Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Lindsay Dong and Version 1 by Patrícia C. Pires.
Most drugs used for the treatment of depression, anxiety and related disorders have low absorption, high metabolism, low brain targeting and/or low water solubility, which can make it hard to formulate them at high strength and can also lead to decreased bioavailability. Incorporating these drugs into nanometric emulsions can solve these issues. Nanometric emulsions were able to increase drug strength up to 20,270-fold (compared to aqueous solubility). The formulations showed droplet size, polydispersity index, zeta potential, viscosity, osmolality, pH, in vitro drug release and ex vivo drug permeation as adequate for the intended effect and administration route.
- anxiety
- brain delivery
- depression
- microemulsion
- nanoemulsion
1. Introduction
Depressive disorders are some of the most prevalent, impairing and costly illnesses, having recently been estimated to affect more than 246 million people worldwide [1,2,3][1][2][3]. Although they can be divided according to subtype and level of severity, these disorders are generally characterized by a depressed mood or general loss of pleasure or interest, usually accompanied by symptoms such as feelings of guilt, worthlessness or hopelessness; low self-esteem; indecisiveness or difficulty in concentrating or thinking; fatigue; psychomotor agitation or retardation; change in appetite; insomnia or hypersomnia; mood swings; and, in most severe cases, recurrent thoughts of death or suicidal ideation (Figure 1).
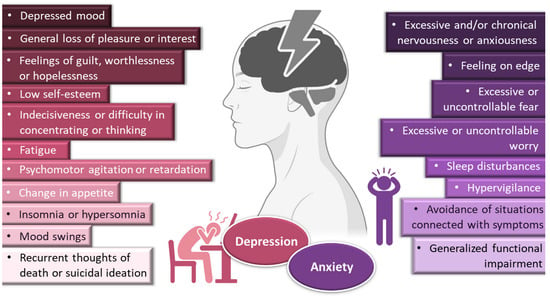
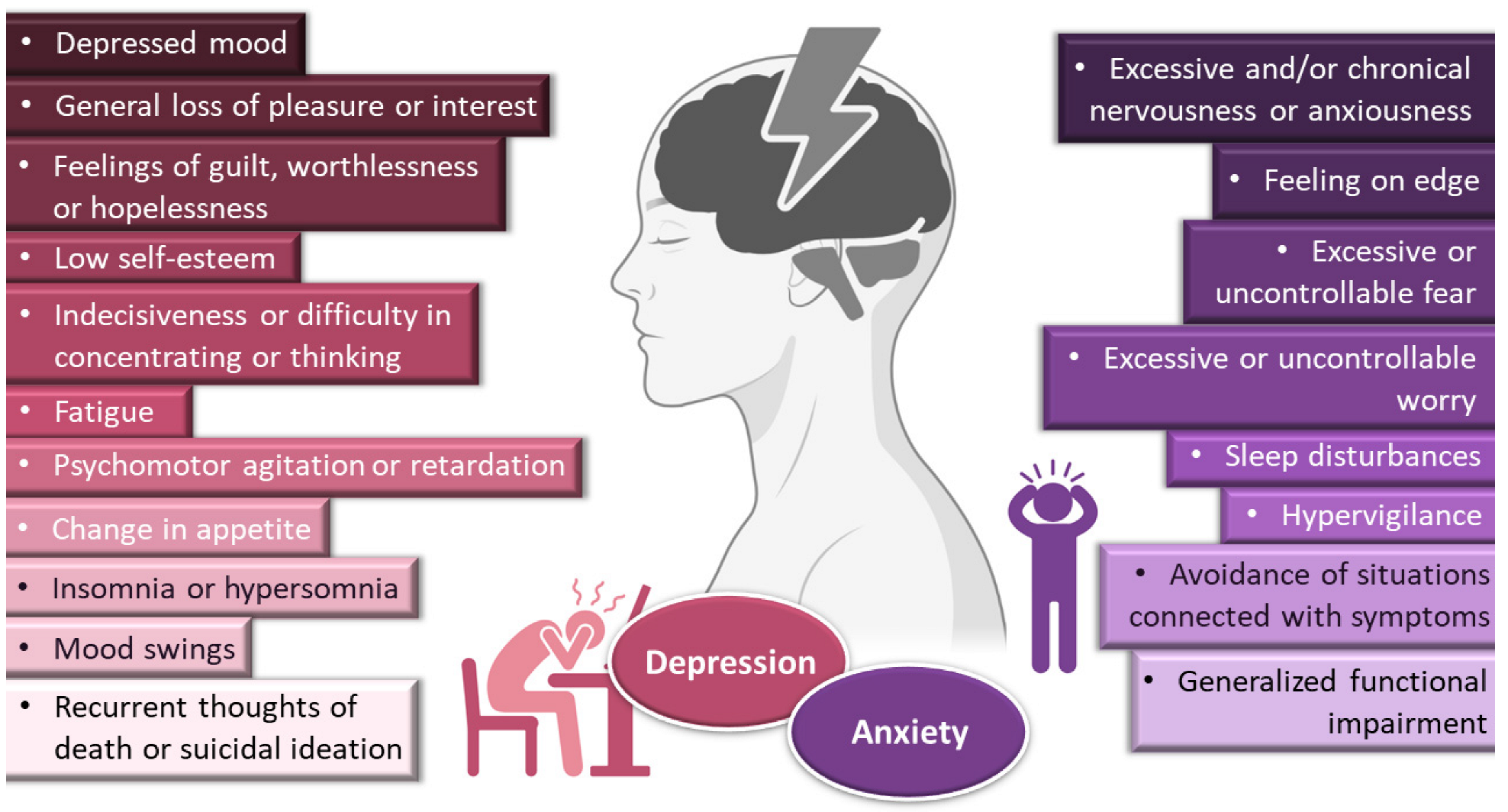
Figure 1.
General symptoms of depression and anxiety disorders. Drawn with BioRender (no copyright required).
Depression frequently coexists with other mental health disorders. There is a significantly increased risk of developing a comorbid depressive disorder when someone already has an anxiety disorder [1,5,9,10,11][1][4][5][6][7]. Anxiety disorders are also among the most common mental disorders, having been recently estimated to globally affect more than 265 million people. They are associated with substantial functional