Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Conner Chen and Version 1 by Elena Kaznacheyeva.
Quinazoline derivatives are a large pool of natural and synthetic compounds. The first derivatives of quinazoline were synthesized at the end of the 19th century. one quinazoline derivative (4-N-[2-(4-phenoxyphenyl)ethyl]quinazoline-4,6-diamine)—EVP4593 (also marked as QNZ) was originally synthesized in 2003 as a modulator of the nuclear factor kappa B (NF-κB) signal transduction pathway. Since that time, EVP4593 has been widely used as a blocker of NF-κB signaling (Sigma-Aldrich, cat #481417). Further it has been reported the ability of EVP4593 to affect store-operated calcium channels. Here we discussed molecular mechanisms underlying the EVP4593 functioning in the cells.
- quinazoline derivatives
- QNZ
- EVP4593
- calcium signaling
- NF-κB signaling
1. EVP4593 and NF-κB Signaling
The nuclear factor kappa B (NF-κB) signaling pathway is one of the general signal transduction pathways that mediate gene expression of proinflammatory and antiapoptotic factors, cell proliferation, differentiation, adhesion, migration and angiogenesis [14][1]. The hyperactivation of NF-κB signaling is a hallmark of many types of cancer [14][1] and inflammatory-associated pathologies [14,15][1][2]. Additionally, the chronic inflammation associated with upregulation of NF-κB signaling is a common feature of multiple neurodegenerative diseases [16][3]. Thus, the search for selective blockers of NF-κB signaling is an attractive task for both anticancer and anti-neurodegenerative disorders’ drug discovery. In 2003, Tobe et al. synthesized a novel pool of quinazoline derivatives that could act as antagonists of the NF-κB signaling pathway [8,9][4][5]. Analyzing various quinazoline core derivatives, Tobe et al. revealed the most effective compound, EVP4593 (4-N-[2-(4-phenoxyphenyl)ethyl]quinazoline-4,6-diamine), blocking NF-κB signaling at nanomolar concentrations (IC50 11 nM) [9][5]. However, even almost 20 years later, the specific molecular target for EVP4593 remains unclear. It has been established that the EVP4593 does not directly influence any key protein involved in NF-κB signaling, including protein