Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Patrícia C. Pires and Version 2 by Lindsay Dong.
Most drugs used for the treatment of depression, anxiety and related disorders have low absorption, high metabolism, low brain targeting and/or low water solubility, which can make it hard to formulate them at high strength and can also lead to decreased bioavailability. Incorporating these drugs into nanometric emulsions can solve these issues. Nanometric emulsions were able to increase drug strength up to 20,270-fold (compared to aqueous solubility). The formulations showed droplet size, polydispersity index, zeta potential, viscosity, osmolality, pH, in vitro drug release and ex vivo drug permeation as adequate for the intended effect and administration route.
- anxiety
- brain delivery
- depression
- microemulsion
- nanoemulsion
1. Introduction
Depressive disorders are some of the most prevalent, impairing and costly illnesses, having recently been estimated to affect more than 246 million people worldwide [1][2][3][1,2,3]. Although they can be divided according to subtype and level of severity, these disorders are generally characterized by a depressed mood or general loss of pleasure or interest, usually accompanied by symptoms such as feelings of guilt, worthlessness or hopelessness; low self-esteem; indecisiveness or difficulty in concentrating or thinking; fatigue; psychomotor agitation or retardation; change in appetite; insomnia or hypersomnia; mood swings; and, in most severe cases, recurrent thoughts of death or suicidal ideation (Figure 1).
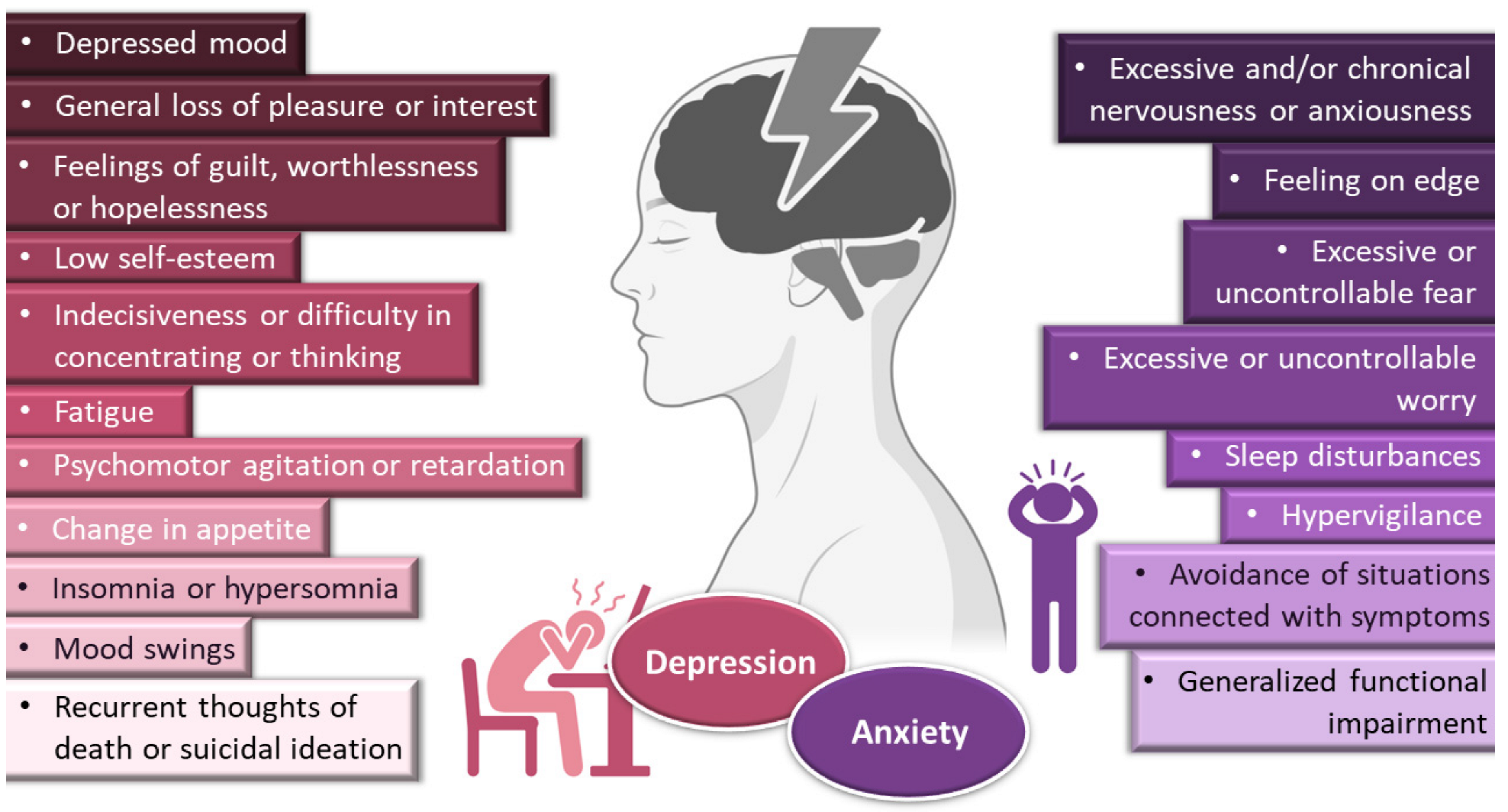
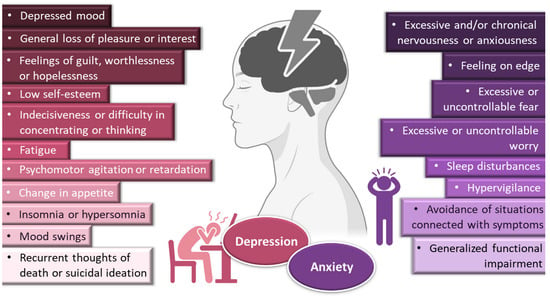
Figure 1.
