Several pathophysiological mechanisms such as oxidative stress, systemic inflammation, increased proteolysis, autophagy and apoptosis, and signaling pathways were shown to be involved in the process of muscle mass loss in limb muscles of cachectic patients with chronic conditions and in animal models.
Whether prolonged periods of immobilization may impair to a greater extent the muscle mass loss and dysfunction elicited by cancer-induced cachexia needs to be further investigated. Investigation of to what extent each particular condition: either cancer cachexia or prolonged immobilization contributes to the wasting process of muscle mass also remains to be clarified.
- muscle unloading model
- cancer-cachexia model
- muscle damage
- muscle proteolysis and autophagy
1.
Note:All the information in this draft can be edited by authors. And the entry will be online only after authors edit and submit it.
Muscle InProtroductioneolysis and Autophagy
Skeletal muscle wasting is associated with chronic diseases including cancer. Cancer-induced cachexia is an invalidating condition that negatively influences the patients’ prognosis regardless of the status of the underlying tumor [1][2][3][4][5][6][1–6]. Muscle mass loss also takes place during periods of immobilization such as prolonged bed rest, microgravity, denervation, and critical illness [7][8][9][10][11][7–11]. The loss of muscle mass impairs muscle contractile function and atrophy mainly characterized by muscle weakness. Poor muscle performance entails an impairment in the patients’ daily life activities with a negative impact on their quality of life [8][12][13][14][15][16][8,12–16]. Furthermore, muscle mass loss due to cancer may be further aggravated by periods of muscle disuse. The specific contribution of each condition to the final muscle loss remains to be fully elucidated.