impairment, which leads to decreased work productivity and quality of life [2,9,12][2][5][8]. Aside from generalized anxiety disorder, there is a wide spectrum of related disorders (such as obsessive-compulsive, posttraumatic stress, panic and social anxiety disorders), but in general symptoms can include feeling exceptionally or chronically nervous, anxious or on edge; having excessive or uncontrollable fear and worry; sleep disturbances and hypervigilance; and constant avoidance of situations that relate to the previously mentioned symptoms (Figure 1) [9,11,13,14][5][7][9][10]. Patients with anxiety disorders also have a higher prevalence of other diseases, such as cardiovascular, respiratory and gastrointestinal conditions [9,12][5][8]. Treatment of anxiety and related disorders includes psychological and pharmacological options, and the choice again depends on patient related factors, such as severity of illness, prior treatment, comorbid disorders, patient preference and motivation, etc. [9][5]. The first-line pharmacological options are similar to those prescribed for depressive disorders: either selective serotonin reuptake inhibitors (escitalopram, fluoxetine, fluvoxamine, paroxetine, sertraline) or serotonin and norepinephrine reuptake inhibitors (duloxetine, venlafaxine), for being generally better tolerated and safer than other treatments [9,12][5][8]. Other options include noradrenergic and specific serotonergic antidepressants, tricyclic antidepressants, monoamine oxidase inhibitors, and reversible inhibitors of monoamine oxidase A [9,11][5][7]. Benzodiazepines can also be used, but as adjunctive short-term therapy, since they can cause dependency, sedation and cognitive impairment (especially with prolonged use) [9,12][5][8]. Some anticonvulsants and atypical antipsychotics have also demonstrated efficacy, but are generally recommended as second-line, third-line, or adjunctive therapies (due to side effects). Given the variety in treatment options, again it should be a case-by-case approach, taking into consideration efficacy versus safety, the specific characteristics of the anxiety disorder, comorbid conditions and treatment duration [9][5].