kinase C (PKC), IκB kinase (IKK) complex and transcriptional factor NF-κB (Figure 1) [10][6]. Therefore, it was assumed that the molecular target of the EVP4593 should be located upstream of NF-κB signaling. The key upstream activators of NF-κB signaling are receptors of interleukins, tumor necrosis factor, lipopolysaccharides and calcium ions [14][1]. It is well-known that calcium influx through store-operated calcium (SOC) channels triggers the activation of the NF-κB cascade [9,10,11][5][6][7] (Figure 1). Moreover, established store-operated calcium entry (SOCE) blockers SKF96365 [17][8] and BTP2 [18][9] were reported to suppress NF-κB activity. The hypothesis that EVP4593 affects SOC channels, thus modulating NF-κB signaling, was proven for the first time in 2011 by Wu et al. [10][6].
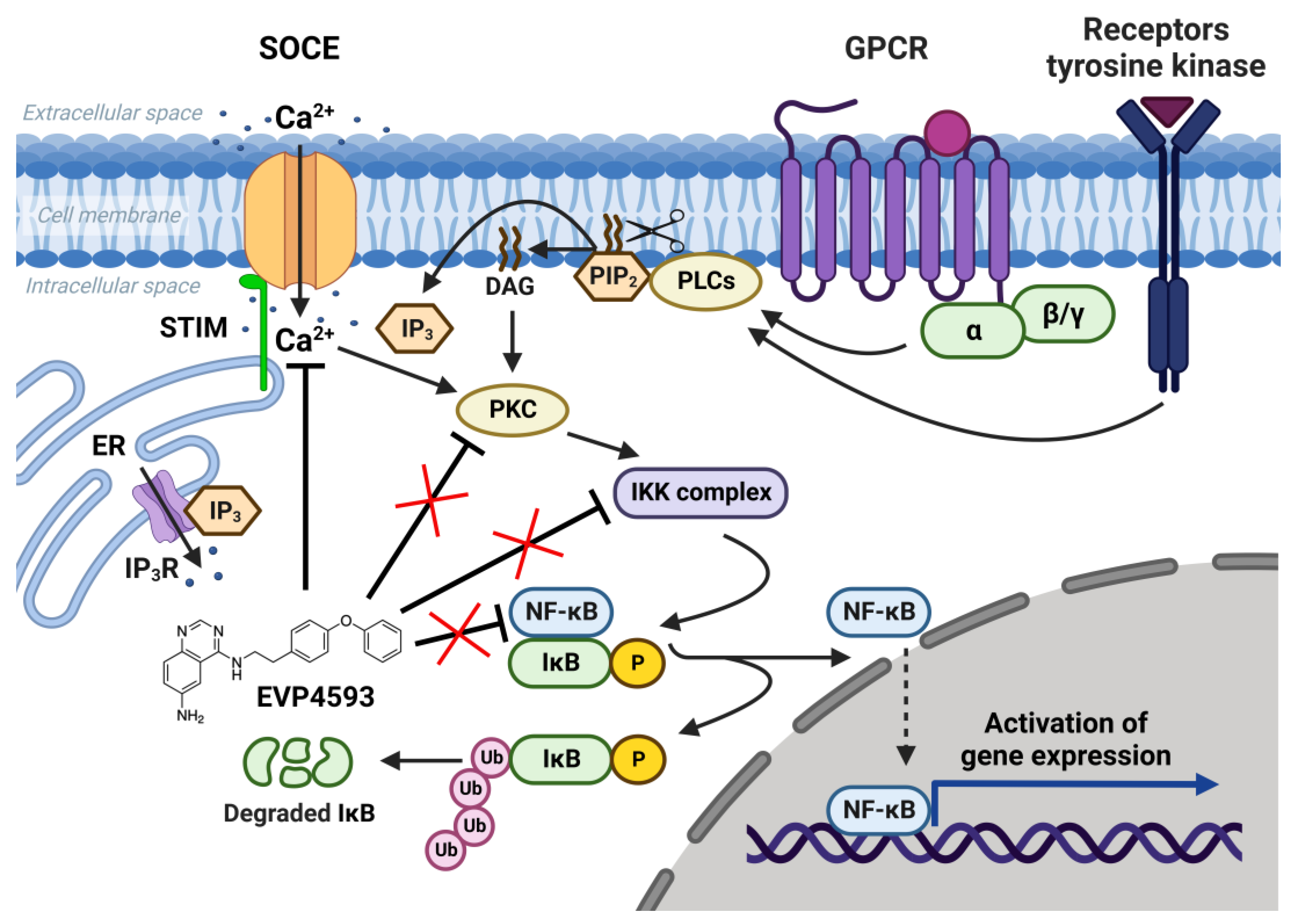
Figure 1. NF-κB signaling pathway and SOCE. SOC channels are activated as a result of intracellular calcium store depletion caused by the activation of inositol-1,4,5-trisphosphate receptor (IP3R). Calcium influx through SOC channels activates protein kinase C (PKC), which activates the IκB kinase (IKK) complex containing the kinases IKKα and IKKβ and the regulatory protein IKKγ. Activated IKK complex phosphorylates inhibitory protein IκB and recruits NF-κB dimers (p50, p52, p65/RelA, RelB and c-Rel). NF-κB free from inhibitory protein IκB migrate to the nucleus and initiate gene expression. EVP4593 does not directly affect any key protein of the NF-κB signal pathway but inhibits SOCE, which is required for NF-κB activation.
2. EVP4593 and SOCE
In 2011, it was shown for the first time that EVP4593 at a concentration of 300 nM can reversibly block SOC channels and reduce pathologically elevated SOCE in an Huntington’s disease (HD) neuroblastoma cell model, expressing full-length mutant huntingtin (138Q), and in striatal neurons isolated from HD-specific YAC128 mice [10][6]. The current–voltage relationships of the registered SOC currents corresponded to the SOC channels formed by the transient receptor potential canonical (TRPC) proteins [10][6]. Moreover, suppression of TRPC1 significantly reduced SOC currents in SK-N-SH cells, modeling HD; at that point, the