Several pathophysiological mechanisms such as oxidative stress, systemic inflammation, increased proteolysis, autophagy and apoptosis, and signaling pathways were shown to be involved in the process of muscle mass loss in limb muscles of cachectic patients with chronic conditions [17][18][19][20][17–20] and in animal models [18][19][21][22][18,19,21,22]. On the other hand, mechanisms related to muscle protein synthesis and regeneration may also be altered in cancer-induced cachexia and muscle wasting.
Our group and others have demonstrated that in the gastrocnemius of mice exposed to different time-points of unilateral hindlimb immobilization, markers of muscle proteolysis and injury were increased [23][24][25][23–25]. Moreover, a decline in the number of progenitor muscle cells along with an upregulation of markers of activated satellite cells during a 7-day period of unloading of the limb muscles in mice was also demonstrated [26][27][26,27]. Whether the prolonged periods of immobilization may impair to a greater extent the muscle mass loss and dysfunction elicited by cancer-induced cachexia needs to be further investigated. Investigation of to what extent each particular condition—either cancer cachexia or prolonged immobilization—contributes to the wasting process of muscle mass also remains to be clarified.
2. Results
2.1. Non-immobilized Controls versus Either Unloading or LC-Cachexia Conditions
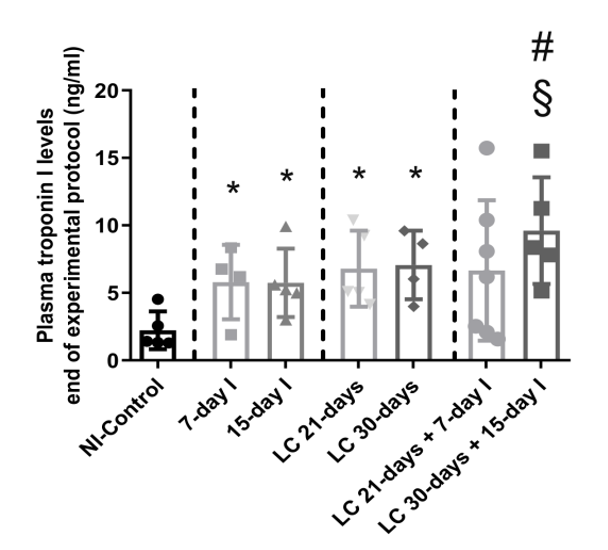
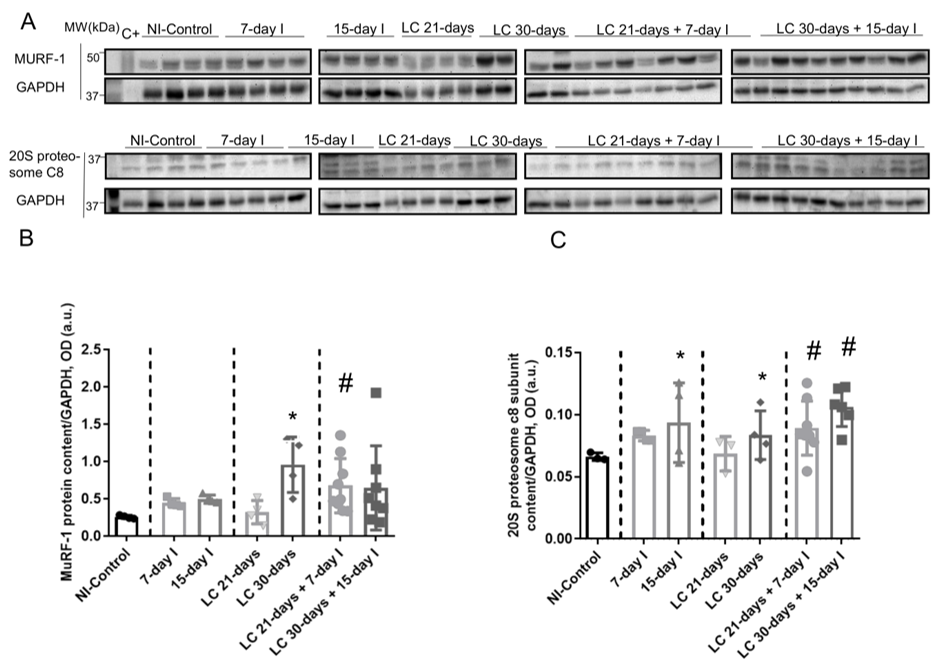
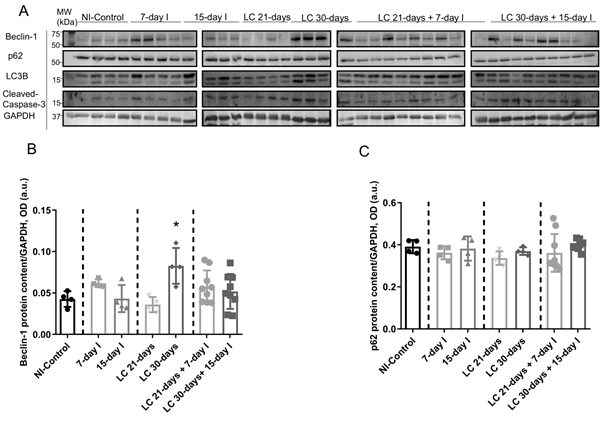
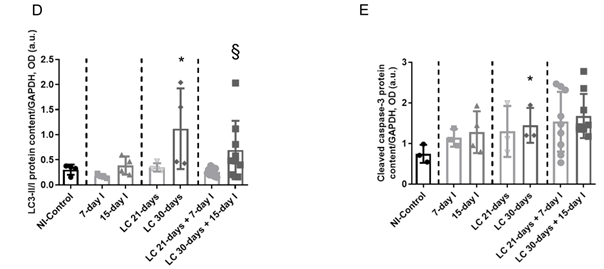
Compared to NI-control animals, in unloaded and LC-cachexia mice, plasma troponin I levels significantly increased (Figure 13). Levels of MuRF-1 and proteasome were significantly greater in the 15-day I unloaded and LC 30-days animals than in NI-control mice (Figure 24A–C). No significant differences were seen in mice of the 7-day I and LC 21-days groups compared to NI-control mice for any of these markers (Figure 24A–C). Also, beclin-1, p62, LC3-II/I, and cleaved caspase-3 protein levels did not significantly differ (Figure 3A-35A-5E). Moreover, beclin-1, LC3-II/I, and cleaved caspase-3 protein levels were significantly higher in muscles of the LC 30-days than in NI-controls, while no differences were detected in p62 protein levels between them (Figure 3A-35A-5E).
