AlDox 和硼替佐米 [ 11 ]。Gd (III)-DOTA-Mal 复合物也成功连接到 AaLS 蛋白笼第 108 位的半胱氨酸残基 [ 26 ]。硒代半胱氨酸 (Sec) 在空气中被氧化成硒代半胱氨酸二聚体 (Sec2)。使用具有一个半胱氨酸的 AaLS 突变体,Sec 被封装在 AaLS 每个亚基的序列位置 122,Sec2 有效地形成与 AaLS 的 Sec 硫化物连接偶联,而不改变衣壳的原始结构 [ 27]. 使用谷胱甘肽或二硫苏糖醇等还原剂,封装的 Sec 可以从 AaLS–ICSec 中定量释放 [ 27 ]。直径为 20 nm 的基于铁蛋白的纳米载体利用内部突变的半胱氨酸残基来包裹 SOD [ 28 ]。2.3.2. 蛋白质介导的封装策略
Spytag (ST)/Spycatcher (SC) 共价连接策略:蛋白笼 NP 的结构不受蛋白笼 NP 外部 ST 融合表达的影响,SC 可以靶向识别 ST 并创建共价异肽键 [ 31 ]]. After modifying SC on the drug, the drug can be encapsulated onto protein cage NPs; protein cage NPs have the same formation process and structure as wild viruses, but they are not infectious and can be used for vaccine production. Seasonal influenza A virus (IAV) often undergoes gene mutations or gene recombination, resulting in the need for frequent updates of IAV and the use of P22 VLPs as a vector for constructing vaccines, which have different globular head structural domains of hemagglutinin (HA) proteins, allowing for rapid updates of IAV. Expression of SP at the C-terminus of the CP of P22 VLPs allows ST to be expressed outside of VLPs and ST/SC to bind covalently, resulting in a P22-HAhead that activates antigen-specific immune responses in mice (Figure 2c), and exhibiting enhanced immunogenicity and potential for antigen delivery [29,32]. AaLS also uses the irreversible covalent isopeptide bond formed by ST/SC to modify the epidermal growth factor receptor (EGFR) to the outside of the protein cage, and each AaLS nanoparticle successfully attaches to an average of approximately 30 TRAIL and EGFRAfb molecules [25].
A sortase-mediated ligation strategy. Sortases catalyze the formation of peptide bonds between the threonine of proteins containing LPETG amino acid sequences at the C-terminus and the glycine of proteins containing polyglycine sequences at the N-terminus [33]. The fusion expression of a sequence LPETG at the C-terminus of the coat protein of P22 VLPs, modified with polyglycine at the N-terminal end of the drug, similarly allows stable loading of different drugs onto protein cage NPs, modifications on the outer side of the protein cage NPs that facilitate the encapsulation of some large protein or protein structural domains [33] (Figure 2d). The covalent bonds formed after chemical modification require the participation of organic solvents, which to a certain extent can cause some damage to the proteins and is detrimental to the encapsulation of the drug, while the covalent bonds formed by using peptides or proteins can be done in a mild buffer system, which can reduce the loss of activity to the biological material [33].
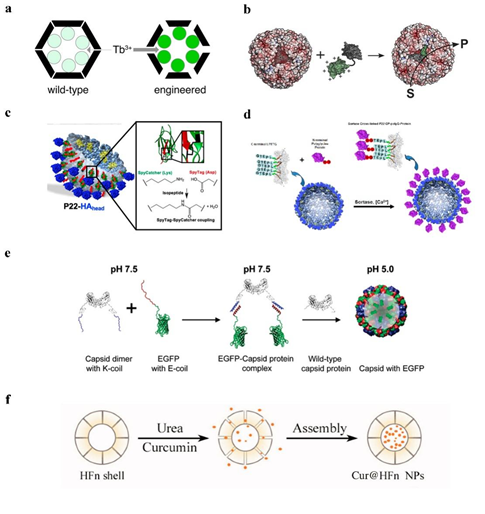
Figure 2. The strategy of encapsulating drugs with protein cage NPs. (a) TB3+ diffuses into encapsulin [19]. Reprinted with permission from Ref. [19] Copyright 2018, American Chemical Society. (b) Positively charged green fluorescent protein GFP (+36) could be encapsulated into AfFtn [21]. Reprinted with permission from Ref. [21] Copyright 2021, Wiley‐VCH Verlag GmbH & Co. KGaA, Weinheim. (c) SC/ ST create a covalent isopeptide bond to encapsulate HAhead [32]. Reprinted with permission from Ref. [32] Copyright 2020, American Chemical Society. (d) To encapsulate HAhead, LPETG amino acid sequences and polyglycine establish a covalent connection [33]. Reprinted with permission from Ref. [33] Copyright 2017, American Chemical Society. (e) EGFP encapsulation into CCMV VLPs under pH control [34]. Reprinted with permission from Ref. [34] Copyright 2009, American Chemical Society. (f) Urea-regulated HFn disassembly/ reassembly [35].
2.4. Disassembly/Reassembly
Certain protein cages have the capacity to regulate the breakdown and reassembly of cage-like structures under particular circumstances. For instance, ferritin may be collected from plants, animals, and microbes and is an intracellular spherical protein that stores iron. To load pharmaceuticals into the ferritin cage’s cavities, the protein can be automatically formed into cage-like structures with cavities, and the breakdown and reassembly of the protein cage can be regulated by the pH value and urea concentration [36]. Additionally, the pH value can control the capsid structure of the cowpea chlorotic mottle virus (CCMV).
1. pH-regulated drug encapsulation strategy for protein cages
The ferritin cage can be dissociated in phosphate buffer with a pH of 2.5, and then adriamycin is added. The protein shell is put back together, and the drug is successfully encapsulated in the protein cage after the pH is raised to 7.4 using NaOH (Figure 2e); however, if the pH of the solution is directly raised from 2.5 to 7.4, drug and protein aggregation will occur, having irreversible effects on proteins. The drug encapsulation of pH-regulated ferritin cages was subsequently enhanced, and it was discovered that adriamycin was more soluble and stable at pH 3–4. Additionally, pH from 2.4 to 4 may be more advantageous for ferritin synthesis than pH from 2.4 to 7.4, and glycine-acetate buffer (pH 2.5) was also discovered to have greater protein recovery.