Yet, despite pharmacological treatment options for depressive and anxiety disorders being many, a great number of these drugs (including the grand majority of new drug candidates) have low water solubility, which can make it hard to formulate them at high strengths in liquid preparations [15][11]. This problem can be tackled by formulating these molecules into solid forms, with oral tablets being the most common option, but dose adjustment can sometimes be difficult, and inappropriate tablet splitting can lead to dose intake variation, which in turn can result in a reduction in treatment efficacy or exacerbation of adverse effects. Moreover, swallowing these formulations can be challenging, especially in the younger population (children and adolescents) or older individuals (particularly if having diseases linked to dysphagia, such as stroke, Parkinson’s, Alzheimer’s or cancer) [16,17][12][13]. Intravenous treatments require liquid solutions, but drug solubilization is usually achieved either by pH adjustments in the formulation, which if very low or very high could be potentially harmful, or using great amounts of organic cosolvents or surfactants, which are potentially toxic excipients, having been reported to cause hemotoxicity and hypersensitivity reactions (pruritus, erythema, rash or urticaria) [15][11]. Moreover, in these types of formulations, drugs are highly susceptible to metabolism, which can occur in all administration routes, but especially systemic ones, due to hepatic first-pass metabolism, being aggravated in oral administration, due to additional gastrointestinal degradation [18][14]. Additionally, in general, the treatment of central nervous systems disorders can be compromised by the very low permeability of the blood-brain barrier, which restricts the transport of most drug molecules, and this is especially relevant for the most common administration routes, in which the drug is transported to the brain from the bloodstream (oral and intravenous) [18,19][14][15].