Figure 13. Mean values and standard deviation of the variable plasma troponin I (ng/mL) of the different study groups of mice. Definition of abbreviations: ng, nanogram; ml, milliliter; NI, non-immobilized; I, immobilization; LC, lung cancer. Statistical significance is represented as follows: * p < 0.05 between 7-day I, 15-day I, LC 21-days, LC 30-days animals, and the NI-control mice; # p < 0.05 between any group of LC-cachexia mice and their respective group of mice bearing the two conditions (LC-cachexia and unloading); § p < 0.05 between any group of unloaded mice and their respective group of mice bearing the two conditions (LC-cachexia and unloading).
Figure 24. (A) Representative immunoblots of MuRF-1, 20S proteasome alpha subunit C8, and GAPDH proteins in the gastrocnemius muscle of all study groups of mice. (B) Mean values and standard deviation of MuRF-1 protein content in the gastrocnemius muscle of the different study groups of mice, as measured by optical densities in arbitrary units (OD, a.u.). Statistical significance is represented as follows: * p < 0.05 between 7-day I, 15-day I, LC 21-days, LC 30-days animals, and the NI-control mice; # p < 0.05 between any group of LC-cachexia mice and their respective group of mice bearing the two conditions (LC-cachexia and unloading). (C) Mean values and standard deviation of 20S proteasome c8 subunit protein content in the gastrocnemius muscle of the different study groups of mice, as measured by optical densities in arbitrary units (OD, a.u.). Statistical significance is represented as follows: * p < 0.05 between 7-day I, 15-day I, LC 21-days, LC 30-days animals, and the NI-control mice; # p < 0.05 between any group of LC-cachexia mice and their respective group of mice bearing the two conditions (LC-cachexia and unloading). Definition of abbreviations: MuRF-1, muscle RING-finger protein-1; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; MW, molecular weight; kDa, kilodalton; C+, positive control; NI, non-immobilized; I, immobilization; LC, lung cancer.
2.2. LC-Cachexia+Unloading versus Unloading Conditions
Compared to unloaded (7-day I and 15-day I) mice, in gastrocnemius of animals with the two conditions (LC-cachexia and unloading), plasma troponin I levels were significantly higher in LC 30-days + 15-day I animals than in 15-day I mice (+67% change, Figure 13). Levels of MuRF-1 and proteasome did not significantly differ between LC-cachexia+unloaded animals compared to non-cachectic unloaded mice (Figure 24A–C). Also, beclin-1, p62, LC3-II/I and cleaved caspase-3 protein levels did not significantly differ, while LC3-II/I levels (only in LC 30-days + 15-day I) significantly increased (+80% change, Figure 3A-35A-5E). Also, the levels of the markers beclin-1, p62, LC3-II/I protein, and cleaved caspase-3 did not significantly differ among the study groups (Figure 3A-35A-5E).
2.3. LC-Cachexia + Unloading versus LC-Cachexia
Compared to LC-cachexia animals, in mice with the two conditions (LC-cachexia and unloading), plasma troponin I levels were significantly increased in LC 30-days + 15-day I mice compared to LC 30-days rodents (+36% change, Figure 13). MuRF-1 and proteasome levels were significantly greater (only in LC 21-days + 7-day I, +72% change and +30% and +25% change in LC 21-days + 7-day I and LC 30-days + 15-day I, respectively for each marker) in both groups of LC-cachexia+unloaded rodents compared to both groups of LC-cachexia animals (Figure 24A–C).
FigureFigure 5: 3. (A)
Representative immunoblots of beclin-1, p62, LC3B, cleaved caspase-3 and GAPDH proteins in the gastrocnemius muscle of all study groups of mice.
(B) Mean values and standard deviation of beclin-1 protein content in the gastrocnemius muscle of the different study groups of mice, as measured by optical densities in arbitrary units (OD, a.u.). Statistical significance is represented as follows: *, p < 0.05 between 7-day I, 15-day I, LC 21-days, LC 30-days animals and the NI-control mice.