2.4. 拆卸/重新组装
某些蛋白质笼具有在特定情况下调节笼状结构的分解和重组的能力。例如,铁蛋白可以从植物、动物和微生物中收集,是一种储存铁的细胞内球形蛋白质。为了将药物装载到铁蛋白笼的空腔中,蛋白质可以自动形成带有空腔的笼状结构,蛋白质笼的分解和重组可以通过 pH 值和尿素浓度来调节 [ 36 ]。此外,pH 值可以控制豇豆褪绿斑驳病毒 (CCMV) 的衣壳结构。 蛋白质笼的 pH 调节药物封装策略 铁蛋白笼可在pH为2.5的磷酸盐缓冲液中解离,然后加入阿霉素。将蛋白壳放回原处,用NaOH将pH值升至7.4后,药物成功包封在蛋白笼中;但是,如果将溶液的pH值直接从2.5提高到7.4,药物和蛋白质就会发生聚集,对蛋白质产生不可逆的影响。随后增强了 pH 调节铁蛋白笼的药物包封,发现阿霉素在 pH 3-4 时更易溶解和稳定。 蛋白质笼的尿素调节药物封装策略 铁蛋白笼的 PH 调节药物包封技术得到改进;然而,最近的一项研究表明,酸性 pH 值分解会在铁蛋白笼表面产生无法修复的孔洞,导致更多人选择较温和的尿素来控制铁蛋白笼的分解和重组 [ 38 ]。HFn 在 8 M 尿素中完全解离,然后添加附着在铁蛋白上的药物。梯度去除尿素,然后将组件放回原处,形成一个包裹药物的铁蛋白笼 [ 39 ]。2.5. 融合表达
利用蛋白质纳米笼装载肽类药物,从而以纯生物学方式包封药物,可以成功避免包封过程中使用化学试剂造成的活性损失。将多肽或酶等蛋白质药物装载到蛋白质纳米笼内部,也可以保护药物不发生变性和降解,提高药物的稳定性和有效性。 P22 VLPs are guided by scaffolding protein (SP) to assemble 420 coat protein (CP) into cage-like structures, and there are two commonly used methods for expressing drug fusions on the P22 protein cage [40]. 1. Using an SP fusion technique that does not interfere with the self-assembly of the SP-guided CP, one capable of encasing the target protein within the P22 capsid, and fusing the target gene to the N-terminal end of a shortened SP [41]. It can be used, for instance, to encapsulate certain peptide medications and enzymes. Such encapsulation can enhance the local concentration of the drug protein within a specified range, and the local concentration of the drug protein can reach more than 300 mg/mL while still maintaining the original action of the drug [42]. 2. 使用CP融合技术,每个VLP可以容纳特定数量的药物,这些药物与P22 VLPs(420种药物)的CP有遗传联系。例如,P22 CP 的 N 端融合表达肽药物,但肽药物被包裹在蛋白笼内,组蛋白蛋白酶 b 附着在药物上。组织蛋白酶 B(Cathepsin B,CTB)在肿瘤细胞中高表达,可调节肿瘤部位肽类药物的释放[ 47 ]。 铁蛋白双腔纳米笼 (DCNC) 由双腔短铁蛋白 (sFt) 构建体组成,该构建体在 N 末端融合,应该是多价凝块靶向肽 (CLT),以及一个大蛋白C 末端的微纤溶酶 (μPn)。CLT 靶向凝块中的纤维蛋白-纤连蛋白复合物,μPn 可有效溶解血栓中的凝块,并可将 DCNC 靶向肿瘤部位以改善肿瘤内药物递送[ 48、49 ]。 AaLS 纳米笼也已用于递送肽药物。periostin 肽 (PP) 与 AaLS 亚基的 C 末端融合允许药物在 AaLS 纳米笼表面表达,并且检测到 AaLS-PP 的促血管生成活性高于游离 PP。因此,AaLS 可以成为转运多肽类药物的良好载体 [ 50 ]。3. 蛋白笼纳米粒子的应用
3.1. 抗肿瘤
Due to the improvement of the procedure for disassembling and reconstituting pH-regulated ferritin cages, the ferritin cages were broken down in glycine-acetate buffer (pH 2.5) and then adriamycin was added to encourage the creation of ferritin cages at a pH of 4. After aspirating the free medication, the buffer was changed to 7.4 before continuing. One ferritin cage can contain up to 28 adriamycin molecules thanks to this mechanism’s ability to restore ferritin cage complexes and increase the drug-loading capacity of ferritin cages [5,35].
Drugs can be encapsulated in the interior of the capsid of the CCMV. The capsid was disassembled into 90 capsid protein dimers at px 用 H 7.5, and when the pH was reduced to 5.0, the capsid structure reorganized. This reversible disassembly and reorganization were employed to encapsulate a variety of medicines [37].
2. Urea-regulated drug encapsulation strategy for protein cages
PH-regulated drug encapsulation techniques for ferritin cages have been improved; however, a recent study has demonstrated that acidic pH breakdown produces irreparable holes in the surface of ferritin cages, leading more individuals to opt for the milder urea to manage ferritin cage disassembly and reconstitution [38] (Figure 2f). Complete dissociation of HFn in 8 M urea was followed by the addition of the drug, which was attached to ferritin. The gradient removed the urea, and the assembly was then put back together to create a ferritin cage encapsulating the medication [39].
2.5. Fusion Expression
The use of protein nanocages to load peptide drugs, thereby encapsulating drugs in a purely biological manner, can successfully avoid the loss of activity caused by chemical reagents used in the encapsulation process. Loading protein drugs, such as peptides or enzymes, into the interior of protein nanocages can also protect the drugs from denaturation and degradation, improving the drug’s stability and effectiveness.
2.5.1. P22 VLPs
P22 VLPs are guided by scaffolding protein (SP) to assemble 420 coat protein (CP) into cage封装,这最大限度地减少了健康器官中的药物泄漏,并表明 HFn-like structures, and there are two commonly used methods for expressing drug fusions on the P22 protein cage [40].
1. Using an SP fusion technique that does not interfere with the self-assembly of the SP-guided CP, one capable of encasing the target protein within the P22 capsid, and fusing the target gene to the N-terminal end of a shortened SP [41]. It can be used, for instance, to encapsulate certain peptide medications and enzymes. Such encapsulation can enhance the local concentration of the drug protein within a specified range, and the local concentration of the drug protein can reach more than 300 mg/mL while still maintaining the original action of the drug [42]. There are several common strategies for drug fusion expression on SP of P22 VLPs:
(1) Fusion at the N-terminal end of one SP to express a target gene, enabling the medication to be contained inside the P22 VLPs [43] (Figure 3a). (2) Simultaneous production of two or more target proteins at one SP’s N-terminus. Directly existing target proteins can also occur in a cascade reaction [44] (Figure 3b). (3) Two separate target genes are expressed through fusion at the N-terminal ends of two different SPs, causing them to co-assemble in a protein cage [45] (Figure 3c). It has recently been reported in the literature that an in vitro assembly method for cargo encapsulation in P22 VLPs has been designed which can control the amount of cargo encapsulation. By adjusting the ratio of encapsulated cargo to wild-type SP, this method can quantitatively encapsulate drugs into protein cages, and wild-type SP and misfolded proteins can escape the cages [46].
2. Using a CP fusion technique, each VLP can hold a specific quantity of medicines that are genetically linked to the CP of P22 VLPs (420 drugs). For instance, the N-terminal fusion of P22 CP expresses peptide drugs, but the peptide drugs are encapsulated inside the protein cage, and the histone proteinase b is attached to the drugs. Cathepsin B (CTB) is highly expressed in tumor cells, and it can regulate the release of peptide drugs in the tumor site [47].
2.5.2. Ferritin Double-Chambered Nanocage (DCNC)
The ferritin double-chambered nanocage (x 在小鼠肿瘤中的 DCNC) is made up of a double chambered short-length ferritin (sFt) construct that has a fusion at the N-terminal end that should be multivalent clot-targeting peptides (CLT), and a big protein microplasmin (μPn) at the C-terminal end. CLT target fibrin–fibronectin complexes in clots, and μPn effectively dissolves clots in thrombi and can target DCNC to tumor sites to improve intra-tumor drug delivery [48,49].
2.5.3. AaLS
Recently, AaLS nanocages have also been used to deliver peptide drugs. The fusion of periostin peptide (PP) to the C-terminus of the AaLS subunit allows drug ex 浓度高于游离 Doxpression on the surface of AaLS nanocages, and the pro-angiogenic activity of AaLS-PP was detected to be higher than that of free PP. Therefore, AaLS can be a good carrier for transporting peptide drugs [50].