remaining currents were almost insensitive to further inhibition by EVP4593. Nevertheless, EVP4593 failed to inhibit SOC currents in SK-N-SH cells with overexpressed TRPC1 [10][6]. Therefore, it was concluded that EVP4593 affects heteromeric channels containing TRPC1 as one of the subunits but not homomeric TRPC1 channels. Besides SOC channels’ inhibitory activity, EVP4593 was shown to delay a progression of a motor phenotype in the fly model of HD [10][6]. Thus, SOC channels (especially TRPC1-containing) have become a potential molecular target for EVP4593, and EVP4593 was established as a promising pharmacological substance for HD treatment.
At the same time, the future studies of HD-specific induced pluripotent stem cells (iPSCs)-based GABAergic medium spiny neurons (MSNs) obtained from adult onset HD patients (40–47 glutamine residues in the polyglutamine tract of mutant huntingtin) demonstrated that both Orai- and TRPC-contained SOC channels were sensitive to treatment by 100 nM EVP4593 [19][10]. Thus, it has been supposed that the molecular target for EVP4593 may represent a common regulator of SOC channels, such as STIM1 or STIM2 proteins (Figure 2).
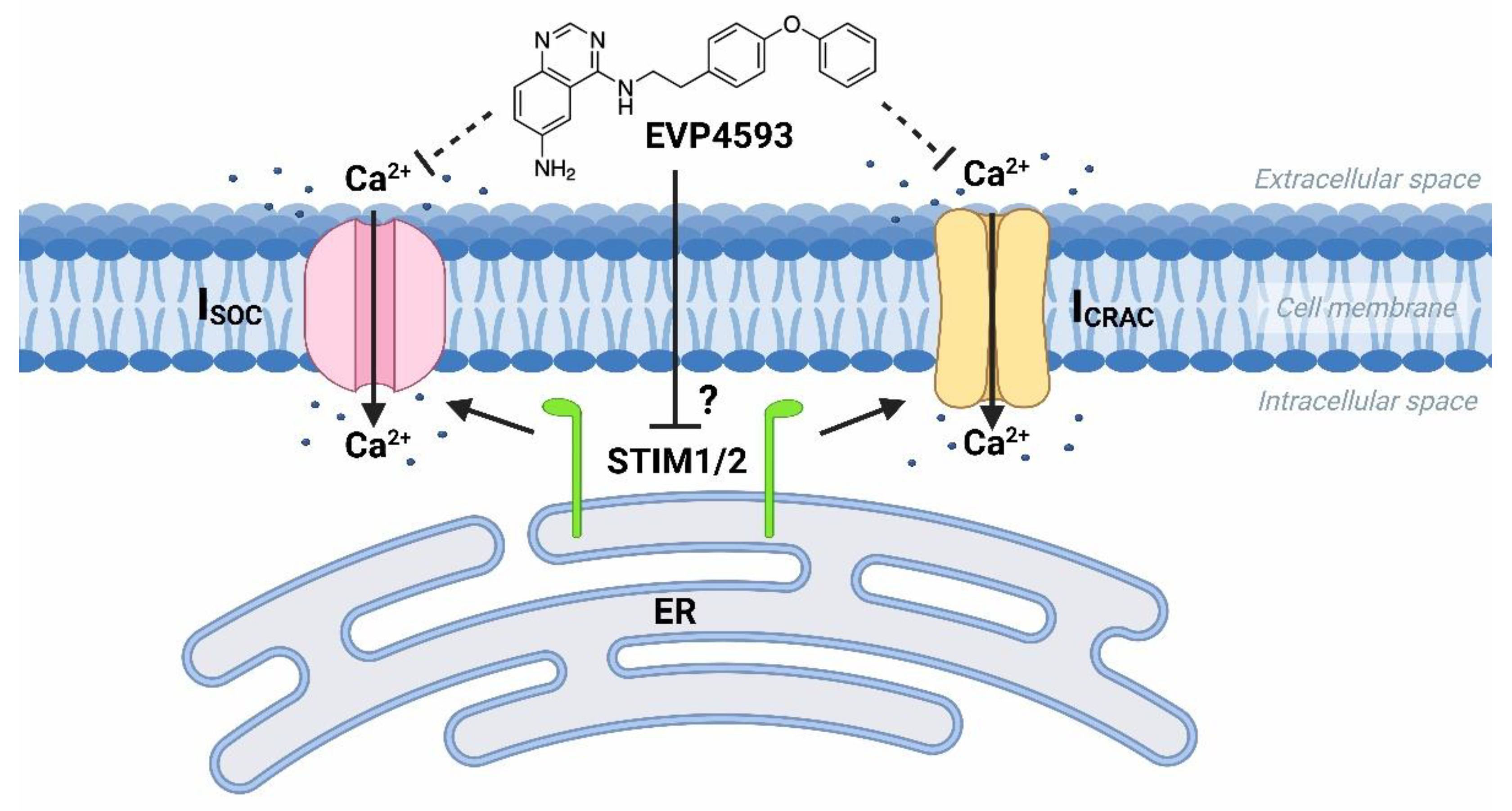
Figure 2. EVP4593 inhibits both Orai- and TRPC-contained store-operated calcium channels. EVP4593 blocks currents through both types of SOC channels that are Orai-contained (ICRAC) and TRPC-contained (ISOC).
Additional experimental data obtained by using fluorescent calcium imaging (the method’s description is available in [20][11]) indicated that EVP4593 could not discriminate between STIM1 and STIM2 in HEK293 cells, affecting SOC in both STIM1 and STIM2 knockout cell lines (Figure 3).
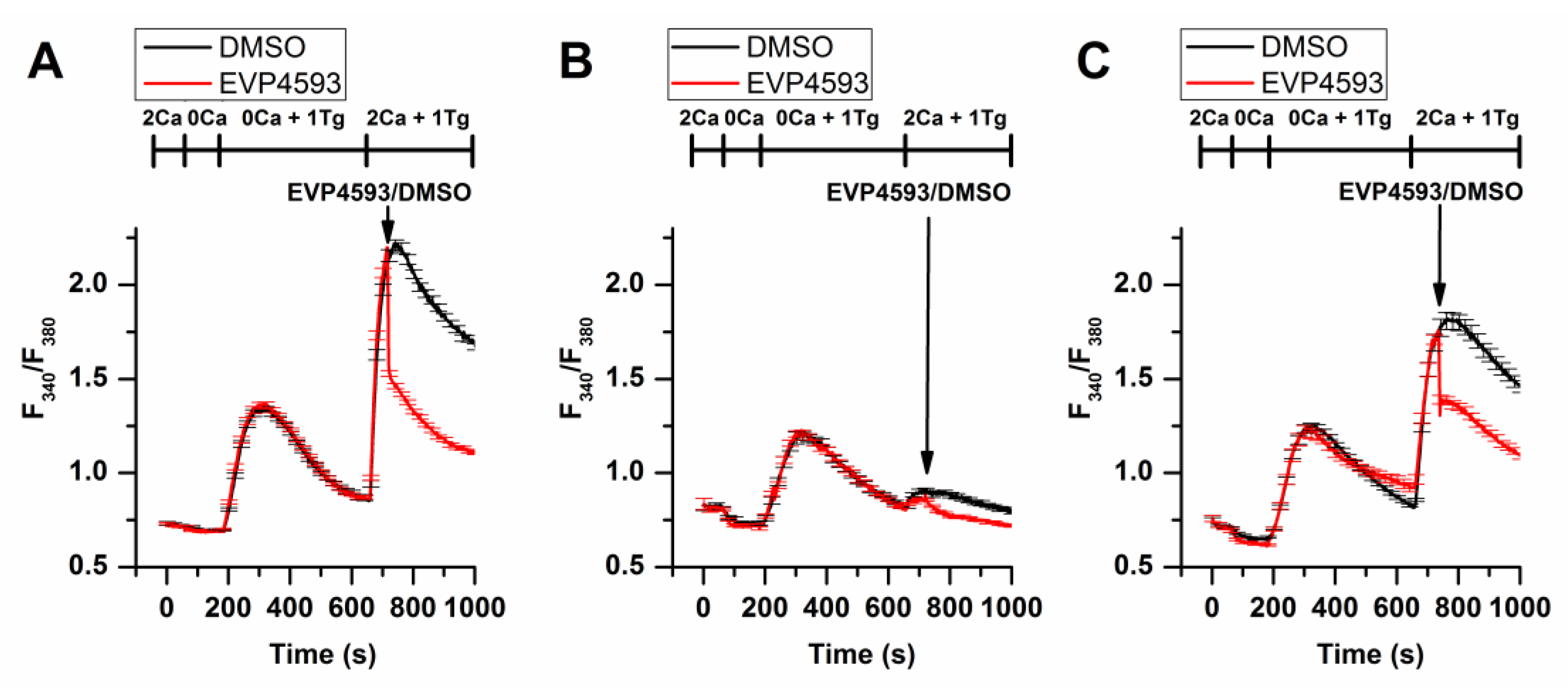
Figure 3. EVP4593 suppresses SOCE in both STIM1 (STIM1KO) and STIM2 (STIM2KO) knockout HEK293 cell lines. Normalized relative fluorescence of Fura-2AM associated with cytosolic calcium level in HEK293 (A), HEK293 STIM1KO (B) and HEK STIM2KO (C) cells during thapsigargin-induced calcium response. The arrow indicates the supply of 1µM EVP4593 or 0.1% DMSO, respectively. The used solutions are indicated above the curves. The curves are plotted as the mean ± SEM.