Mean values and standard deviation of beclin-1 protein content in the gastrocnemius muscle of the different study groups of mice, as measured by optical densities in arbitrary units (OD, a.u.). Statistical significance is represented as follows: *, p<0.05 between 7-day I, 15-day I, LC 21-days, LC 30-days animals and the NI-control mice.
(C)
Mean values and standard deviation of p62 protein content in the gastrocnemius muscle of the different study groups of mice, as measured by optical densities in arbitrary units (OD, a.u.).
(D) Mean values and standard deviation of LC3B protein content in the gastrocnemius muscle of the different study groups of mice, as measured by optical densities in arbitrary units (OD, a.u.). Statistical significance is represented as follows: *, p < 0.05 between 7-day I, 15-day I, LC 21-days, LC 30-days animals and the NI-control mice; §, p < 0.05 between any group of unloaded mice and their respective group of mice bearing the two conditions (LC-cachexia and unloading).
Mean values and standard deviation of LC3B protein content in the gastrocnemius muscle of the different study groups of mice, as measured by optical densities in arbitrary units (OD, a.u.). Statistical significance is represented as follows: *, p<0.05 between 7-day I, 15-day I, LC 21-days, LC 30-days animals and the NI-control mice; §, p<0.05 between any group of unloaded mice and their respective group of mice bearing the two conditions (LC-cachexia and unloading).
(E) Mean values and standard deviation of cleaved caspase-3 protein content in the gastrocnemius muscle of the different study groups of mice, as measured by optical densities in arbitrary units (OD, a.u.). Statistical significance is represented as follows: *, p < 0.05 between 7-day I, 15-day I, LC 21-days, LC 30-days animals and the NI-control mice. Definition of abbreviations: p62, nucleoporin 62; LC3B, light chain 3 isoform B; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; MW, molecular weight; kDa, kilodalton; NI, non-immobilized; I, immobilization; LC, lung cancer.
Mean values and standard deviation of cleaved caspase-3 protein content in the gastrocnemius muscle of the different study groups of mice, as measured by optical densities in arbitrary units (OD, a.u.). Statistical significance is represented as follows: *, p<0.05 between 7-day I, 15-day I, LC 21-days, LC 30-days animals and the NI-control mice. Definition of abbreviations: p62, nucleoporin 62; LC3B, light chain 3 isoform B; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; MW, molecular weight; kDa, kilodalton; NI, non-immobilized; I, immobilization; LC, lung cancer.
3. Dicussion
Further studies will have to disentangle the biological mechanisms whereby disuse muscle atrophy enhances the loss of muscle mass and function in cancer cachexia models, both in patients and animals. As far as we are concerned, the current investigation addresses a novel relevant question that has been answered using novel experimental approaches in mice. Besides, the experimental conditions carried out herein reproduce to a great extent clinical scenarios of cancer-induced cachectic patients who may be exposed to prolonged periods of bed rest to receive different therapies (surgery, chemotherapy, etc.).
A significantly greater decline in gastrocnemius weight and limb strength was also observed in the cachectic mice exposed to hindlimb unloading than in non-immobilized cachectic mice. In addition, atrophy of fast-twitch myofibers along with higher levels of muscle damage including systemic troponin levels were also seen in cancer cachectic mice exposed to hindlimb unloading (especially the 15-day cohort). Moreover, protein levels of E3 ligases, particularly atrogin-1, proteasome content, total protein ubiquitination, and autophagy were also significantly larger in the cancer cachectic mice exposed to unloading, even in animals bearing to the shorter cohort.
References
- Tang, J.; Curull, V.; Ramis-Cabrer, D.; Duran, X.; Rodríguez-Fuster, A.; Espases, R.A.; Barreiro, E. EL Peso Corporal Preoperatorio y la Albúmina Predicen la Supervivencia en Pacientes con Neoplasias de Pulmón Resecables: EL Papel de la Epoc. Bronconeumol. 2020, doi:10.1016/j.arbres.2020.07.021.
- Gea, J.; Martínez-Llorens, J. Muscle Dysfunction in Chronic Obstructive Pulmonary Disease: Latest Developments. Bronconeumol. 2019, 55, 237–238, doi:10.1016/j.arbres.2018.07.016.