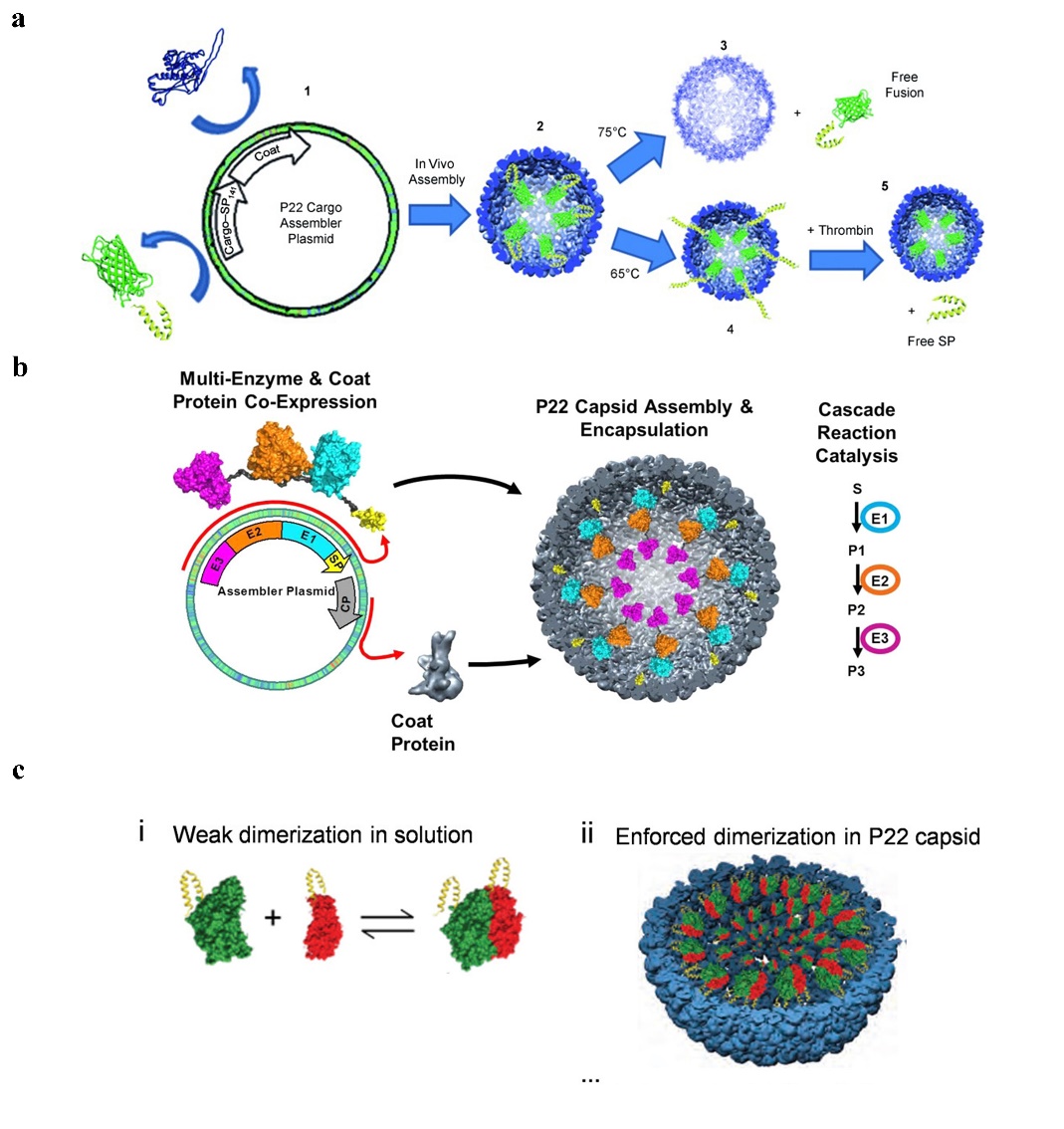
Figure 3. The给药 [ strategy of drug39 gusion expression on SP of P22 VLPs. (a) P22 self-assembly with coat protein and cargo-scaffold-protein fusion. Reprinted with permission from Ref. [51]. Copyright 2011, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. (b) Continuous expression of P22 VLPs with three enzymes under the control of the same promoter. Reprinted with permission from Ref. [44]. Copyright。这表明这种蛋白笼 2014, American Chemical Society. (c) Two plasmids separately containing the hydrogenase cargo (hyaA-SP and hyaB-SP) and coat protein (CP) under the control of different promoters, to produce P22 capsids packed with both subunits of EcHyd-1. Reprinted with permission from Ref. [42]. Copyright 2022, American Chemical Society.
3. Application of Protein Cage Nanoparticles
An ideal nanomedicine carrier should be able to target specific cells, provide large concentrations of therapeutic drugs, have remarkable physical and chemical properties, be biocompatible, and be easy to disintegrate in vivo [36,52].
This makes protein cage NPs the药物递送方法具有良好的安全性。乳腺癌的主要治疗方法一直是细胞毒性化疗,使用蛋白笼 ideal delivery mechanism for peptides, proteins, nucleic acids, and small molecules. In this article, we discuss the use of protein cage NPs for targeting to various tumor areas for tumor therapy, siRNA administration, peptide and protein drug delivery, and contrast techniques. We have investigated numerous encapsulation methodologies for protein cage NPs vectors.
3.1. Anti-Tumor
Targeted peptides are frequently utilized in the design of antitumor medications because highly efficient antitumor drugs should be directed specifically to tumor cells and delivered into tumor tissue to destroy tumor cells and avoid damaging normal cells. Protein cage NPs are protein-based nanocages, and by utilizing genetic engineering, it is quite simple to add targeting peptides to the surface of the protein cages. The RGD4C peptide was inserted into the AaLS sequence between positions 70 and 71, or the SP94 peptide was inserted into the C-terminus at the outer surface of AaLS. The RGD4C peptide specifically binds to the integrin avb3 receptor, which is overexpressed on the surface of endothelial cells in tumor vessels, and the SP94 peptide binds to the surface receptor on hepatocellular carcinoma cells. Fluorescent proteins can be linked to RGD4C-AaLS and SP94-AaLS to create diagnostic cancer probes. Bortezomib (BTZ) was linked to SP94-AaLS to effectively inhibit proteasome activity in hepatocellular carcinoma cells. AlDox, which can be released when pH is 5.5, was linked to RGD4C-AaLS to have a high cytotoxic effectNP 载体递送可以成功地防止细胞毒性药物对正常组织的损伤 on cancer cells that express high levels of the integrin [11]. The SP94 peptide is inserted into the encapsulin protein cage nanoparticle from Thermotoga maritima, which can also be targeted to liver cancer cells for cancer diagnosis or treatment [1229 ]]. Dox and curcumin can be released from the protein cage NPs in an acid-responsive manner with a pH range乳腺癌细胞通常过度表达生长因子 of 5.0–6.0. Targeting peptides and anticancer medications have recently been coupled to AaLS protein cages by the ST/SC creation of heteropeptide covalent connections, according to a research team [25]. The TNF-related apoptosis-inducing ligand (TRAIL) and control of EGFR downstream signaling, together with the overexpression of the epidermal EGFR in some tumor cells, have substantial effects on inducing apoptosis in tumor cells. GRP78 is an SP94 peptide receptor that was created on the surface of Pyrococcus furiosus ferritin Fn by genetic engineering. To create a nano-drug delivery system that targets hepatocellular carcinoma cells, the doxorubicin (Dox) was encapsulated into the cage using urea-controlled disassembly and reassembly of hccFn [38].