3. EVP4593 and Mitochondrial Complex I
In addition to the well-studied effects of EVP4593 on SOCE and NF-κB signaling, Robin Krishnathas and colleagues identified mitochondrial complex I as a novel target for EVP4593 [21][12]. EVP4593 has been shown to exhibit strong inhibitory activity against mitochondrial complex I at nanomolar concentration. Molecular docking predictions illustrated that EVP4593 may incorporate into the quinone binding site, thus inhibiting mitochondrial complex I [22][13]. However, EVP4593 targeting mitochondrial complex I did not explain the inhibitory effect on SOCE and NF-κB signaling. Perhaps there are some independent targets for EVP4593. It should be noted that the velocity of the EVP4593 effects detected in experiments does not unequivocally conclude which target is primary from between mitochondrial complex I or SOC channels.
Mitochondrial complex I dysfunctions are also associated with oncogenesis and some neurodegenerative disorders [23,24,25][14][15][16]. Thus, EVP4593 can be successfully used in the treatment of cancer and neurodegenerative diseases, with a suitable overlap in effects on SOCE and mitochondrial complex I.
4. EVP4593 and mTOR Signaling
mTOR (Mechanistic/Mammalian Target of Rapamycin) signaling controls a wide range of processes involved in cellular metabolism and plays a crucial role in autophagy regulation [26,27][17][18]. Aberrant mTOR signaling is associated with a number of pathologies. Hyperactivated mTOR signaling marks some types of cancer and promotes proliferation and tumor progression [26][17]. mTOR signaling inhibitors are considered promising anticancer drugs [28][19]. Indeed, the promising therapeutic strategy is the suppression of the mTOR signaling pathway and, consequently, the enhancement of autophagy [27][18]. Ran Marciano, Manu Prasad and colleagues firstly found that EVP4593 inhibited the mTOR pathway in tumor cells growing under glucose starvation but not under normal conditions [29][20]. This fact indicates the absence of direct interaction of EVP4593 with the components of mTOR signaling. At the same time, low levels of phosphorylated-4EBP1, phosphorylated-P70S6K and phosphorylated-S6RP under EVP4593 treatment indicates inhibition of the mTORC1 pathway [29][20]. Since EVP4593 may inhibit mitochondrial complex I, the tumor cells pretreated by EVP4593 may have a significantly lower level of ATP under glucose deprivation, which leads to an inhibition of the AMPK/mTORC1 pathway and suppression of tumor progression. Additionally, EVP4593 inhibits the NF-ĸB pathway that promotes survival under glucose starvation (as well as other NF-ĸB inhibitors) [30][21]. Thus, EVP4593 promotes the selective death of the most glucose-dependent tumor cells.
The upregulation and involvement of the mTOR signaling pathway in neurodegenerative pathologies, including Huntington’s disease, is excellently discussed in the review by Professor Henry Querfurth [27][18]. The autophagy induction by mTOR signaling antagonists is accompanied by the removal of mutant protein aggregates and may protect neurons from degeneration [27][18]. Moreover, it was also reported that other NF-κB inhibition may reduce the number of autophagosomes [32][22]. Possible controversies can be potentially solved because the reduced number of autophagosomes may indicate the high efficacy of autophagy, whereas a large number of autophagosomes may be a result of autophagy block and the accumulation of “pre-autophagosomes”.
The connection between mTORC1 signaling and SOCE is interesting. The data suggest that mTORC1 signaling is a positive regulator of SOCE [33,34,35][23][24][25]. Additionally, SOCE inhibition led to enhanced autophagy probably through the Akt/mTOR signaling pathway [36,37][26][27]. Furthermore, mTORC1 is a positive regulator of NF-κB signaling [38][28]. mTORC1 may phosphorylate IKKα/β and induce the transcriptional activity of NF-κB [38][28]. Resveratrol, which is known to inhibit NF-κB and mTOR signaling, reduce SOCE in preincubation experiments and also enhance autophagy [39][29]. However, the role of calcium signaling in the regulation of autophagy remains complicated [40][30]. Therefore, for the long-term effect of EVP4593, the following axis can be formed: inhibition of mitochondrial complex I—inhibition of the AMPK/mTORC1 pathway (or Akt/mTOR pathway)—inhibition NF-κB signaling—inhibition of SOCE (Figure 4). Nevertheless, EVP4593 also demonstrates an acute inhibitory SOCE effect confirmed by patch-clamp experiments and fluorescent calcium imaging [10,19,31,41][6][10][31][32] (Figure 3 and Figure 4).