General symptoms of depression and anxiety disorders. Drawn with BioRender (no copyright required).
Depression frequently coexists with other mental health disorders. There is a significantly increased risk of developing a comorbid depressive disorder when someone already has an anxiety disorder [1][4][5][6][7][1,5,9,10,11]. Anxiety disorders are also among the most common mental disorders, having been recently estimated to globally affect more than 265 million people. They are associated with substantial functional impairment, which leads to decreased work productivity and quality of life [2][5][8][2,9,12]. Aside from generalized anxiety disorder, there is a wide spectrum of related disorders (such as obsessive-compulsive, posttraumatic stress, panic and social anxiety disorders), but in general symptoms can include feeling exceptionally or chronically nervous, anxious or on edge; having excessive or uncontrollable fear and worry; sleep disturbances and hypervigilance; and constant avoidance of situations that relate to the previously mentioned symptoms (Figure 1) [5][7][9][10][9,11,13,14]. Patients with anxiety disorders also have a higher prevalence of other diseases, such as cardiovascular, respiratory and gastrointestinal conditions [5][8][9,12]. Treatment of anxiety and related disorders includes psychological and pharmacological options, and the choice again depends on patient related factors, such as severity of illness, prior treatment, comorbid disorders, patient preference and motivation, etc. [5][9]. The first-line pharmacological options are similar to those prescribed for depressive disorders: either selective serotonin reuptake inhibitors (escitalopram, fluoxetine, fluvoxamine, paroxetine, sertraline) or serotonin and norepinephrine reuptake inhibitors (duloxetine, venlafaxine), for being generally better tolerated and safer than other treatments [5][8][9,12]. Other options include noradrenergic and specific serotonergic antidepressants, tricyclic antidepressants, monoamine oxidase inhibitors, and reversible inhibitors of monoamine oxidase A [5][7][9,11]. Benzodiazepines can also be used, but as adjunctive short-term therapy, since they can cause dependency, sedation and cognitive impairment (especially with prolonged use) [5][8][9,12]. Some anticonvulsants and atypical antipsychotics have also demonstrated efficacy, but are generally recommended as second-line, third-line, or adjunctive therapies (due to side effects). Given the variety in treatment options, again it should be a case-by-case approach, taking into consideration efficacy versus safety, the specific characteristics of the anxiety disorder, comorbid conditions and treatment duration [5][9].
Yet, despite pharmacological treatment options for depressive and anxiety disorders being many, a great number of these drugs (including the grand majority of new drug candidates) have low water solubility, which can make it hard to formulate them at high strengths in liquid preparations [11][15]. This problem can be tackled by formulating these molecules into solid forms, with oral tablets being the most common option, but dose adjustment can sometimes be difficult, and inappropriate tablet splitting can lead to dose intake variation, which in turn can result in a reduction in treatment efficacy or exacerbation of adverse effects. Moreover, swallowing these formulations can be challenging, especially in the younger population (children and adolescents) or older individuals (particularly if having diseases linked to dysphagia, such as stroke, Parkinson’s, Alzheimer’s or cancer) [12][13][16,17]. Intravenous treatments require liquid solutions, but drug solubilization is usually achieved either by pH adjustments in the formulation, which if very low or very high could be potentially harmful, or using great amounts of organic cosolvents or surfactants, which are potentially toxic excipients, having been reported to cause hemotoxicity and hypersensitivity reactions (pruritus, erythema, rash or urticaria) [11][15]. Moreover, in these types of formulations, drugs are highly susceptible to metabolism, which can occur in all administration routes, but especially systemic ones, due to hepatic first-pass metabolism, being aggravated in oral administration, due to additional gastrointestinal degradation [14][18]. Additionally, in general, the treatment of central nervous systems disorders can be compromised by the very low permeability of the blood-brain barrier, which restricts the transport of most drug molecules, and this is especially relevant for the most common administration routes, in which the drug is transported to the brain from the bloodstream (oral and intravenous) [14][15][18,19].
