Oxygenated polyunsaturated fatty acids (oxylipins) are bioactive molecules established as important mediators during inflammation. Different classes of oxylipins have been found to have opposite effects, e.g., pro-inflammatory prostaglandins and anti-inflammatory resolvins. Production of the different classes of oxylipins occurs during distinct stages of development and resolution of inflammation. Chronic inflammation is involved in the progression of many pathophysiological conditions and diseases such as non-alcoholic fatty liver disease, insulin resistance, diabetes, and obesity. Determining oxylipin profiles before, during, and after inflammatory-related diseases could provide clues to the onset, development, and prevention of detrimental conditions.
- eicosanoid
- inflammation
- diagnosis
- progression
- mass spectrometry
1. Introduction
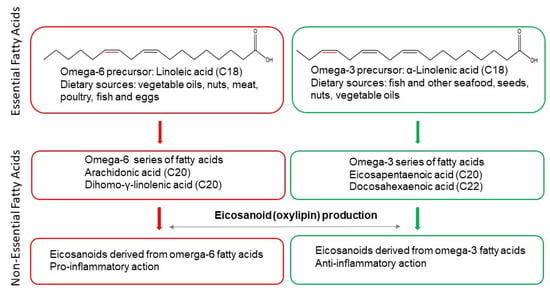
2. Non-Alcoholic Fatty Liver Disease (NAFLD)
3. Obesity and Diabetes
Apart from NAFLD, chronic inflammation is associated with obesity which in turn contributes to the development of insulin resistance and type 2 diabetes (T2D). The plasma level of three eicosanoids (unknown eicosanoid (EIC 62), 8-iso-prostaglandin A1 (8-iso-PGA1), and 12-hydroxy-5,8,10-heptadecatrienoic acid (12-HHTrE) was shown to predict incident T2D [31][48] and the resolvin D2/LTB4 ratio, and may serve as a biomarker of prognosis for ischemic stroke [32][49]. An analysis of clinical samples revealed significant differences in the levels of four oxylipins (PGF2α, PGE2, 15-keto-PGE2, and 13,14-dihydro-15-keto-PGE2) between T2D patients and the corresponding lean and obese control subjects, with the combination of PGF2α and 15-keto-PGE2 having the most predictive value [33][50]. Targeted lipidomics analysis of human urine samples indicated that metabolite products of PGD and PGE are associated with low-grade chronic inflammation in obesity [34][51]. Upregulated PGE2 production by β-cells may have a role in the β-cells’ adaptation response to obesity and insulin resistance in T2D when PGE2 and its receptor EP3 are highly expressed [35][52]. However, the levels of other oxylipins such as the SPMs and hydroxy-DHA metabolites were shown to be lower in obesity and white adipose tissue inflammation [36][55]. Animal studies revealed that increasing the bioavailability of SPMs such as Maresin-1 minimises inflammation and mediates therapeutic actions [37][56]. It is not surprising therefore that eicosanoids are seen as potential targets to combat obesity. One such approach suggests seeking ways to increase the energy expenditure of thermogenic tissues such as brown and brite adipose tissue. Both 5-HETE and 5,6-EET have been reported to be consistently associated with the abundance of those tissues, though further studies are needed to determine whether those eicosanoids are candidates that affect thermogenic capacity [38][57].4. Technological Advancements
The importance of oxylipins in physiological and pathophysiological processes is underscored by their role in the ability of cells to acquire different functional phenotypes depending on the microenvironment. Omega-3 and omega-6 PUFA-derived oxylipins can modulate the inflammatory phenotype of cells [39][64] by acting as ligands for receptors such as the peroxisome proliferator-activated receptor PPAR [40][65] and prostaglandin E2 (PGE2) receptor PTGER4 [41][66].
The eicosanoid class comprises hundreds of structurally and stereochemically distinct species derived either enzymatically or non-enzymatically from a handful of precursors. It is therefore not surprising that within the group of eicosanoids can be found isomers (e.g., PGE2 and PGF2α) with different biological functions [14]. In addition to their diversity, eicosanoids occupy a small mass range and are found in low nanomolar concentrations in biological samples (e.g., human plasma and murine bone marrow-derived macrophages [42][67]). Thus, their detection and evaluation require methods that are sensitive, selective, and reproducible.
Mass spectrometry is a powerful technique for identifying and quantifying known and unknown analytes, and offers high specificity and selectivity. Technical advances have given mass spectrometry a range of tools to obtain information at the molecular level from samples, such as determining the molecular weight of an analyte, its separation from isomeric and isobaric species, its chemical formula, its molecular structure, and structural information [43][44][45][68,69,70]. Mass spectrometry can be used to investigate a wide range of classes of molecules, including those with small and large molecular weights, volatile and non-volatile, polar and non-polar [46][47][48][71,72,73].
Applications using mass spectrometry are either targeted or untargeted. Untargeted approaches attempt to measure as many compounds as possible in a sample, and can be used to discover unanticipated changes between experimental groups. This information can then be used to generate and test hypotheses, for example by applying targeted approaches which offer a selection of the best possible conditions for the detection, identification, and (absolute or relative) quantitation of particular analytes of choice, and is usually used in follow-up experiments. Targeted approaches may use multiple reaction monitoring (MRM) methods which use the knowledge of the mass of the ionised analyte and its corresponding fragments formed during the mass spectrometry analysis [49][50][74,75].
For lipidomics, liquid chromatography-mass spectrometry (LC-MS) is commonly used. LC separates molecules depending on their hydrophobicity, molecular size, and polarity, and covers a broad range of non-polar and weakly polar analytes [51][52][76,77]. Another separation method that is gaining popularity in metabolomics is ion mobility coupled with mass spectrometry (IMS-MS; IM-MS) (IMS) [42][53][54][55][56][57][58][67,78,79,80,81,82,83].
Recently, supercritical fluid chromatography (SFC) has gained traction as an alternative technique to LC in lipidomics due to its high efficiency [59][60][92,93]. Briefly, SFC uses supercritical fluid such as CO2 as a mobile phase [61][94], which results in high separation efficiency and short separation time [62][63][95,96]. SFC-MS/MS methods have been reported to detect inflammation-related lipids including oxylipins in rats [64][97], as well as for the simultaneous measurement of five AA-derived metabolites (PGD2, PGE2, PGF2α, 6KetoPGF1α and LTB4) in biological samples [65][98]. Thus, SFC coupled to MS is a promising approach for the interrogation of the lipidome.
Novel ionisation approaches have appeared with the development of ambient mass spectrometry, characterised by direct sampling and ionisation of the analytes with no or minimal sample preparation [66][99]. Ambient ionisation MS has been successfully employed to rapidly differentiate bacterial species based on their lipid profiles [67][100]. Mass spectrometry imaging (MSI) developments allow more detailed investigations of biological questions such as the biochemical origin of lipid spatial distribution [68][101]. Examples of MSI techniques include secondary ion mass spectrometry (SIMS), Desorption electrospray ionisation (DESI) and matrix-assisted desorption/ionisation (MALDI). Lipid characterisation is now possible due to technological advances in SIMS [69][102]. DESI-MSI can record 2D distributions of polar lipids in tissue slices at ambient conditions (at atmospheric pressure) [70][103], and has been developed for the simultaneous imaging of polar and non-polar lipids in mouse brain tissue [71][104]. Improvements in MALDI resolution resulted in the identification and localisation of lipids within the kidney, as well as the localisation of lipid droplets with lesion-specific macrophages [72][105]. Thus, further method developments could provide the means to image eicosanoids in a variety of biological samples.