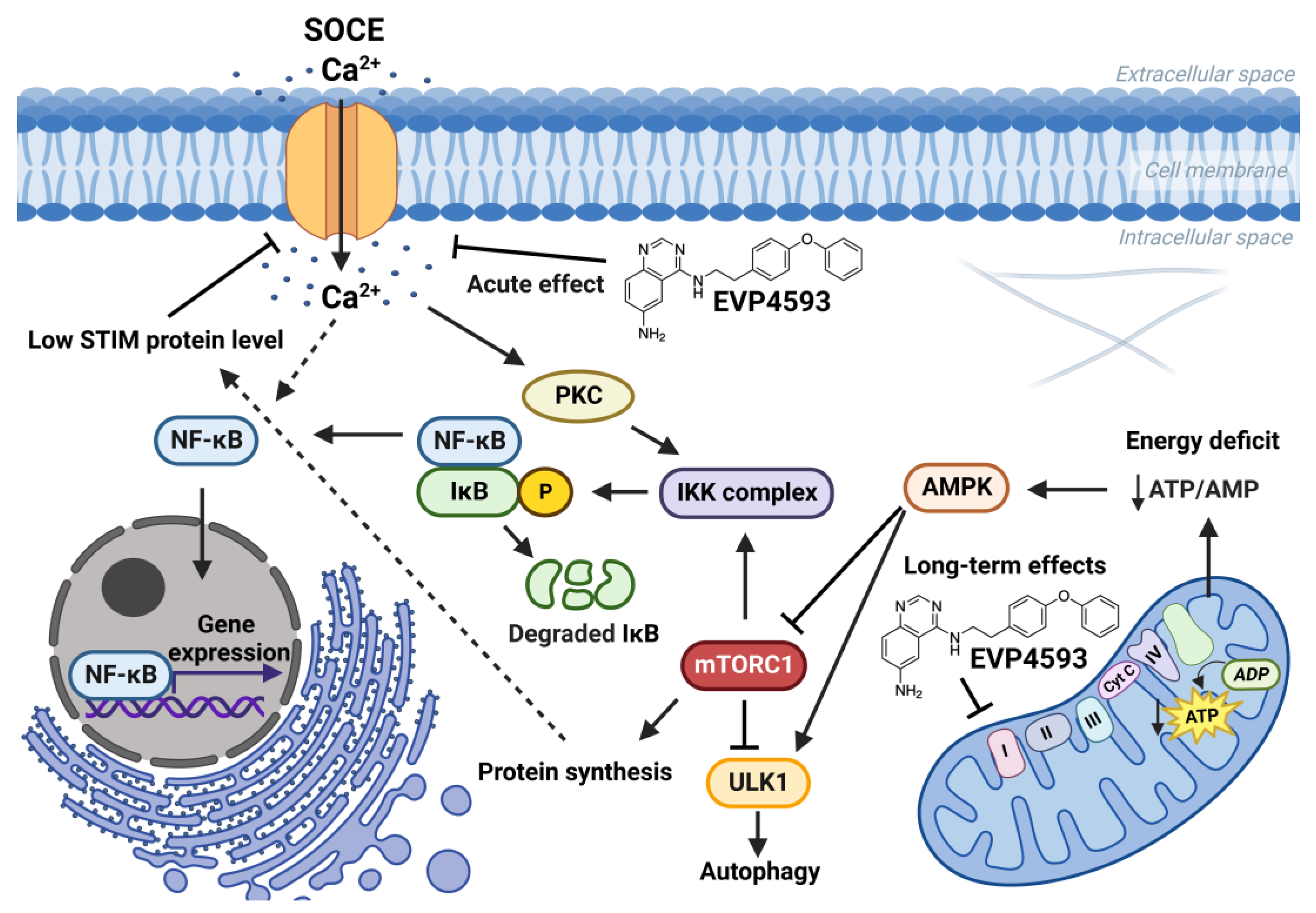

Figure 4. Effects of EVP4593 on cell signaling. EVP4593 has both acute and long-term effects. The acute effect is to directly inhibit SOCE. Long-term effects may be associated with the inhibition of the mitochondrial complex I. Energy deficit induced by EVP4593 inhibits the AMPK/mTORC1 pathway or activates Unc-51 such as autophagy activating kinase 1 (ULK1), which leads to enhanced autophagy and suppressed protein synthesis. Additionally, EVP4593 can negatively affect NF-κB signaling by inhibiting SOCE or mTORC1 signaling. Consequently, EVP4593 also suppresses the expression of NF-κB -dependent genes. The lower levels of STIM proteins due to EVP4593 action may also result in reduced SOCE.
5. EVP4593 and Gene Expression
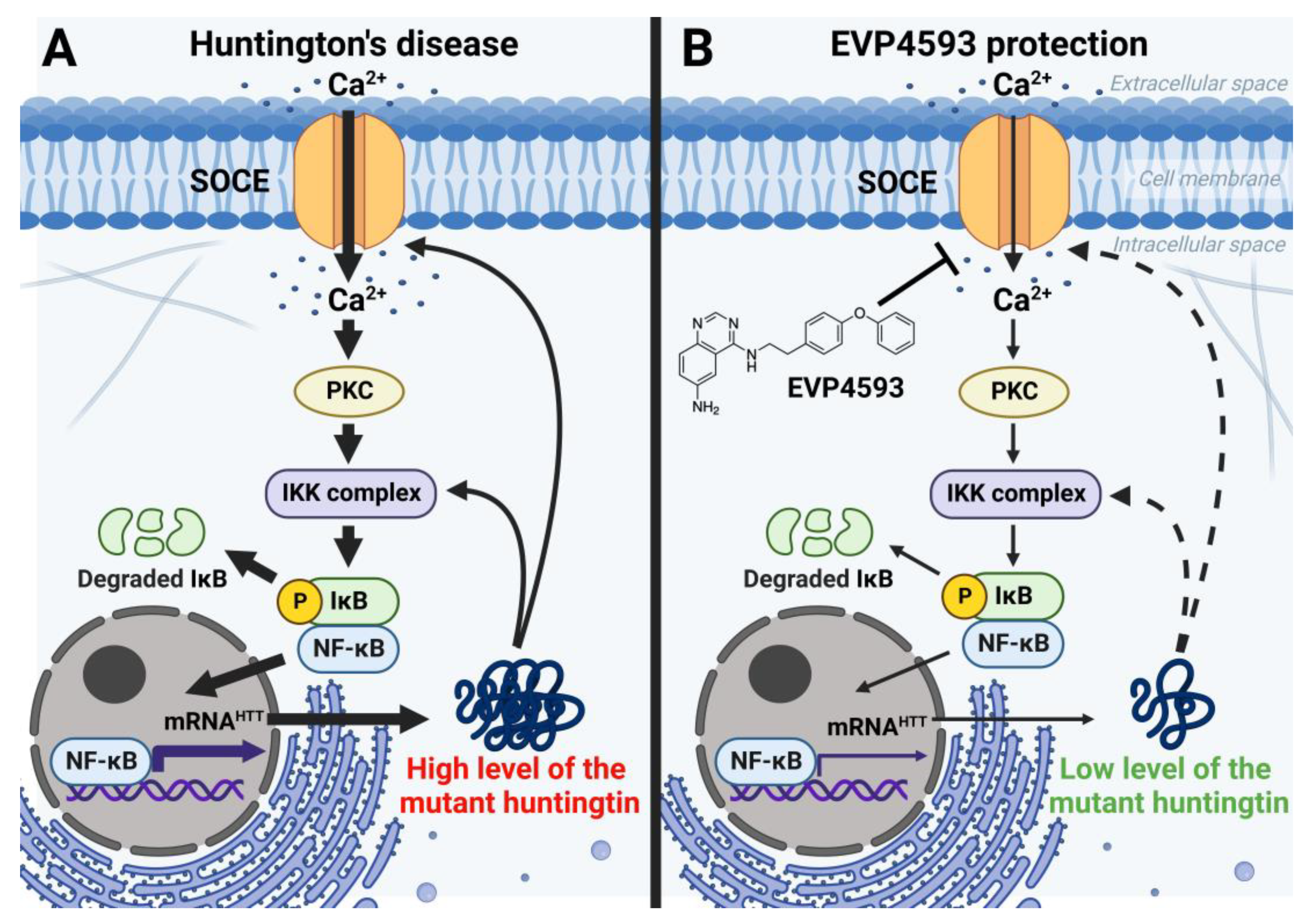
In addition to an acute inhibitory effect of EVP4593 on SOCE, the long-term effects of incubation cells with EVP4593 have also been observed. The modulation of gene expression by EVP4593 is obvious since the expression of many genes depends on NF-κB. Indeed, it was found that incubation of HD-specific MSNs with 300 nM EVP4593 for 24–48 h reduced excessive levels of the huntingtin protein [42][33]. These data were not surprising because the expression of the huntingtin gene depends on NF-κB [43][34] (Figure 5). Notably, modulation of huntingtin expression may have therapeutic applications. It has been shown that even a 10% decrease in mutant huntingtin level has a neuroprotective effect, while even a 90% decrease in normal huntingtin level has no pathological effect [44][35].
Figure 5. EVP4593 reduces the level of the huntingtin protein. Huntingtin gene expression is regulated by NF-κB-dependent promoter/enhancer. (A) Elevated SOCE in HD induces hyperactivation of NF-κB signaling, resulting in a high level of the huntingtin protein. Mutant huntingtin enhances calcium influx through SOC channels and potentiates the IKK complex. Thus, a pathological vicious circle is formed. (B) EVP4593 attenuates pathologically enhanced SOCE and decreases NF-κB-dependent huntingtin production.