Dox was encapsulated with EGFR 和 HFn, which minimized drug leakage in healthy organs and showed that HFn-Dox had a higher Dox concentration in mice tumors than free Dox administration [39]. This indicates that this protein cage NPs drug delivery method has a good safety profile. The primary treatment for breast cancer has been cytotoxic chemotherapy, and the use of protein cage NP vector delivery can successfully prevent cytotoxic drug damage to normal tissues [29]. Breast cancer cells often overexpress the surface receptors for the growth factors EGFR and HER2. Breast cancer cells are attracted to drug-carrying R2 的表面受体。乳腺癌细胞被携带药物的 P22 VLPs, which can then be used to diagnose the disease by fusing fluorescent tags to 吸引,然后可以通过将荧光标签融合到 EGFRAfb and和 HER2Afb and covalently binding them to the SP suggested by P并将它们共价结合到 P22 VLPs. The cytotoxic protein small Singlet Oxygen Generator, an encapsulated protein cage that successfully targets breast cancer cells and causes their death, was loaded onto the surface of T. maritima encapsulin and fused to the genetically 建议的 SP 来诊断疾病。细胞毒性蛋白小单线态氧发生器,一种成功靶向乳腺癌细胞并导致其死亡的封装蛋白笼,被加载到T. maritima封装蛋白的表面,并与转基因抗体蛋白 modified antibody protein DARPin9.29. Additionally, this team discovered that an 融合。此外,该团队还发现,一种 18.4 kDa protein can的蛋白质可以直接融合到包囊蛋白的表面,而不会改变其稳定性或结构 [ be fused directly to the surface of encapsulin without altering its stability or structure [5353 ]]. This这一发现使得在封装蛋白表面表达一些大蛋白变得更加容易,并开辟了更多的应用可能性。通过共价键将 discovery makes it easier to express some big proteins on the surface of encapsulin and opens up more application possibilities. Loading TNF-related apoptosis-inducing ligand TNF 相关凋亡诱导配体 (TRAIL) onto AaLS by covalent bonding and binding them to proapoptotic death receptors overexpressed in cancer cells is an effective protein therapy strategy for cancer [25].
The加载到 AaLS 上,并将它们与癌细胞中过表达的促凋亡死亡受体结合,是一种有效的癌症蛋白质治疗策略 [ targeting25 effectiveness of protein cage NPs at tumor locations has recently received increased focus, and P22 VLPs modified with polyethylene glycol were chosen to investigate the targeting effectiveness in colorectal cancer models. In order to increase the duration of the drug’s circulation in vivo, polyethylene glycol on the surface of protein cage NPs is modified to keep it from being detected in blood vessels. The 7-day tumor model displayed the highest accumulation of P22 VLPs after 24 h of treatment compared to the 15-day and 21-day models in this investigation, and it was discovered that P22 VLPs accumulated mostly in tumor-associated macrophages, suggesting that P22 VLPs could be developed for the detection of early tumorigenic stages [54].
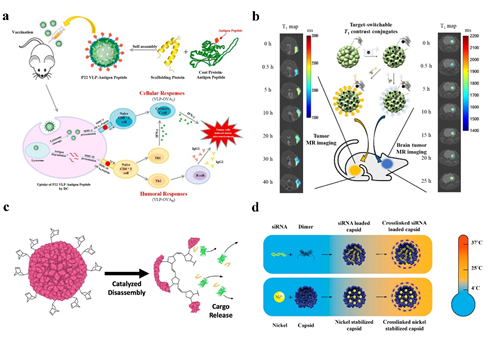
Cancer immunotherapy has also been practiced with P22 VLPs. The production of ovalbumin (OVA), OVAB, and OVAT epitopes fused to the C-terminus of P22 VLPs capsid protein efficiently stimulated the cytotoxic lymphocyte (CTL) immune response in mice [55] (Figure 4a). The results of this work will serve as the basis for the creation of a preventive tumor vaccine.
3.2. siRNA Delivery
Negatively charged siRNA can be contained within CCMV due to the arginine structural domain’s facilitation of electrostatic interactions with negatively charged biomolecules. A protein cage NPs stable at 37 °C can be built for in vitro siRNA delivery using DTSSP crosslinker to couple lysine on the particle surface [14] (Figure 4d). Forkhead box transcription factor (FOXA1) was used as a therapeutic target in the application of CCMV delivery of siRNA, and cell penetrating peptides (CPPs), L17E, were covalently coupled to the surface of CCMV loaded with siRNA, effectively silencing FOXA1, the target gene of mcf-7 in breast cancer cells [56].
It was discovered that both CCMV and BMV VLPs could internalize into mcf-7 cells without targeting ligands and that CCMV could elicit an immunogenic response in raw264.7-blue macrophage cells, although BMV scarcely elicited one. Similar to CCMV, the plant virus bromemosaic virus (BMV) may effectively load siRNA, and the positively charged N-terminal portion of the BMV coat protein can be effectively loaded with siRNA [57]. It was possible to successfully release siRNA into tumor cells to silence genes by employing VLP-siGFP nanovectors, which had a mass ratio of 1:6 (siGFP/CP). As a result, BMV might be a better vector for siRNA delivery [56].
3.3. Peptide and Protein Delivery
Cancer, cardiovascular, and metabolic illnesses can all be effectively treated with peptide medications. The solubility, stability, and membrane permeability of peptide drugs, however, make them less desirable as carriers for the delivery of peptide drugs. Among these carriers, protein nanocages are excellent for the delivery of peptide drugs because they can successfully target and release peptide drugs to the focal site precisely. By way of illustration, the peptide drug for P22 VLPs were fused to the N-terminus of P22 CP and two different peptides, NuBCP-9 peptide and KLAK peptide, were linked by CTB cleavable linkers. The two peptides acted by different mechanisms and may have synergistic effects to kill tumor cells. Between the peptide and the CP as well as between the two peptides, CTB cleavable linkers are placed (Figure 4c). When this nano-drug delivery system is directed to the tumor site, the peptide medication is precisely released at the tumor site for tumor therapy in the presence of CTB, which is abundantly expressed in the tumor environment [47]. Enzymes like green fluorescent protein have been effectively put inside the protein shell by CCMV [34], P22 VLPs [51], and other protein cage NPs. The enzyme substrate can diffuse freely into the interior of the VLP through a 2 nm pore in the VLP coat. P22 VLPs have successfully encapsulated dehydrogenase D [58] within the protein cage and have been reported to be able to encapsulate fusion proteins up to 180 kDa in size without affecting the structure of the P22 VLPs [59].
3.4. Imaging
One of the most used techniques for clinical diagnosis is magnetic resonance imaging (MRI). Contrast agents (CA) are utilized to increase the resolution of MR images when it is insufficient, and both T1-weighted and T2-weighted (CAs) are frequently used [60].