Incorporating drugs into a nanosystem can be an effective strategy to tackle these issues. Nanosystems (also known as nanocarriers) are colloidal structures with a mean diameter of less than 500 nm. Among their many advantages, they allow: the enhancement of drug solubilization; metabolic and chemical degradation drug protection; the reduction of high plasma protein binding; increased permeability through biological membranes; and the promotion of brain bioavailability, which is especially useful for diseases with a brain etiology [19,20,21,22,23][15][16][17][18][19] (Figure 2). The many types of nanosystems can be divided into four main categories: polymeric nanocarriers, such as polymeric nanoparticles and micelles; lipid nanoparticles, such as solid lipid nanoparticles or nanostructured lipid carriers; liposomes and their derived counterparts, such as niosomes, ethosomes, transfersomes, cubosomes and phytosomes; and nanometric emulsions, such as nanoemulsions and microemulsions [18,24,25,26][14][20][21][22]. Yet, despite all mentioned advantages, most of these nanosystems have several drawbacks, such as a low encapsulation efficiency; reduced physical stability; requiring the use of organic solvents during preparation; requiring complex and time-consuming preparation methods; and having non-biocompatible components [27,28,29][23][24][25] (Figure 2).
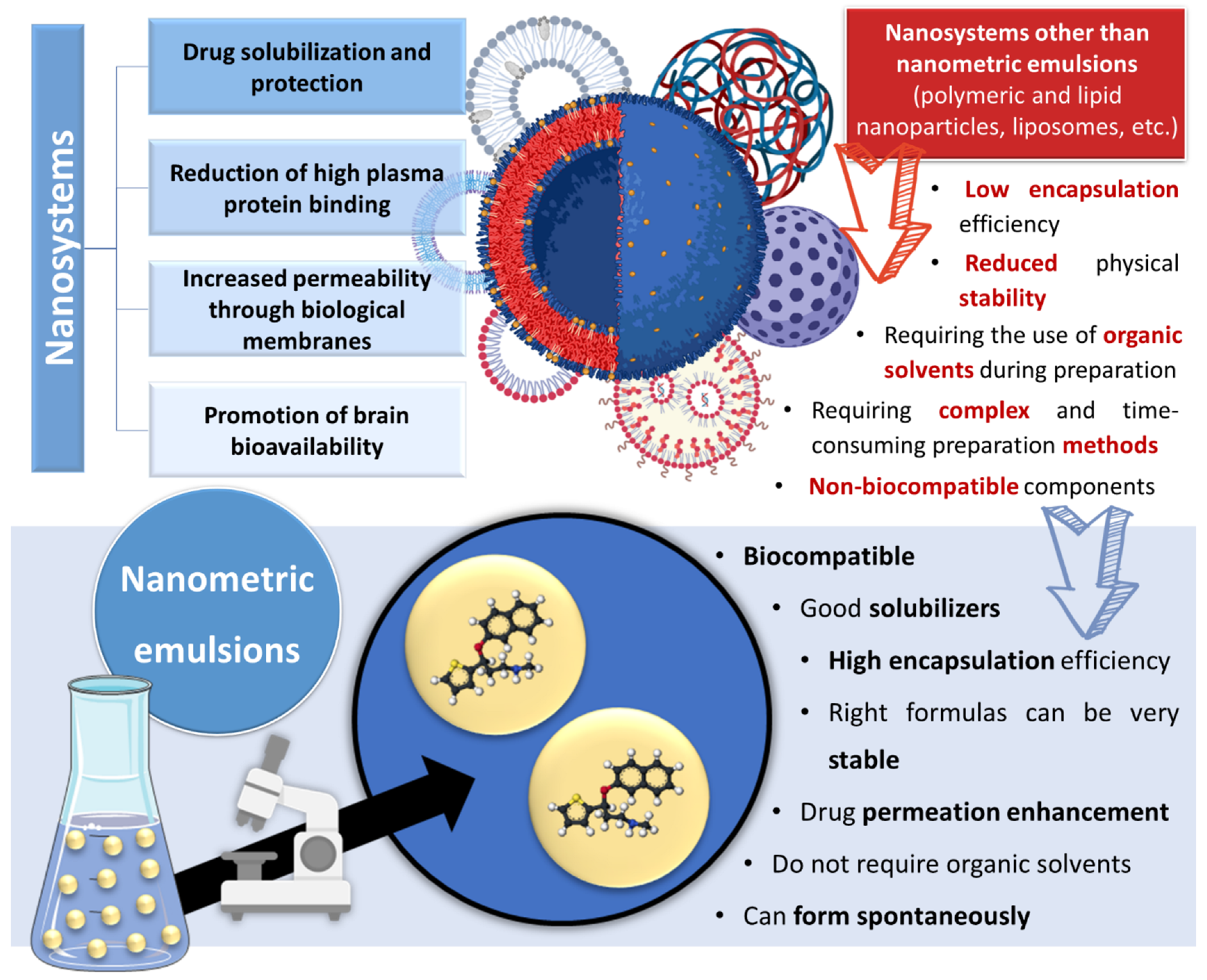

Figure 2. Advantages and disadvantages of drug encapsulation into nanosystems, with a focus on the superiority of nanometric emulsions. Drawn with BioRender (no copyright required).
However, nanometric emulsions can surpasses all of these drawbacks. Being colloidal liquid-in-liquid dispersions, they are usually made of a water phase, an oil, a surfactant, a cosurfactant and/or a cosolvent. They can be classified according to droplet size, between nanoemulsions (20,200 nm) or microemulsions (10–100 nm), although the size range can differ between scholars. Regarding what concerns the differences in their characteristics, while microemulsions have thermodynamic stability, nanoemulsions have a relatively high kinetic stability, and both have higher surface area and