- González-Marrón, A.; Martín-Sánchez, J.C.; Garcia-Alemany, F.; Martínez-Martín, E.; Matilla-Santander, N.; Cartanyà-Hueso, À.; Vidal, C.; García, M.; Martínez-Sánchez, J.M. Estimation of the risk of lung cancer in women participating in a population-based breast cancer screening program. Bronconeumol. 2020, 56, 277–281, doi:10.1016/j.arbr.2020.03.001.
- Rocha, P.; Arriola, E. Immunotherapy is Here to Stay: A New Treatment Paradigm in Lung Cancer. Bronconeumol. 2019, 55, 124–125, doi:10.1016/j.arbres.2018.08.012.
- Schmidt, S.F.; Rohm, M.; Herzig, S.; Berriel Diaz, M. Cancer Cachexia: More Than Skeletal Muscle Wasting. Trends Cancer 2018, 4, 849–860.
- Chalela, R.; Gea, J.; Barreiro, E. Immune phenotypes in lung cancer patients with COPD: Potential implications for immunotherapy. Thorac. Dis. 2018, 10, doi:10.21037/jtd.2018.06.143.
- Barreiro, E. Models of disuse muscle atrophy: Therapeutic implications in critically ill patients. Transl. Med. 2018, 6, 29–29, doi:10.21037/atm.2017.12.12.
- Barreiro, E.; Bustamante, V.; Cejudo, P.; Gáldiz, J.B.; Gea, J.; de Lucas, P.; Martínez-Llorens, J.; Ortega, F.; Puente-Maestu, L.; Roca, J.; et al. Guidelines for the Evaluation and Treatment of Muscle Dysfunction in Patients with Chronic Obstructive Pulmonary Disease. Bronconeumol. 2015, 51, doi:10.1016/j.arbr.2015.04.027.
- Man, W.D.-C.; Kemp, P.; Moxham, J.; Polkey, M.I. Skeletal muscle dysfunction in COPD: Clinical and laboratory observations. Sci. 2009, 117, 251–264, doi:10.1042/CS20080659.
- Maltais, F.; Decramer, M.; Casaburi, R.; Barreiro, E.; Burelle, Y.; Debigaŕe, R.; Richard Dekhuijzen, P.N.; Franssen, F.; Gayan-Ramirez, G.; Gea, J.; et al. An official American thoracic society/european respiratory society statement: Update on limb muscle dysfunction in chronic obstructive pulmonary disease. J. Respir. Crit. Care Med. 2014, 189, doi:10.1164/rccm.201402-0373ST.
- Shrikrishna, D.; Patel, M.; Tanner, R.J.; Seymour, J.M.; Connolly, B.A.; Puthucheary, Z.A.; Walsh, S.L.F.; Bloch, S.A.; Sidhu, P.S.; Hart, N.; et al. Quadriceps wasting and physical inactivity in patients with COPD. Respir. J. 2012, 40, 1115–1122, doi:10.1183/09031936.00170111.
- Laveneziana, P.; Verges, S.; Barreiro, E. Relevance of Respiratory Muscle Function Assessment in Respiratory Disease. Bronconeumol. 2020, 56, 549–550.
- Gea, J.; Pascual, S.; Castro-Acosta, A.; Hernández-Carcereny, C.; Castelo, R.; Márquez-Martín, E.; Montón, C.; Palou, A.; Faner, R.; Furlong, L.I.; et al. The BIOMEPOC Project: Personalized Biomarkers and Clinical Profiles in Chronic Obstructive Pulmonary Disease. Bronconeumol. 2019, 55, 93–99, doi:10.1016/j.arbres.2018.07.026.
- Jaitovich, A.; Barreiro, E. Skeletal muscle dysfunction in chronic obstructive pulmonary disease what we know and can do for our patients. J. Respir. Crit. Care Med. 2018, 198, doi:10.1164/rccm.201710-2140CI.