The most widely used T1 CA are paramagnetic gadolinium (Gd(III)), but investigations have indicated that Gd is potentially cytotoxic and that using Gd-based contrast agents may result in systemic nephrotoxicity, necessitating complexation with chelating agents [26] (Figure 4b). Due to the protein cage NP’s distinctive spherical hollow form, biodegradability, simplicity of mutagenesis, and chemical coupling changes, it is possible to fix the contrast agent on the protein cage NP and reduce the release rate of the contrast agent. Recombinant human heavy chain ferritin with Gd tagging was designed to target T1 contrast agents (HFn-Gd) [4]. the cavity of the P22 “wiffleball” encapsulates the DTPA-Gd complex with a greater resolution than free DTPA-Gd, and with 1900 Gd。
3.2. siRNA递送
由于精氨酸结构域促进与带负电荷的生物分子的静电相互作用,因此带负电荷的 siRNA 可以包含在 CCMV 中。可以构建在 37 °C 下稳定的蛋白笼 NP,用于使用 DTSSP 交联剂在颗粒表面偶联赖氨酸 [ 14 ] 进行体外 siRNA 递送。Forkhead box transcription factor (FOXA1) 被用作 CCMV siRNA 递送的治疗靶点,细胞穿透肽 (CPPs) L17E 共价偶联到载有 siRNA 的 CCMV 表面,有效沉默 FOXA1,靶点乳腺癌细胞中的 mcf-7 基因 [ 56 ]。3.3. 肽和蛋白质递送
癌症、心血管和代谢疾病都可以用肽药物有效治疗。然而,肽药物的溶解度、稳定性和膜渗透性使得它们不太适合作为递送肽药物的载体。在这些载体中,蛋白质纳米笼非常适合肽类药物的递送,因为它们可以成功地将肽类药物精确靶向并释放到焦点位点。举例来说,P22 VLP 的肽药物融合到 P22 CP 的 N 末端,两种不同的肽,NuBCP-9 肽和 KLAK 肽,通过 CTB 可切割接头连接。这两种肽通过不同的机制发挥作用,可能具有协同杀伤肿瘤细胞的作用。在肽和 CP 之间以及两个肽之间,放置了 CTB 可切割接头。3.4. 成像
临床诊断中最常用的技术之一是磁共振成像 (MRI)。造影剂 (CA) 用于在分辨率不足时增加 MR 图像的分辨率,并且经常使用 T1 加权和 T2 加权 (CA) [ 60 ]。 最广泛使用的 T1 CA 是顺磁性钆 (Gd(III)),但研究表明 Gd 具有潜在的细胞毒性,使用基于 Gd 的造影剂可能导致全身性肾毒性,需要与螯合剂络合 [ 26 ]。由于蛋白笼NP独特的球形空心形态、可生物降解、诱变简单、化学偶联变化等特点,可以将造影剂固定在蛋白笼NP上,降低造影剂的释放速率。带有 Gd 标记的重组人重链铁蛋白旨在靶向 T1 造影剂 (HFn-Gd) [ 4 ]。P22“wiffleball”的空腔封装了 DTPA-Gd 复合物,其分辨率高于游离 DTPA-Gd,并且具有 1900 Gd3+ included in each P22 VLP [61]. Gadolinium-tetraazacyclododecane tetraacetic acid (GdDOTA) is attached to CCMV by reaction with lysine residues on the surface of the viral capsid [62]. The Gd(III)-DOTA complex was chemically attached to the surface of AaLS to create a targeted T1 contrast agent after a tumor-targeting peptide was changed on its surface using ST/SC to generate a covalent heteropeptide connection. The utilization of flavoprotein (AfFtnAA) nanocages with a slow iron release rate, high iron loading capacity, and thermal stability encapsulates magnetic nanostructures (MNS) based on superparamagnetic iron oxide and metal ferrite NPs to produce increased contrast in MR imaging [63].
4. Conclusions and Discussion
The study of nanomaterial drug delivery systems has attracted more attention recently, which has aided in the creation of protein cage nanomaterials. Protein cage nanomaterials offer new opportunities for developing sophisticated protein nanomaterial drug delivery systems because of their favorable biocompatibility, lack of toxicity, and ideal internal cavity shape, even on the surface of the protein cage NPs, for drug delivery. Numerous studies have also demonstrated that protein cage NPs can effectively protect protease activity and that, in contrast to free enzymes, enzymes encapsulated in protein cage NPs can maintain enzyme activity at high concentrations, with drug protein concentrations occasionally reaching 300 mg/mL [42,65]. This work comprehensively describes the primary ways for encapsulating pharmaceuticals in low-cost and easily accessible protein cage NPs, how they are released, and their prospective applications for the biomedical field (Table 2), such as applications in the delivery of antitumor drugs and vaccines, siRNA, peptides and protein-based drugs, and applications in Biologic imaging techniques.
This work enumerates the protein cage NPs produced by eukaryotic or prokaryotic expression systems. The majority of these NPs are produced in large quantities by E. coli expression purification, which is easy to use and inexpensive to run. As a result, many drug carriers with high purity can be produced, and even protein cage NPs for encapsulating drugs can be produced directly, minimizing the loss of carriers and drugs during the encapsulation process. Protein cage NPs are convenient for targeting modification. Targeting drug-loaded protein cage NPs to a specific location before releasing the drug can effectively improve drug utilization and even prevent the release of some toxic drugs during delivery, reducing the harm the drug causes to healthy tissues and cells. For a variety of drug delivery applications, including targeted tumor cell treatment, contrast, siRNA, and peptides, protein cage NPs hold great promise. Although VLPs have shown great efficacy, there are still limitations to their clinical use as drug delivery tools. HFn nanocages are naturally present in humans and do not elicit inflammatory or immune responses [36]; however, most Protein cage NPs, like other NPs, are cleared by phagocytic and dendritic cell-mediated clearance. The CD40L/CD47 Self-peptide can bind to SIRP-α and inhibit macrophage phagocytosis [66,67], the tobacco mosaic virus’s immunogenicity has been decreased using serum albumin (SA) [56], and PEGylation can shield the surface of Protein cage NPs, allowing NPs to go undetected in the vasculature, prolonging body circulation, and inhibiting macrophage phagocytosis, but it is also a major challenge in application [54,68,69].
Protein cage NPs are used to encapsulate peptides and protein-based drugs, some enzymes involved in biosynthesis, and multi-enzyme cascade reactions for cancer therapy. Protein cage NPs can be modified both internally and externally to mimic the cellular environment, particularly large inner diameter protein cage NPs such as P22 VLPs, developed as biosynthetically active nanoreactors. With the help of further in-depth research on the safety, stability, toxicity, and pharmacokinetics of novel protein cage NPs, we hope that they will be successfully tested in preclinical and clinical settings. We also hope that this review will give researchers new ideas and insights for developing novel protein cage NPs for drug delivery.
Table 2. Protein cage NPs for drug encapsulation and delivery.