free energy than macroemulsions, which makes them more physically stable in comparison. They can also be classified according to the nature of their internal and external phases, as: oil-in-water (o/w) or water-in-oil (w/o), if they are biphasic (most common); or oil-in-water-in-oil or water-in-oil-in-water, if they are triphasic (Figure 3) [18,30,31][14][26][27].
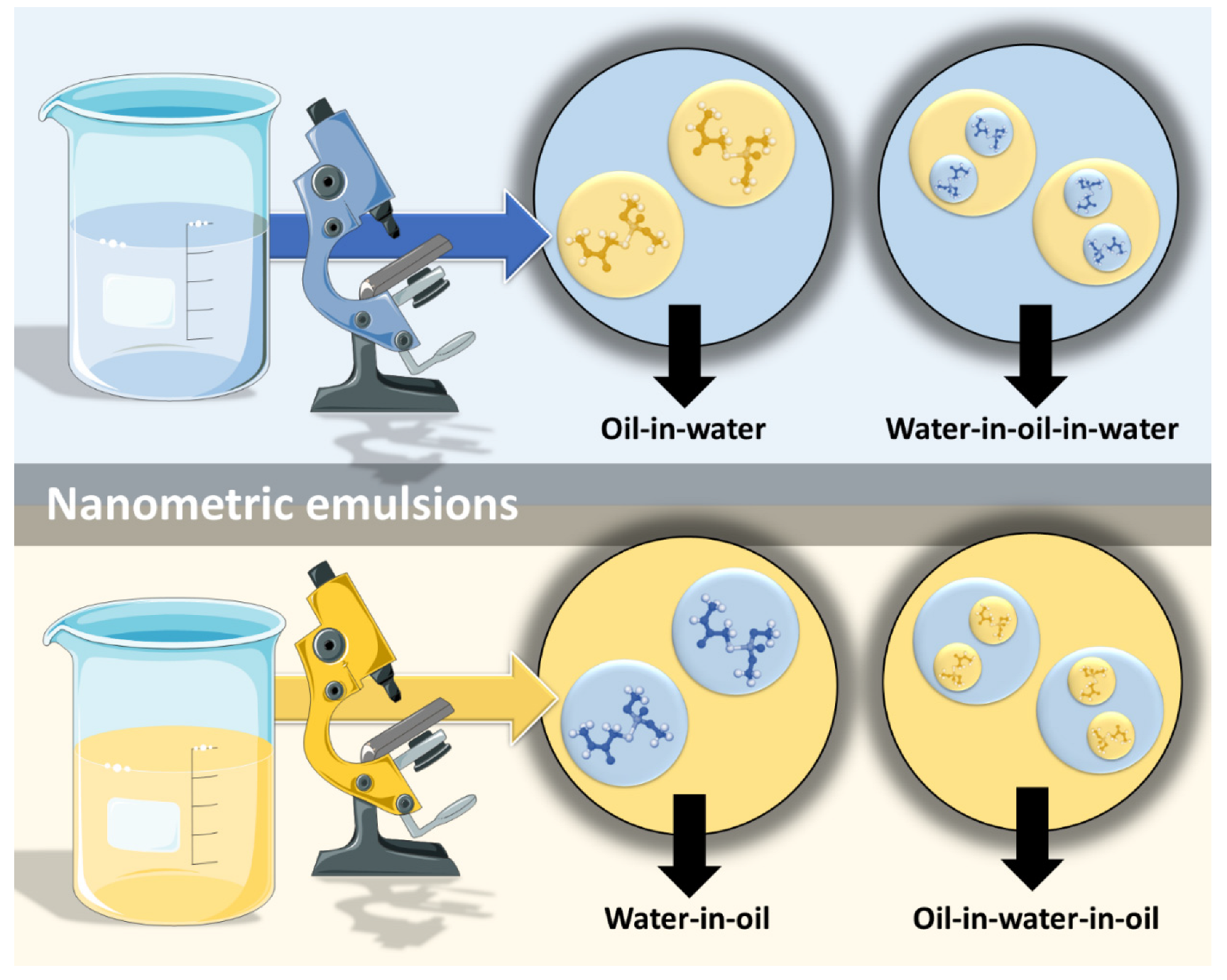

Figure 3. Types of nanometric emulsions, according to the nature of their internal and external phases. Drawn with BioRender (no copyright required).
2. Nanometric Emulsions
2.1. Nanometric Emulsions through Intravenous Administration—The Fastest Way to Achieve Systemic Drug Delivery
Intravenous administration involves injecting drugs directly into the bloodstream, which results in the fastest systemic drug delivery, being ideal for the treatment of acute and emergency situations. Moreover, since it bypasses any physical, chemical or biological barrier that might hinder drug absorption, it leads to the highest systemic bioavailability (theoretically 100%) among all delivery routes [32][28]. Nevertheless, this type of administration has its disadvantages, mostly due to the invasiveness of the injection, which can cause substantial discomfort or pain, and consequently decrease patient compliance, also having an associated risk of injury (and sometimes even infection) at the administration site. The intravenous route also requires trained personnel and, consequently, hospitalization, which is a major limitation [32,33][28][29]. In what concerns formulation characteristics, intravenous preparations should be sterile, isotonic (osmolality around 290 mOsmol/kg) and euhydric (physiological pH), in order to avoid local damage on vascular endothelium and circulating blood cells. These preparations should also have a low viscosity (up to 15 or 20 cP), since they should be easily drawn into a syringe and injected from it, and high viscosity intravenous formulations have been linked to blood viscosity increase, and consequently cardio or cerebrovascular adverse events [34][30].