What is interesting is that proteins, encoded by NF-κB-independent genes, may also change their levels upon treatment by EVP4593. SOC channels’ activator STIM2, which has a high level associated with excessive SOCE in HD-specific neurons [42][33], can also be downregulated by the application of EVP4593, thus protecting neurons from toxic calcium influx. Another research group reported that excessive STIM2-dependent SOC channels’ activity appears to lead to spine loss in YAC128 (HD mice model) MSN [45][36], confirming the key role of STIM2 at a high level in HD pathogenesis and establishing STIM2 as a promising target for anti-HD drugs. On the other hand, it is extremely important that EVP4593 reduces excessive STIM2 levels to control values since it has been reported that downregulation of STIM2 can be dangerous because STIM2-dependent stability of mushroom spines was shown to be a mechanism of hippocampal synaptic loss in a mice model of Alzheimer’s disease [46][37]. Curiously, despite the expression of the STIM2 encoding gene, it does not depend on NF-κB; another blocker of NF-κB signaling, wogonin, can also reduce STIM2 levels [47][38]. Wogonin also inhibits the mTOR pathway and can have a similar effect as rapamycin on the STIM protein level [48][39].
References
- Zhang, Q.; Lenardo, M.J.; Baltimore, D. 30 Years of NF-κB: A Blossoming of Relevance to Human Pathobiology. Cell 2017, 168, 37–57.
- Jamornwan, S.; Chokpanuwat, T.; Uppakara, K.; Soodvilai, S.; Saengsawang, W. Anti-Inflammatory Activity of Panduratin A against LPS-Induced Microglial Activation. Biomedicines 2022, 10, 2587.
- Srinivasan, M.; Lahiri, D.K. Significance of NF-κB as a pivotal therapeutic target in the neurodegenerative pathologies of Alzheimer’s disease and multiple sclerosis. Expert. Opin. Ther. Targets 2015, 19, 471–487.
- Tobe, M.; Isobe, Y.; Tomizawa, H.; Nagasaki, T.; Takahashi, H.; Hayashi, H. A novel structural class of potent inhibitors of NF-kappa B activation: Structure-activity relationships and biological effects of 6-aminoquinazoline derivatives. Bioorg. Med. Chem. 2003, 11, 3869–3878.
- Tobe, M.; Isobe, Y.; Tomizawa, H.; Nagasaki, T.; Takahashi, H.; Fukazawa, T.; Hayashi, H. Discovery of quinazolines as a novel structural class of potent inhibitors of NF-kappa B activation. Bioorg. Med. Chem. 2003, 11, 383–391.
- Wu, J.; Shih, H.P.; Vigont, V.; Hrdlicka, L.; Diggins, L.; Singh, C.; Mahoney, M.; Chesworth, R.; Shapiro, G.; Zimina, O.; et al. Neuronal store-operated calcium entry pathway as a novel therapeutic target for Huntington’s disease treatment. Chem. Biol. 2011, 18, 777–793.