|
Protein Cage Nps Type |
Cargo |
Encapsulation Method |
Surface Modification |
Release Method |
Application |
References |
|
P22 |
Alexa Fluor 647 |
Covalent modification |
polyethylene glycol 1000 (PEG1K) |
- |
Anti-tumor (targeting efficiency) |
[54] |
|
CD47 peptide/CD40L |
Covalent modification |
decoration protein |
- |
None |
[66] |
|
|
AlDo |
Covalent modification |
S133C or K118C EGFRAfb and HER2Afb targeting peptides |
pH = 5.0 |
Anti-tumor (human breast cancer cells) |
[29] |
|
|
HAhead |
Covalent modification |
None |
Antigen-antibody reaction |
IAV vaccine |
[32] |
|
|
NuBCP-9 peptide and KLAK peptide |
Fusion expression |
None |
Cathepsin B cleavage |
Peptide and protein delivery Anti-tumor |
[47] |
|
|
GCL/GshF |
Fusion expression |
None |
Enzyme activity |
GSH-deficient |
[70] |
|
|
Cytochrome P450 enzymes/Protoporphyrin IX |
Fusion expression |
PEG (EST) |
Enzyme prodrug therapy |
Anti-tumor (breast tumor cells) |
[71] |
|
|
DTPA−Gd |
Covalent modification |
K118C |
As contrast agents |
Imaging |
[61] |
|
|
Fn |
Dox/Camptothecin |
Diffusion |
hydrophobic peptides at the C-terminus of HFn |
pH = 5.0 |
Anti-tumor (human glioblastoma cells) |
[6] |
|
Dox |
Diffusion |
engineering 4-fold channel-nicked HFn |
Acid-responsive |
Anti-tumor (cervical cancer cells and breast tumor cells) |
[18] |
|
|
Fn |
GFP(+36) |
Electrostatic adsorption |
AfFtn WT/K150A, R151A |
- |
None |
[21] |
|
SOD |
Covalent modification |
tF1iC (E131C) |
Enzyme activity |
Anti-inflammatory effect |
[28] |
|
|
Dox |
Disassembly/reassembly(pH) |
None |
- |
None |
[5] |
|
|
Dox |
Disassembly/reassembly(Urea) |
SP94 targeting peptides |
pH = 5.0 |
Anti-tumor (hepatocellular carcinoma) |
[38] |
|
|
Dox |
Disassembly/reassembly(Urea) |
None |
pH = 5.0 |
Anti-tumor (human colon cancer cells) |
[39] |
|
|
Curcumin |
Disassembly/reassembly(Urea) |
None |
Acid-responsive |
Anti-tumor (breast cancer cells) |
[35] |
|
|
multivalent microplasmin |
Fusion expression |
Fibrin clot-targeting peptides |
Enzyme activity |
Anti-tumor |
[49] |
|
|
Gd-DTPA |
Covalent modification |
None |
As contrast agents |
Imaging (breast cancer and pancreatic cancer) |
[4] |
|
|
Fe |
Covalent modification |
AfFtn K150A/R151A |
As contrast agents |
Imaging (macrophage cell) |
[63] |
|
|
AaLS |
Gd(III)-DOTA |
Covalent modification |
HER2 or EGFR targeting peptides |
As contrast agents |
Imaging (tumor cells) |
[26] |
|
Sec |
Covalent modification |
C37A/E122C |
Glutathione or dithiothreitol. |
Anti-tumor (human cancer cell lines) |
[27] |
|
|
AlDox |
Covalent modification |
R108C RGD4C and SP94 targeting peptides |
pH = 5.5 |
Anti-tumor (hepatocellular carcinoma) |
[11] |
|
|
TRAIL |
Covalent modification |
R108C EGFRAfb targeting peptides |
Ligand-receptor interaction |
Anti-tumor (human epidermoid cancer cell) |
[25] |
|
|
Periostin peptide |
Fusion expression |
None |
Ligand-receptor interaction |
Pro-angiogenic activity (Peripheral artery disease) |
[50] |
|
|
CCMV |
siRNA |
Electrostatic adsorption |
DTSSP cross-linker |
Transfection |
siRNA Delivery |
[14] |
|
siRNA |
Disassembly/reassembly(pH/salt) |
M-lycotoxin peptide L17E |
Transfection |
siRNA Delivery |
[56] |
|
|
Encapsulin |
GCaMP |
Fusion expres-sion |
Amino acid changes and deletions in pore-forming loop region |
- |
None |
[19] |
|
AlDox |
Covalent modification |
123C SP94 targeting peptides |
pH = 5.5 |
Anti-tumor Imaging (hepatocellular carcinoma) |
[12] |
References
- Xie, S.; Ai, L.; Cui, C.; Fu, T.; Cheng, X.; Qu, F.; Tan, W. Functional Aptamer-Embedded Nanomaterials for Diagnostics and Therapeutics. ACS Appl. Mater. Interfaces 2021, 13, 9542–9560.
- Bhaskar, S.; Lim, S. Engineering protein nanocages as carriers for biomedical applications. NPG Asia Mater. 2017, 9, e371.
- Kant, R.; Rayaprolu, V.; McDonald, K.; Bothner, B. Curating viscoelastic properties of icosahedral viruses, virus-based nanomaterials, and protein cages. J. Biol. Phys. 2018, 44, 211–224.
- Cai, Y.; Wang, Y.; Zhang, T.; Pan, Y. Gadolinium-Labeled Ferritin Nanoparticles as T1 Contrast Agents for Magnetic Resonance Imaging of Tumors. ACS Appl. Nano Mater. 2020, 3, 8771–8783.
- Kilic, M.A.; Ozlu, E.; Calis, S. A novel protein-based anticancer drug encapsulating nanosphere: apoferritin-doxorubicin complex. J. Biomed. Nanotechnol. 2012, 8, 508–514.
- Wang, Z.; Zhao, Y.; Zhang, S.; Chen, X.; Sun, G.; Zhang, B.; Jiang, B.; Yang, Y.; Yan, X.; Fan, K. Re-engineering the inner surface of ferritin nanocage enables dual drug payloads for synergistic tumor therapy. Theranostics 2022, 12, 1800–1815.
- Comas-Garcia, M.; Colunga-Saucedo, M.; Rosales-Mendoza, S. The Role of Virus-Like Particles in Medical Biotechnology. Mol. Pharm. 2020, 17, 4407–4420.
- Tagit, O.; de Ruiter, M.V.; Brasch, M.; Ma, Y.; Cornelissen, J.J.L.M. Quantum dot encapsulation in virus-like particles with tuneable structural properties and low toxicity. RSC Adv. 2017, 7, 38110–38118.
- Choi, B.; Kim, H.; Choi, H.; Kang, S. Protein Cage Nanoparticles as Delivery Nanoplatforms. Adv. Exp. Med. Biol. 2018, 1064, 27–43.
- Chen, H.; Tan, X.; Fu, Y.; Dai, H.; Wang, H.; Zhao, G.; Zhang, Y. The development of natural and designed protein nanocages for encapsulation and delivery of active compounds. Food Hydrocoll. 2021, 121, 107004.
- Min, J.; Kim, S.; Lee, J.; Kang, S. Lumazine synthase protein cage nanoparticles as modular delivery platforms for targeted drug delivery. RSC Adv. 2014, 4, 48596–48600.
- Moon, H.; Lee, J.; Min, J.; Kang, S. Developing genetically engineered encapsulin protein cage nanoparticles as a targeted delivery nanoplatform. Biomacromolecules 2014, 15, 3794–3801.
- Llauro, A.; Luque, D.; Edwards, E.; Trus, B.L.; Avera, J.; Reguera, D.; Douglas, T.; Pablo, P.J.; Caston, J.R. Cargo-shell and cargo-cargo couplings govern the mechanics of artificially loaded virus-derived cages. Nanoscale 2016, 8, 9328–9336.
- Pretto, C.; van Hest, J.C.M. Versatile Reversible Cross-Linking Strategy to Stabilize CCMV Virus Like Particles for Efficient siRNA Delivery. Bioconjug. Chem. 2019, 30, 3069–3077.
- Beren, C.; Cui, Y.; Chakravarty, A.; Yang, X.; Rao, A.L.N.; Knobler, C.M.; Zhou, Z.H.; Gelbart, W.M. Genome organization and interaction with capsid protein in a multipartite RNA virus. Proc. Natl. Acad. Sci. USA 2020, 117, 10673–10680.
- Hashemi, K.; Ghahramani Seno, M.M.; Ahmadian, M.R.; Malaekeh-Nikouei, B.; Bassami, M.R.; Dehghani, H.; Afkhami-Goli, A. Optimizing the synthesis and purification of MS2 virus like particles. Sci. Rep. 2021, 11, 19851.
- Ruszkowski, M.; Strugala, A.; Indyka, P.; Tresset, G.; Figlerowicz, M.; Urbanowicz, A. Cryo-EM reconstructions of BMV-derived virus-like particles reveal assembly defects in the icosahedral lattice structure. Nanoscale 2022, 14, 3224–3233.