Incorporating drugs into a nanosystem can be an effective strategy to tackle these issues. Nanosystems (also known as nanocarriers) are colloidal structures with a mean diameter of less than 500 nm. Among their many advantages, they allow: the enhancement of drug solubilization; metabolic and chemical degradation drug protection; the reduction of high plasma protein binding; increased permeability through biological membranes; and the promotion of brain bioavailability, which is especially useful for diseases with a brain etiology [15][16][17][18][19][19,20,21,22,23] (Figure 2). The many types of nanosystems can be divided into four main categories: polymeric nanocarriers, such as polymeric nanoparticles and micelles; lipid nanoparticles, such as solid lipid nanoparticles or nanostructured lipid carriers; liposomes and their derived counterparts, such as niosomes, ethosomes, transfersomes, cubosomes and phytosomes; and nanometric emulsions, such as nanoemulsions and microemulsions [14][20][21][22][18,24,25,26]. Yet, despite all mentioned advantages, most of these nanosystems have several drawbacks, such as a low encapsulation efficiency; reduced physical stability; requiring the use of organic solvents during preparation; requiring complex and time-consuming preparation methods; and having non-biocompatible components [23][24][25][27,28,29] (Figure 2).
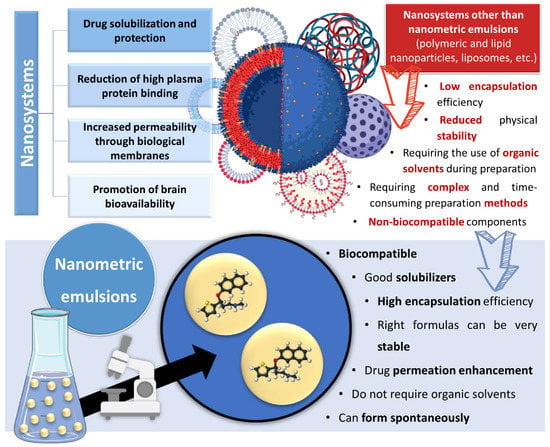

Figure 2. Advantages and disadvantages of drug encapsulation into nanosystems, with a focus on the superiority of nanometric emulsions. Drawn with BioRender (no copyright required).
However, nanometric emulsions can surpasses all of these drawbacks. Being colloidal liquid-in-liquid dispersions, they are usually made of a water phase, an oil, a surfactant, a cosurfactant and/or a cosolvent. They can be classified according to droplet size, between nanoemulsions (20,200 nm) or microemulsions (10–100 nm), although the size range can differ between scholars. Regarding what concerns the differences in their characteristics, while microemulsions have thermodynamic stability, nanoemulsions have a relatively high kinetic stability, and both have higher surface area and free energy than macroemulsions, which makes them more physically stable in comparison. They can also be classified according to the nature of their internal and external phases, as: oil-in-water (o/w) or water-in-oil (w/o), if they are biphasic (most common); or oil-in-water-in-oil or water-in-oil-in-water, if they are triphasic (Figure 3) [14][26][27][18,30,31].

Figure 3. Types of nanometric emulsions, according to the nature of their internal and external phases. Drawn with BioRender (no copyright required).
2. Nanometric Emulsions
2.1. Nanometric Emulsions through Intravenous Administration—The Fastest Way to Achieve Systemic Drug Delivery
Intravenous administration involves injecting drugs directly into the bloodstream, which results in the fastest systemic drug delivery, being ideal for the treatment of acute and emergency situations. Moreover, since it bypasses any physical, chemical or biological barrier that might hinder drug absorption, it leads to the highest systemic bioavailability (theoretically 100%) among all delivery routes [28][32]. Nevertheless, this type of administration has its disadvantages, mostly due to the invasiveness of the injection, which can cause substantial discomfort or pain, and consequently decrease patient compliance, also having an associated risk of injury (and sometimes even infection) at the administration site. The intravenous route also requires trained personnel and, consequently, hospitalization, which is a major limitation [28][29][32,33]. In what concerns formulation characteristics, intravenous preparations should be sterile, isotonic (osmolality around 290 mOsmol/kg) and euhydric (physiological pH), in order to avoid local damage on vascular endothelium and circulating blood cells. These preparations should also have a low viscosity (up to 15 or 20 cP), since they should be easily drawn into a syringe and injected from it, and high viscosity intravenous formulations have been linked to blood viscosity increase, and consequently cardio or cerebrovascular adverse events [30][34].