- Barreiro, E. Impact of Physical Activity and Exercise on Chronic Obstructive Pulmonary Disease Phenotypes: The Relevance of Muscle Adaptation. Bronconeumol. 2019, 55, 613–614, doi:10.1016/j.arbres.2019.04.024.
- Barreiro, E.; Bustamante, V.; Curull, V.; Gea, J.; López-Campos, J.L.; Munoz, X. Relationships between chronic obstructive pulmonary disease and lung cancer: Biological insights. Thorac. Dis. 2016, 8, doi:10.21037/jtd.2016.09.54.
- Gordon, B.S.; Kelleher, A.R.; Kimball, S.R. Regulation of muscle protein synthesis and the effects of catabolic states. J. Biochem. Cell Biol. 2013, 45, 2147–2157.
- Chacon-Cabrera, A.; Fermoselle, C.; Salmela, I.; Yelamos, J.; Barreiro, E. MicroRNA expression and protein acetylation pattern in respiratory and limb muscles of Parp-1-/-and Parp-2-/-mice with lung cancer cachexia. Biophys. Acta Gen. Subj. 2015, 1850, doi:10.1016/j.bbagen.2015.09.020.
- Chacon-Cabrera, A.; Mateu-Jimenez, M.; Langohr, K.; Fermoselle, C.; García-Arumí, E.; Andreu, A.L.; Yelamos, J.; Barreiro, E. Role of PARP activity in lung cancer-induced cachexia: Effects on muscle oxidative stress, proteolysis, anabolic markers, and phenotype. Cell. Physiol. 2017, 232, doi:10.1002/jcp.25851.
- Bonaldo, P.; Sandri, M. Cellular and molecular mechanisms of muscle atrophy. DMM Dis. Model. Mech. 2013, 6, 25–39.
- Chacon-Cabrera, A.; Fermoselle, C.; Urtreger, A.J.; Mateu-Jimenez, M.; Diament, M.J.; de Kier Joffé, E.D.B.; Sandri, M.; Barreiro, E. Pharmacological Strategies in Lung Cancer-Induced Cachexia: Effects on Muscle Proteolysis, Autophagy, Structure, and Weakness. Cell. Physiol. 2014, 229, 1660–1672, doi:10.1002/jcp.24611.
- Salazar-Degracia, A.; Blanco, D.; Vilà-Ubach, M.; de Biurrun, G.; de Solórzano, C.O.; Montuenga, L.M.; Barreiro, E. Phenotypic and metabolic features of mouse diaphragm and gastrocnemius muscles in chronic lung carcinogenesis: Influence of underlying emphysema. Transl. Med. 2016, 14, 244, doi:10.1186/s12967-016-1003-9.
- Lang, S.M.; Kazi, A.A.; Hong-Brown, L.; Lang, C.H. Delayed recovery of skeletal muscle mass following hindlimb immobilization in mTOR heterozygous mice. PLoS ONE 2012, 7, e38910, doi:10.1371/journal.pone.0038910.
- Chacon-Cabrera, A.; Lund-Palau, H.; Gea, J.; Barreiro, E. Time-Course of muscle mass loss, damage, and proteolysis in gastrocnemius following unloading and reloading: Implications in chronic diseases. PLoS ONE 2016, 11, doi:10.1371/journal.pone.0164951.
- Chacon-Cabrera, A.; Gea, J.; Barreiro, E. Short- and Long-Term Hindlimb Immobilization and Reloading: Profile of Epigenetic Events in Gastrocnemius. Cell. Physiol. 2017, 232, doi:10.1002/jcp.25635.
- Arentson-Lantz, E.J.; English, K.L.; Paddon-Jones, D.; Fry, C.S. Fourteen days of bed rest induces a decline in satellite cell content and robust atrophy of skeletal muscle fibers in middle-aged adults. Appl. Physiol. 2016, 120, 965–975, doi:10.1152/japplphysiol.00799.2015.
- Guitart, M.; Lloreta, J.; Mañas-Garcia, L.; Barreiro, E. Muscle regeneration potential and satellite cell activation profile during recovery following hindlimb immobilization in mice. Cell. Physiol. 2018, 233, doi:10.1002/jcp.26282.