- Ahn, B.; Lee, S.G.; Yoon, H.R.; Lee, J.M.; Oh, H.J.; Kim, H.M.; Jung, Y. Four-fold Channel-Nicked Human Ferritin Nanocages for Active Drug Loading and pH-Responsive Drug Release. Angew. Chem. Int. Ed. Engl. 2018, 57, 2909–2913.
- Williams, E.M.; Jung, S.M.; Coffman, J.L.; Lutz, S. Pore Engineering for Enhanced Mass Transport in Encapsulin Nanocompartments. ACS Synth. Biol. 2018, 7, 2514–2517.
- Wilkerson, J.W.; Yang, S.O.; Funk, P.J.; Stanley, S.K.; Bundy, B.C. Nanoreactors: Strategies to encapsulate enzyme biocatalysts in virus-like particles. New Biotechnol. 2018, 44, 59-63.
- Tetter, S.; Hilvert, D. Enzyme Encapsulation by a Ferritin Cage. Angew. Chem. Int. Ed. Engl. 2017, 56, 14933–14936.
- Pulsipher, K.W.; Bulos, J.A.; Villegas, J.A.; Saven, J.G.; Dmochowski, I.J. A protein-protein host-guest complex: Thermostable ferritin encapsulating positively supercharged green fluorescent protein. Protein Sci. 2018, 27, 1755–1766.
- Kraj, P.; Selivanovitch, E.; Lee, B.; Douglas, T. Polymer Coatings on Virus-like Particle Nanoreactors at Low Ionic Strength-Charge Reversal and Substrate Access. Biomacromolecules 2021, 22, 2107–2118.
- Smith, M.T.; Hawes, A.K.; Bundy, B.C. Reengineering viruses and virus-like particles through chemical functionalization strategies. Curr. Opin. Biotechnol. 2013, 24, 620–626.
- Jun, H.; Jang, E.; Kim, H.; Yeo, M.; Park, S.G.; Lee, J.; Shin, K.J.; Chae, Y.C.; Kang, S.; Kim, E. TRAIL & EGFR affibody dual-display on a protein nanoparticle synergistically suppresses tumor growth. J. Control Release 2022, 349, 367–378.
- Kim, H.; Jin, S.; Choi, H.; Kang, M.; Park, S.G.; Jun, H.; Cho, H.; Kang, S. Target-switchable Gd(III)-DOTA/protein cage nanoparticle conjugates with multiple targeting affibody molecules as target selective T1 contrast agents for high-field MRI. J. Control Release 2021, 335, 269–280.
- Wang, S.; Al-Soodani, A.T.; Thomas, G.C.; Buck-Koehntop, B.A.; Woycechowsky, K.J. A Protein-Capsid-Based System for Cell Delivery of Selenocysteine. Bioconjug. Chem. 2018, 29, 2332–2342.
- Shuvaev, V.V.; Khoshnejad, M.; Pulsipher, K.W.; Kiseleva, R.Y.; Arguiri, E.; Cheung-Lau, J.C.; LeFort, K.M.; Christofidou-Solomidou, M.; Stan, R.V.; Dmochowski, I.J.; et al. Spatially controlled assembly of affinity ligand and enzyme cargo enables targeting ferritin nanocarriers to caveolae. Biomaterials 2018, 185, 348–359.
- Kim, H.; Choi, H.; Bae, Y.; Kang, S. Development of target-tunable P22 VLP-based delivery nanoplatforms using bacterial superglue. Biotechnol. Bioeng. 2019, 116, 2843–2851.
- Horowitz, E.D.; Finn, M.G.; Asokan, A. Tyrosine cross-linking reveals interfacial dynamics in adeno-associated viral capsids during infection. ACS Chem. Biol. 2012, 7, 1059–1066.
- Si, M.; Xu, Q.; Jiang, L.; Huang, H. SpyTag/SpyCatcher Cyclization Enhances the Thermostability of Firefly Luciferase. PLoS ONE 2016, 11, e0162318.
- Sharma, J.; Shepardson, K.; Johns, L.L.; Wellham, J.; Avera, J.; Schwarz, B.; Rynda-Apple, A.; Douglas, T. A Self-Adjuvanted, Modular, Antigenic VLP for Rapid Response to Influenza Virus Variability. ACS Appl. Mater. Interfaces 2020, 12, 18211–18224.
- Patterson, D.; Schwarz, B.; Avera, J.; Western, B.; Hicks, M.; Krugler, P.; Terra, M.; Uchida, M.; McCoy, K.; Douglas, T. Sortase-Mediated Ligation as a Modular Approach for the Covalent Attachment of Proteins to the Exterior of the Bacteriophage P22 Virus-like Particle. Bioconjug. Chem. 2017, 28, 2114–2124.
- Minten, I.J.; Hendriks, L.J.; Nolte, R.J.; Cornelissen, J.J. Controlled encapsulation of multiple proteins in virus capsids. J. Am. Chem. Soc. 2009, 131, 17771–17773.
- Ji, P.; Wang, X.; Yin, J.; Mou, Y.; Huang, H.; Ren, Z. Selective delivery of curcumin to breast cancer cells by self-targeting apoferritin nanocages with pH-responsive and low toxicity. Drug Deliv. 2022, 29, 986–996.
- Zhang, J.; Cheng, D.; He, J.; Hong, J.; Yuan, C.; Liang, M. Cargo loading within ferritin nanocages in preparation for tumor-targeted delivery. Nat. Protoc. 2021, 16, 4878–4896.
- de Ruiter, M.V.; Putri, R.M.; Cornelissen, J. CCMV-Based Enzymatic Nanoreactors. Methods Mol. Biol. 2018, 1776, 237–247.
- Jiang, B.; Zhang, R.; Zhang, J.; Hou, Y.; Chen, X.; Zhou, M.; Tian, X.; Hao, C.; Fan, K.; Yan, X. GRP78-targeted ferritin nanocaged ultra-high dose of doxorubicin for hepatocellular carcinoma therapy. Theranostics 2019, 9, 2167–2182.
- Liang, M.; Fan, K.; Zhou, M.; Duan, D.; Zheng, J.; Yang, D.; Feng, J.; Yan, X. H-ferritin-nanocaged doxorubicin nanoparticles specifically target and kill tumors with a single-dose injection. Proc. Natl. Acad. Sci. USA 2014, 111, 14900–14905.
- Kelly, P.; Anand, P.; Uvaydov, A.; Chakravartula, S.; Sherpa, C.; Pires, E.; O’Neil, A.; Douglas, T.; Holford, M. Developing a Dissociative Nanocontainer for Peptide Drug Delivery. Int. J. Environ. Res. Public Health 2015, 12, 12543–12555.
- Gonzalez-Davis, O.; Chauhan, K.; Zapian-Merino, S.J.; Vazquez-Duhalt, R. Bi-enzymatic virus-like bionanoreactors for the transformation of endocrine disruptor compounds. Int. J. Biol. Macromol. 2020, 146, 415–421.
- Wang, Y.; Douglas, T. Bioinspired Approaches to Self-Assembly of Virus-like Particles: From Molecules to Materials. Acc. Chem. Res. 2022, 55, 1349–1359.
- Patterson, D.P.; Rynda-Apple, A.; Harmsen, A.L.; Harmsen, A.G.; Douglas, T. Biomimetic antigenic nanoparticles elicit controlled protective immune response to influenza. ACS Nano 2013, 7, 3036–3044.
- Patterson, D.P.; Schwarz, B.; Waters, R.S.; Gedeon, T.; Douglas, T. Encapsulation of an enzyme cascade within the bacteriophage P22 virus-like particle. ACS Chem. Biol. 2014, 9, 359–365.