2.2. Transdermal Administration of Nanometric Emulsions—Overcoming the Skin Barrier
Transdermal administration delivers drugs across the skin’s layers to the blood circulatory system. It can be preferred over the parenteral route for being non-invasive, thus circumventing its associated issues, such as needle phobia [33,36][29][31]. When compared to the oral route, it has the advantages of avoiding hepatic first-pass metabolism and gastrointestinal degradation, which can increase drug bioavailability, and not needing repeated dosing, which can increase patient compliance, also being suitable for patients for whom the oral route is not eligible (situations such as unconsciousness or vomiting) [21,32,33,36][17][28][29][31]. By providing sustained drug plasma levels, transdermal delivery is especially suitable for drugs that need relatively constant plasma levels and prolonged duration of the therapeutic effect [21,32][17][28]. It is also associated with more uniform pharmacokinetic drug profiles, with fewer peaks, thus minimizing the risk of toxic side effects [33][29]. Nevertheless, in order to be feasible candidates for delivery by transdermal administration, drugs should have certain characteristics, such as being highly potent, small in size (<500 Da), and having a log p-value between 1 and 3 (lipophilic) [21,32,33][17][28][29]. Moreover, transdermal delivery is associated with overall poor drug permeation through the skin barrier, which can consequently lead to low bioavailability [36][31]. Hence, some methods can be used to temporarily and reversibly modify the skin barrier: physical methods, such as iontophoresis, electroporation and ultrasound; or chemical methods, such as the use of excipients with absorption enhancing capability (e.g., fatty acids, surfactants, terpenes and solvents). Nevertheless, these methods should be used with caution, since they could cause toxicity and skin irritation [21,32][17][28].2.3. Oral Delivery of Nanometric Emulsions—Overcoming the Problems Related to the Most Common Route
Whenever possible to use, non-invasive administration methods are usually the best option for chronic therapy. Within them, the oral route is the most common, being conventionally chosen to deliver the great majority of small molecular weight drugs [32,33][28][29]. The ease of self-administration, painlessness and cost-effectiveness associated with this route all lead to high patient compliance [32,39][28][32]. Moreover, drugs can have access to a large surface area available for absorption to the systemic circulation, with the possibility for sustained and controlled delivery [39][32]. Nevertheless, orally delivered drugs need to deal with multiple levels of barriers. Prior to absorption, the harsh environment of the gastrointestinal tract can lead to chemical and enzymatic drug degradation, and after absorption the first-pass hepatic metabolism can also significantly reduce drug bioavailability.2.4. Intranasal Nanometric Emulsions—A Direct Route to the Brain
Intranasal administration is promising for the treatment of affections with a brain etiology due to allowing (at least part of) the drug to reach the brain directly by neuronal transport. This also makes it possible for drugs to simultaneously (at least partially) avoid the blood-brain barrier, the harsh environment of the gastrointestinal tract and the hepatic first-pass metabolism. Therefore, it can not only increase brain drug bioavailability and minimize systemic adverse events, but also generate a short onset of action, which is a must in emergency situations. Moreover, the intranasal route is non-invasive and the formulations can be easily administrated by the patients themselves or a caregiver, hence not requiring hospitalization. Additionally, it is a good alternative to the oral route for patients with symptoms such as vomiting, increased salivation, or inability to swallow. Nasal liquid or semisolid preparations should have non-irritant components, a pH between 5.0 and 6.5 (similar to the nasal mucosa’s), and be isotonic to slightly hypertonic. The limitations associated with this administration route include requiring a low administration volume (150–200 μL for humans, therefore requiring relatively potent drugs), the possibility of the formulation’s residence time in the nasal cavity being short (which could be tackled by increasing the formulation’s viscosity or adding a mucoadhesive polymer), and the presence of degrading enzymes and efflux transporters in the nasal cavity [18,20,46][14][16][33].3. Conclusions
The development of nanometric emulsions to encapsulate antidepressant and anxiolytic drugs has proven to be effective in increasing both drug strength and delivery, especially for lipophilic molecules. This happens not only due to small droplet size and the possibility of encapsulation of said molecules, but also due to the use of excipients with solubilizing capacity and permeation enhancing properties, such as surfactants, cosolvents and cyclodextrins. Furthermore, formulation characterization is not complete without determining and reporting droplet size, PDI, zeta potential, viscosity, osmolality and pH, which are all factors that could influence their in vivo performance and/or safety. Formulation stability studies are also recommended in order to know the time during which a selected formula will keep its properties. In vitro drug release, ex vivo drug permeation and specific biochemical estimations are not as indispensable, but might provide useful information that could help explain, deepen the knowledge or predict the outcomes of in vivo studies. On the other hand, in vivo animal pharmacokinetic and/or pharmacodynamic experiments are essential in order to assess the full potential of a developed formulation, and without them that assessment is left incomplete. Safety studies should also be more frequently performed, since even if a certain formulation is therapeutically effective, it is not promising unless it has a reasonably favorable efficacy/safety ratio. Hence, although the number of studies that have been performed so far is still small, which presents a limitation for drawing generalized conclusions, overall, nano and microemulsions have shown to be promising strategies to improve the solubilization and increase the bioavailability of antidepressant and/or anxiolytic drugs, being potential strategies to replace current therapies. More experimental studies should be conducted in the future, including clinical trials, in order to address these formulations’ true medical applicability.References
- American Psychological Association. Clinical Practice Guideline for the Treatment of Depression across Three Age Cohorts; American Psychological Association: Washington, DC, USA, 2019.