- Choi, S.; Kim, J.H.; Roh, E.J.; Ko, M.J.; Jung, J.E.; Kim, H.J. Nuclear factor-kappaB activated by capacitative Ca2+ entry enhances muscarinic receptor-mediated soluble amyloid precursor protein (sAPPalpha) release in SH-SY5Y cells. J. Biol. Chem. 2006, 281, 12722–12728.
- Zhu, M.; Lv, B.; Ge, W.; Cui, Z.; Zhao, K.; Feng, Y.; Yang, X. Suppression of store-operated Ca2+ entry regulated by silencing Orai1 inhibits C6 glioma cell motility via decreasing Pyk2 activity and promoting focal adhesion. Cell Cycle 2020, 19, 3468–3479.
- Dragoni, S.; Laforenza, U.; Bonetti, E.; Lodola, F.; Bottino, C.; Berra-Romani, R.; Carlo Bongio, G.; Cinelli, M.P.; Guerra, G.; Pedrazzoli, P.; et al. Vascular endothelial growth factor stimulates endothelial colony forming cells proliferation and tubulogenesis by inducing oscillations in intracellular Ca2+ concentration. Stem Cells 2011, 29, 1898–1907.
- Vigont, V.; Nekrasov, E.; Shalygin, A.; Gusev, K.; Klushnikov, S.; Illarioshkin, S.; Lagarkova, M.; Kiselev, S.L.; Kaznacheyeva, E. Patient-Specific iPSC-Based Models of Huntington’s Disease as a Tool to Study Store-Operated Calcium Entry Drug Targeting. Front. Pharmacol. 2018, 9, 696.
- Grekhnev, D.A.; Novikova, I.V.; Krisanova, A.V.; Yuskovets, V.N.; Chernov, N.M.; Yakovlev, I.P.; Kaznacheyeva, E.V.; Vigont, V.A. Dithiadiazole derivative 3-(4-nitrophenyl)-5-phenyl-3H-1,2,3,4-dithiadiazole-2-oxide—Novel modulator of store-operated calcium entry. Biochem. Biophys. Res. Commun. 2022, 626, 38–43.
- Krishnathas, R.; Bonke, E.; Dröse, S.; Zickermann, V.; Nasiri, H.R. Identification of 4-N-quinazoline-4,6-diamine as a novel, highly potent and specific inhibitor of mitochondrial complex I. Medchemcomm 2017, 8, 657–661.
- Kurelac, I.; Cavina, B.; Sollazzo, M.; Miglietta, S.; Fornasa, A.; De Luise, M.; Iorio, M.; Lama, E.; Traversa, D.; Nasiri, H.R.; et al. NDUFS3 knockout cancer cells and molecular docking reveal specificity and mode of action of anti-cancer respiratory complex I inhibitors. Open Biol. 2022, 12, 220198.
- Bassal, M.A.; Samaraweera, S.E.; Lim, K.; Benard, B.A.; Bailey, S.; Kaur, S.; Leo, P.; Toubia, J.; Thompson-Peach, C.; Nguyen, T.; et al. Germline mutations in mitochondrial complex I reveal genetic and targetable vulnerability in IDH1-mutant acute myeloid leukaemia. Nat. Commun. 2022, 13, 2614.
- Jain, S.; Hu, C.; Kluza, J.; Ke, W.; Tian, G.; Giurgiu, M.; Bleilevens, A.; Campos, A.R.; Charbono, A.; Stickeler, E.; et al. Metabolic targeting of cancer by a ubiquinone uncompetitive inhibitor of mitochondrial complex I. Cell Chem. Biol. 2022, 29, 436–450.
- Hu, Q.; Wang, G. Mitochondrial dysfunction in Parkinson’s disease. Transl. Neurodegener. 2016, 5, 14.
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976.
- Querfurth, H.; Lee, H.K. Mammalian/mechanistic target of rapamycin (mTOR) complexes in neurodegeneration. Mol. Neurodegener. 2021, 16, 44.
- Occhiuzzi, M.A.; Lico, G.; Ioele, G.; De Luca, M.; Garofalo, A.; Grande, F. Recent advances in PI3K/PKB/mTOR inhibitors as new anticancer agents. Eur. J. Med. Chem. 2022, 246, 114971.
- Marciano, R.; Prasad, M.; Ievy, T.; Tzadok, S.; Leprivier, G.; Elkabets, M.; Rotblat, B. High-Throughput Screening Identified Compounds Sensitizing Tumor Cells to Glucose Starvation in Culture and VEGF Inhibitors In Vivo. Cancers 2019, 11, 156.
- Mauro, C.; Leow, S.C.; Anso, E.; Rocha, S.; Thotakura, A.K.; Tornatore, L.; Moretti, M.; De Smaele, E.; Beg, A.A.; Tergaonkar, V.; et al. NF-KB controls energy homeostasis and metabolic adaptation by upregulating mitochondrial respiration. Nat. Cell Biol. 2011, 13, 1272–1279.
- Fu, J.; Lu, L.; Wang, H.; Hou, Y.; Dou, H. Hirsutella sinensis mycelium regulates autophagy of alveolar macrophages via TLR4/NF-κB signaling pathway. Int. J. Med. Sci. 2021, 18, 1810–1823.
- Ogawa, A.; Firth, A.L.; Smith, K.A.; Maliakal, M.V.; Yuan, J.X. PDGF enhances store-operated Ca2+ entry by upregulating STIM1/Orai1 via activation of Akt/mTOR in human pulmonary arterial smooth muscle cells. Am. J. Physiol. Cell. Physiol. 2012, 302, 405–411.