- Jordan, P.C.; Patterson, D.P.; Saboda, K.N.; Edwards, E.J.; Miettinen, H.M.; Basu, G.; Thielges, M.C.; Douglas, T. Self-assembling biomolecular catalysts for hydrogen production. Nat. Chem. 2016, 8, 179–185.
- Sharma, J.; Douglas, T. Tuning the catalytic properties of P22 nanoreactors through compositional control. Nanoscale 2020, 12, 336–346.
- Wang, J.; Fang, T.; Li, M.; Zhang, W.; Zhang, Z.P.; Zhang, X.E.; Li, F. Intracellular delivery of peptide drugs using viral nanoparticles of bacteriophage P22: covalent loading and cleavable release. J. Mater. Chem. B 2018, 6, 3716–3726.
- Seo, J.; Al-Hilal, T.A.; Jee, J.G.; Kim, Y.L.; Kim, H.J.; Lee, B.H.; Kim, S.; Kim, I.S. A targeted ferritin-microplasmin based thrombolytic nanocage selectively dissolves blood clots. Nanomedicine 2018, 14, 633–642.
- Seo, J.; Do Yoo, J.; Kim, M.; Shim, G.; Oh, Y.K.; Park, R.W.; Lee, B.; Kim, I.S.; Kim, S. Fibrinolytic nanocages dissolve clots in the tumor microenvironment, improving the distribution and therapeutic efficacy of anticancer drugs. Exp. Mol. Med. 2021, 53, 1592–1601.
- Kim, B.R.; Yoon, J.W.; Choi, H.; Kim, D.; Kang, S.; Kim, J.H. Application of periostin peptide-decorated self-assembled protein cage nanoparticles for therapeutic angiogenesis. BMB Rep. 2022, 55, 175–180.
- O’Neil, A.; Reichhardt, C.; Johnson, B.; Prevelige, P.E.; Douglas, T. Genetically programmed in vivo packaging of protein cargo and its controlled release from bacteriophage P22. Angew. Chem. Int. Ed. Engl. 2011, 50, 7425–7428.
- Barwal, I.; Kumar, R.; Kateriya, S.; Dinda, A.K.; Yadav, S.C. Targeted delivery system for cancer cells consist of multiple ligands conjugated genetically modified CCMV capsid on doxorubicin GNPs complex. Sci. Rep. 2016, 6, 37096.
- Van de Steen, A.; Khalife, R.; Colant, N.; Mustafa Khan, H.; Deveikis, M.; Charalambous, S.; Robinson, C.M.; Dabas, R.; Esteban Serna, S.; Catana, D.A., et al. Bioengineering bacterial encapsulin nanocompartments as targeted drug delivery system. Synth. Syst. Biotechnol. 2021, 6, 231–241.
- Zhang, J.; Zhang, W.; Yang, M.; Zhu, W.; Li, M.; Liang, A.; Zhang, H.; Fang, T.; Zhang, X.E.; Li, F. Passive cancer targeting with a viral nanoparticle depends on the stage of tumorigenesis. Nanoscale 2021, 13, 11334–11342.
- Li, W.; Jing, Z.; Wang, S.; Li, Q.; Xing, Y.; Shi, H.; Li, S.; Hong, Z. P22 virus-like particles as an effective antigen delivery nanoplatform for cancer immunotherapy. Biomaterials 2021, 271, 120726.
- Lam, P.; Steinmetz, N.F. Delivery of siRNA therapeutics using cowpea chlorotic mottle virus-like particles. Biomater. Sci. 2019, 7, 3138–3142.
- Nunez-Rivera, A.; Fournier, P.G.J.; Arellano, D.L.; Rodriguez-Hernandez, A.G.; Vazquez-Duhalt, R.; Cadena-Nava, R.D. Brome mosaic virus-like particles as siRNA nanocarriers for biomedical purposes. Beilstein. J. Nanotechnol. 2020, 11, 372–382.
- Patterson, D.P.; Prevelige, P.E.; Douglas, T. Nanoreactors by programmed enzyme encapsulation inside the capsid of the bacteriophage P22. ACS Nano 2012, 6, 5000–5009.
- Patterson, D.P. Encapsulation of Active Enzymes within Bacteriophage P22 Virus-Like Particles. Methods Mol. Biol. 2018, 1798, 11-24.
- Ruggiero, M.; Alberti, D.; Bitonto, V.; Geninatti Crich, S. Ferritin: A Platform for MRI Contrast Agents Delivery. Inorganics 2019, 7.
- Qazi, S.; Liepold, L.O.; Abedin, M.J.; Johnson, B.; Prevelige, P.; Frank, J.A.; Douglas, T. P22 viral capsids as nanocomposite high-relaxivity MRI contrast agents. Mol. Pharm. 2013, 10, 11–17.
- Liepold, L.; Anderson, S.; Willits, D.; Oltrogge, L.; Frank, J.A.; Douglas, T.; Young, M. Viral capsids as MRI contrast agents. Magn. Reason. Med. 2007, 58, 871–879.
- Nandwana, V.; Ryoo, S.R.; Kanthala, S.; Kumar, A.; Sharma, A.; Castro, F.C.; Li, Y.; Hoffman, B.; Lim, S.; Dravid, V.P. Engineered ferritin nanocages as natural contrast agents in magnetic resonance imaging. RSC Adv. 2017, 7, 34892–34900.
- Kelly, M.P.; Napolitano, T.; Anand, P.; Ho, J.S.K.; Jabeen, S.; Kuppan, J.; Manir, S.; Holford, M. Induced Disassembly of a Virus-like Particle under Physiological Conditions for Venom Peptide Delivery. Bioconjug. Chem. 2021, 32, 111–120.
- D, P.P.; Hjorth, C.; Hernandez Irias, A.; Hewagama, N.; Bird, J. Delayed In Vivo Encapsulation of Enzymes Alters the Catalytic Activity of Virus-Like Particle Nanoreactors. ACS Synth. Biol. 2022, 11, 2956–2968.
- Schwarz, B.; Madden, P.; Avera, J.; Gordon, B.; Larson, K.; Miettinen, H.M.; Uchida, M.; LaFrance, B.; Basu, G.; Rynda-Apple, A., et al. Symmetry Controlled, Genetic Presentation of Bioactive Proteins on the P22 Virus-like Particle Using an External Decoration Protein. ACS Nano 2015, 9, 9134–9147.
- Gulati, N.M.; Pitek, A.S.; Czapar, A.E.; Stewart, P.L.; Steinmetz, N.F. The in vivo fates of plant viral nanoparticles camouflaged using self-proteins: overcoming immune recognition. J. Mater. Chem. B 2018, 6, 2204–2216.
- Rohovie, M.J.; Nagasawa, M.; Swartz, J.R. Virus-like particles: Next-generation nanoparticles for targeted therapeutic delivery. Bioeng. Transl. Med. 2017, 2, 43-57.
- Steinmetz, N.F.; Manchester, M. PEGylated viral nanoparticles for biomedicine: the impact of PEG chain length on VNP cell interactions in vitro and ex vivo. Biomacromolecules 2009, 10, 784–792.
- Wang, Y.; Uchida, M.; Waghwani, H.K.; Douglas, T. Synthetic Virus-like Particles for Glutathione Biosynthesis. ACS Synth. Biol. 2020, 9, 3298–3310.
- Chauhan, K.; Hernandez-Meza, J.M.; Rodriguez-Hernandez, A.G.; Juarez-Moreno, K.; Sengar, P.; Vazquez-Duhalt, R. Multifunctionalized biocatalytic P22 nanoreactor for combinatory treatment of ER+ breast cancer. J. Nanobiotechnol. 2018, 16, 17.