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, Regional, and National Incidence, Prevalence, and Years Lived with Disability for 354 Diseases and Injuries for 195 Countries and Territories, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858.
- Zhao, Y.F.; Verkhratsky, A.; Tang, Y.; Illes, P. Astrocytes and Major Depression: The Purinergic Avenue. Neuropharmacology 2022, 220, 109252.
- Kircanski, K.; Joormann, J.; Gotlib, I.H. Cognitive Aspects of Depression. Wiley Interdiscip. Rev. Cogn. Sci. 2012, 3, 301–313.
- Katzman, M.A.; Bleau, P.; Blier, P.; Chokka, P.; Kjernisted, K.; Van Ameringen, M.; Antony, M.M.; Bouchard, S.; Brunet, A.; Flament, M.; et al. Canadian Clinical Practice Guidelines for the Management of Anxiety, Posttraumatic Stress and Obsessive-Compulsive Disorders. BMC Psychiatry 2014, 14, S1.
- Hu, P.; Lu, Y.; Pan, B.-X.; Zhang, W.-H. New Insights into the Pivotal Role of the Amygdala in Inflammation-Related Depression and Anxiety Disorder. Int. J. Mol. Sci. 2022, 23, 11076.
- Penninx, B.W.; Pine, D.S.; Holmes, E.A.; Reif, A. Anxiety Disorders. Lancet 2021, 397, 914–927.
- Nasir, M.; Trujillo, D.; Levine, J.; Dwyer, J.B.; Rupp, Z.W.; Bloch, M.H. Glutamate Systems in DSM-5 Anxiety Disorders: Their Role and a Review of Glutamate and GABA Psychopharmacology. Front. Psychiatry 2020, 11, 548505.
- Won, E.; Kim, Y.K. Neuroinflammation—Associated Alterations of the Brain as Potential Neural Biomarkers in Anxiety Disorders. Int. J. Mol. Sci. 2020, 21, 6546.
- Chesnut, M.; Harati, S.; Paredes, P.; Khan, Y.; Foudeh, A.; Kim, J.; Bao, Z.; Williams, L.M. Stress Markers for Mental States and Biotypes of Depression and Anxiety: A Scoping Review and Preliminary Illustrative Analysis. Chronic Stress 2021, 5, 1–17.
- Patel, D.; Zode, S.S.; Bansal, A.K. Formulation Aspects of Intravenous Nanosuspensions. Int. J. Pharm. 2020, 586, 119555.
- Wening, K.; Breitkreutz, J. Oral Drug Delivery in Personalized Medicine: Unmet Needs and Novel Approaches. Int. J. Pharm. 2011, 404, 1–9.
- Schiele, J.T.; Quinzler, R.; Klimm, H.; Pruszydlo, M.G.; Haefeli, W.E. Difficulties Swallowing Solid Oral Dosage Forms in a General Practice Population: Prevalence, Causes, and Relationship to Dosage Forms. Eur. J. Clin. Pharmacol. 2013, 69, 937–948.
- Pires, P.C.; Santos, A.O. Nanosystems in Nose-to-Brain Drug Delivery: A Review of Non-Clinical Brain Targeting Studies. J. Control. Release 2018, 270, 89–100.
- Gao, H. Progress and Perspectives on Targeting Nanoparticles for Brain Drug Delivery. Acta Pharm. Sin. B 2016, 6, 268–286.
- Pires, P.C.; Melo, D.; Santos, A.O. Intranasal Delivery of Antiseizure Drugs. In Drug Delivery Devices and Therapeutic Systems; Academic Press: Cambridge, MA, USA, 2021; pp. 623–646. ISBN 978-0-12-819838-4.
- Yu, Y.Q.; Yang, X.; Wu, X.F.; Fan, Y.B. Enhancing Permeation of Drug Molecules across the Skin via Delivery in Nanocarriers: Novel Strategies for Effective Transdermal Applications. Front. Bioeng. Biotechnol. 2021, 9, 646554.