- Peng, H.; Liu, J.; Sun, Q.; Chen, R.; Wang, Y.; Duan, J.; Li, C.; Li, B.; Jing, Y.; Chen, X.; et al. mTORC1 enhancement of STIM1-mediated store-operated Ca2+ entry constrains tuberous sclerosis complex-related tumor development. Oncogene 2013, 32, 4702–4711.
- Vaeth, M.; Maus, M.; Klein-Hessling, S.; Freinkman, E.; Yang, J.; Eckstein, M.; Cameron, S.; Turvey, S.E.; Serfling, E.; Berberich-Siebelt, F.; et al. Store-Operated Ca2+ Entry Controls Clonal Expansion of T Cells through Metabolic Reprogramming. Immunity 2017, 47, 664–679.
- Tang, B.D.; Xia, X.; Lv, X.F.; Yu, B.X.; Yuan, J.N.; Mai, X.Y.; Shang, J.Y.; Zhou, J.G.; Liang, S.J.; Pang, R.P. Inhibition of Orai1-mediated Ca2+ entry enhances chemosensitivity of HepG2 hepatocarcinoma cells to 5-fluorouracil. J. Cell. Mol. Med. 2017, 21, 904–915.
- Chen, Y.W.; Chen, Y.F.; Chen, Y.T.; Chiu, W.T.; Shen, M.R. The STIM1-Orai1 pathway of store-operated Ca2+ entry controls the checkpoint in cell cycle G1/S transition. Sci. Rep. 2016, 6, 22142.
- Li, Y.; Yang, L.; Dong, L.; Yang, Z.W.; Zhang, J.; Zhang, S.L.; Niu, M.J.; Xia, J.W.; Gong, Y.; Zhu, N. Crosstalk between the Akt/mTORC1 and NF-κB signaling pathways promotes hypoxia-induced pulmonary hypertension by increasing DPP4 expression in PASMCs. Acta Pharmacol. Sin. 2019, 40, 1322–1333.
- Selvaraj, S.; Sun, Y.; Sukumaran, P.; Singh, B.B. Resveratrol activates autophagic cell death in prostate cancer cells via downregulation of STIM1 and the mTOR pathway. Mol. Carcinog. 2016, 55, 818–831.
- Decuypere, J.P.; Bultynck, G.; Parys, J.B. A dual role for Ca2+ in autophagy regulation. Cell Calcium. 2011, 50, 242–250.
- Nekrasov, E.D.; Vigont, V.A.; Klyushnikov, S.A.; Lebedeva, O.S.; Vassina, E.M.; Bogomazova, A.N.; Chestkov, I.V.; Semashko, T.A.; Kiseleva, E.; Suldina, L.A.; et al. Manifestation of Huntington’s disease pathology in human induced pluripotent stem cell-derived neurons. Mol. Neurodegener. 2016, 11, 27.
- Vigont, V.; Kolobkova, Y.; Skopin, A.; Zimina, O.; Zenin, V.; Glushankova, L.; Kaznacheyeva, E. Both Orai1 and TRPC1 are Involved in Excessive Store-Operated Calcium Entry in Striatal Neurons Expressing Mutant Huntingtin Exon 1. Front. Physiol. 2015, 6, 337.
- Vigont, V.A.; Grekhnev, D.A.; Lebedeva, O.S.; Gusev, K.O.; Volovikov, E.A.; Skopin, A.Y.; Bogomazova, A.N.; Shuvalova, L.D.; Zubkova, O.A.; Khomyakova, E.A.; et al. STIM2 Mediates Excessive Store-Operated Calcium Entry in Patient-Specific iPSC-Derived Neurons Modeling a Juvenile Form of Huntington’s Disease. Front. Cell Dev. Biol. 2021, 9, 625231.
- Bečanović, K.; Nørremølle, A.; Neal, S.J.; Kay, C.; Collins, J.A.; Arenillas, D.; Lilja, T.; Gaudenzi, G.; Manoharan, S.; Doty, C.N.; et al. A SNP in the HTT promoter alters NF-κB binding and is a bidirectional genetic modifier of Huntington disease. Nat. Neurosci. 2015, 18, 807–816.
- Lu, B.; Palacino, J. A novel human embryonic stem cell-derived Huntington’s disease neuronal model exhibits mutant huntingtin (mHTT) aggregates and soluble mHTT-dependent neurodegeneration. FASEB J. 2013, 27, 1820–1829.
- Wu, J.; Ryskamp, D.A.; Liang, X.; Egorova, P.; Zakharova, O.; Hung, G.; Bezprozvanny, I. Enhanced Store-Operated Calcium Entry Leads to Striatal Synaptic Loss in a Huntington’s Disease Mouse Model. J. Neurosci. 2016, 36, 125–141.
- Chernyuk, D.; Zernov, N.; Kabirova, M.; Bezprozvanny, I.; Popugaeva, E. Antagonist of neuronal store-operated calcium entry exerts beneficial effects in neurons expressing PSEN1ΔE9 mutant linked to familial Alzheimer disease. Neuroscience 2019, 410, 118–127.
- Sukkar, B.; Hauser, S.; Pelzl, L.; Hosseinzadeh, Z.; Sahu, I.; Al-Maghout, T.; Bhuyan, A.A.M.; Zacharopoulou, N.; Stournaras, C.; Schöls, L.; et al. Inhibition of Lithium Sensitive Orai1/ STIM1 Expression and Store Operated Ca2+ Entry in Chorea-Acanthocytosis Neurons by NF-κB Inhibitor Wogonin. Cell. Physiol. Biochem. 2018, 51, 278–289.
- Zhu, Y.; Wang, J. Wogonin increases β-amyloid clearance and inhibits tau phosphorylation via inhibition of mammalian target of rapamycin: Potential drug to treat Alzheimer’s disease. Neurol. Sci. 2015, 36, 1181–1188.
More