- Yan, H.; Zhai, B.; Yang, F.; Chen, Z.; Zhou, Q.; Paiva-Santos, A.C.; Yuan, Z.; Zhou, Y. Nanotechnology-Based Diagnostic and Therapeutic Strategies for Neuroblastoma. Front. Pharmacol. 2022, 13, 908713.
- Bernardo, J.; Santos, A.C.; Videira, R.A.; Valentão, P.; Veiga, F.; Andrade, P.B. Trichilia Catigua and Turnera Diffusa Phyto-Phospholipid Nanostructures: Physicochemical Characterization and Bioactivity in Cellular Models of Induced Neuroinflammation and Neurotoxicity. Int. J. Pharm. 2022, 620, 121774.
- Alqosaibi, A.I. Nanocarriers for Anticancer Drugs: Challenges and Perspectives. Saudi J. Biol. Sci. 2022, 29, 103298.
- Nie, X.; Chen, Z.; Pang, L.; Wang, L.; Jiang, H.; Chen, Y.; Zhang, Z.; Fu, C.; Ren, B.; Zhang, J. Oral Nano Drug Delivery Systems for the Treatment of Type 2 Diabetes Mellitus: An Available Administration Strategy for Antidiabetic Phytocompounds. Int. J. Nanomed. 2020, 15, 10215–10240.
- Safta, D.A.; Bogdan, C.; Moldovan, M.L. Vesicular Nanocarriers for Phytocompounds in Wound Care: Preparation and Characterization. Pharmaceutics 2022, 991.
- Pires, P.C.; Rodrigues, M.; Alves, G.; Santos, A.O. Strategies to Improve Drug Strength in Nasal Preparations for Brain Delivery of Low Aqueous Solubility Drugs. Pharmaceutics 2022, 14, 588.
- Tapeinos, C.; Battaglini, M.; Ciofani, G. Advances in the Design of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers for Targeting Brain Diseases. J. Control. Release 2017, 264, 306–332.
- Stefanov, S.R.; Andonova, V.Y. Lipid Nanoparticulate Drug Delivery Systems: Recent Advances in the Treatment of Skin Disorders. Pharmaceuticals 2021, 14, 1083.
- Kumar, A.; Pandey, A.N.; Jain, S.K. Nasal-Nanotechnology: Revolution for Efficient Therapeutics Delivery. Drug Deliv. 2016, 23, 671–683.
- Handa, M.; Tiwari, S.; Yadav, A.K.; Almalki, W.H.; Alghamdi, S.; Alharbi, K.S.; Shukla, R.; Beg, S. Therapeutic Potential of Nanoemulsions as Feasible Wagons for Targeting Alzheimer’s Disease. Drug Discov. Today 2021, 26, 2881–2888.
- Homayun, B.; Lin, X.; Choi, H. Challenges and Recent Progress in Oral Drug Delivery Systems for Biopharmaceuticals. Pharmaceutics 2019, 11, 129.
- Alkilani, A.Z.; McCrudden, M.T.C.; Donnelly, R.F. Transdermal Drug Delivery: Innovative Pharmaceutical Developments Based on Disruption of the Barrier Properties of the Stratum Corneum. Pharmaceutics 2015, 7, 438–470.
- Samiun, W.S.; Ashari, S.E.; Salim, N.; Ahmad, S. Optimization of Processing Parameters of Nanoemulsion Containing Aripiprazole Using Response Surface Methodology. Int. J. Nanomed. 2020, 15, 1585–1594.
- Shukla, T.; Upmanyu, N.; Agrawal, M.; Saraf, S.; Saraf, S.; Alexander, A. Biomedical Applications of Microemulsion through Dermal and Transdermal Route. Biomed. Pharmacother. 2018, 108, 1477–1494.
- Thwala, L.N.; Préat, V.; Csaba, N.S. Emerging Delivery Platforms for Mucosal Administration of Biopharmaceuticals: A Critical Update on Nasal, Pulmonary and Oral Routes. Expert Opin. Drug Deliv. 2017, 14, 23–36.
- Erdó, F.; Bors, L.A.; Farkas, D.; Bajza, Á.; Gizurarson, S. Evaluation of Intranasal Delivery Route of Drug Administration for Brain Targeting. Brain Res. Bull. 2018, 143, 155–170.
More
