Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Sergiu-Emil Georgescu and Version 2 by Sirius Huang.
Feeding farm animals with aflatoxin-contaminated feed can cause various severe toxic effects, leading to increased susceptibility to infectious diseases and increased mortality, weight loss, poor performance and reduced reproductive capability. Following ingestion of contaminated foodstuffs, aflatoxins are metabolized and biotransformed differently in animals.
- mycotoxin
- aflatoxin
- toxicity
- metabolism
- swine
- decontamination
1. Introduction
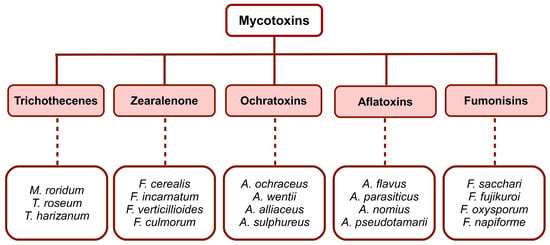
Mycotoxins are toxins produced by certain fungal species. They are classified into five main groups (Figure 1), with specific chemical structures, that occur frequently in foods and feeds, i.e., trichothecenes, zearalenone, ochratoxins, fumonisins and aflatoxins. At the same time, fungi that produce mycotoxins are divided into two groups: those that invade before grain harvesting, a group commonly called field fungi, and those that grow only after harvesting, called storage fungi. Among the field fungi, several types of mycotoxin-producing species can be distinguished. The most important are i. Fusarium graminearum (deoxynivalenol, nivalenol), normally developed on the field plants; ii. Fusarium moniliforme (fumonisins), and sometimes Aspergillus flavus (aflatoxin), present in the case of senile or stressed plants; iii. Penicillium verrucosum (ochratoxin) and A. flavus (aflatoxin) that colonize the plant prior to harvesting, and subsequently predispose the crop to mycotoxin contamination. Mycotoxins are spread in animal feed, cereal crops, vegetables, and animal products. Feeding stuffs for farmed animals are considered as having the highest levels of mycotoxins [1][2][3][4][5][6].
Aflatoxins are a group of secondary metabolites that are produced by several Aspergillus species with increased toxicity and carcinogenic potential. Pigs, poultry and cattle are the most important farm animals affected by aflatoxicosis. The most potent toxicant is AFB1 [7].
Until 1985, the Food and Agriculture Organization reported that approximately 25% of the world’s agricultural production is contaminated with mycotoxins [14]. Taking into consideration the predicted climate change in southeastern Europe, increased cereal contamination with AFB1 and OTA is expected [15]. Contamination with aflatoxins is most predominant in the regions of Africa and Asia, due to climatic conditions that favor the development of aflatoxigenic strains in both field and storage conditions [16][17]. The risks of aflatoxin-contaminated feed depend largely on the age and physiologic status of farm animals.
2. Types of Aflatoxins
Mycotoxins are natural compounds of low molecular weight, up to 500 Da; aflatoxins are considered the most toxic, responsible for a significant decline in agriculture. They represent the most abundant groups found in foodstuffs, oilseeds, cereals, and dairy products [6][18]. All types of aflatoxins are derived from fungal species belonging to the genus Aspergillus and are considered among the most harmful mycotoxins for both animals and humans [19][20][21][22][23].
Aflatoxins are colorless to pale yellow crystalline substances, freely soluble in moderately polar solvents such as chloroform, methanol, dimethyl sulfoxide, with a water solubility of 10–20 μg/mL. In conditions such as under ultraviolet light in the presence of oxygen, extremes of pH < 3 or pH > 10 and oxidizing agents, aflatoxins are unstable. For example, ammonization at high temperatures results in the opening of the lactone ring, generating the decarboxylation of an aflatoxin molecule, an irreversible reaction. Some important physical and chemical properties of aflatoxins are given in Table 1 [20][24][25][26][27][28][29].
Currently, over 20 types of aflatoxins are known and among the best known are B1, B2, G1, G2, M1, M2, aflatoxicol and aflatoxin Q1 (Figure 2). Some of these forms are derivatives or metabolites of animal metabolism. For example, aflatoxin M1 and aflatoxin M2 are the metabolites of aflatoxin B1 and aflatoxin B2 which are found in the milk of lactating mammals fed with aflatoxin-contaminated feed [20][29][30].
Table 1.
Physical and chemical properties of major aflatoxins. Adapted after
.
| Aflatoxin Type | Molecular Formula | Molecular Weight (g /mol) |
Melting Point (°C) | Fluorescence | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| λ Excitation (nm) | λ Emission (nm) | ||||||||||
| B1 | [29] | C | 17 | H | 12 | O | 6 | 312 | 268–269 | 223 | 425 |
| B2 | [29] | C | 17 | H | 14 | O | 6 | 314 | 286–289 | 265 | 425 |
| G1 | [29] | C | 17 | H | 12 | O | 7 | 328 | 244–246 | 243 | 450 |
| G2 | [29] | C | 17 | H | 14 | O | 7 | 330 | 237–240 | 265 | 450 |
| M1 | [33] | C | 17 | H | 12 | O | 7 | 328 | 299 | 365 | 435 |
| M2 | [34] | C | 17 | H | 14 | O | 7 | 330 | 293 | 360 | 450 |
| Aflatoxicol | [32] | C | 17 | H | 14 | O | 6 | 314 | 225 | 325 | 425 |
| Aflatoxin Q1 | [31] | C | 17 | H | 12 | O | 7 | 328 | 250 | 365 | 466 |
2.1. Aflatoxins B1 and B2
Aflatoxin B1 (AFB1) is the most potent carcinogenic mycotoxin naturally produced by Aspergillus species such as A. flavus, A. parasiticus, A. nomius, A. bombycis, A. arachidicola, A. minisclerotigenes, A. ochraceoroseus, A. pseudotamarii and A. rambellii, and it exerts harmful effects on humans and animals. The sensitivity degree and toxicity of AFB1 vary significantly between species, due to differences in its biotransformation. Some animals are considered extremely susceptible to AFB1, especially turkeys, rats, pigs, sheep, and dogs, whereas others such as monkeys, mice and chickens are considered resistant. The LD50 values for aflatoxin B1 are variable, depending on species and sex, with values ranging from 9 to 60 mg of AFB1 per kg of body weight [20][30][35][36][37][38].
Aflatoxin B2 (AFB2) is a blue-fluorescent, toxic secondary metabolite produced by the same species as AFB1, such as A. arachidicola, A. flavus, A. minisclerotigenes, A. nomius and A. parasiticus. This metabolite can be synthesized through multiple sequences that begin with a [2+3]-cycloaddition between quinone and 2,3-dihydrofuran [20][39][40][41].
2.2. Aflatoxins G1 and G2
Aflatoxin G1 (AFG1) and aflatoxin G2 (AFG2) are toxins produced by species of the common soil fungi, A. parasiticus, A. nominus, A. bombyccis, A. arachidicola and A. flavus. The presence of AFG1 is associated with toxicity and hepato-carcinogenicity in human and animal populations, while AFG2 has much lower activity [20][30][42][43].
2.3. Aflatoxins M1 and M2
The aflatoxins M1 (AFM1) and M2 (AFM2) are mammalian bio-conversion products or 4-hydroxy derivatives of AFB1 and AFB2, respectively, produced by A. flavus and A. parasiticus. After entering the body of humans or animals, AFB1 and AFB2 are metabolized by the hepatic microsomal mixed function oxidase system (cytochrome P450) to a reactive epoxide intermediate, but they can be also hydroxylated to the less harmful aflatoxins M1 and M2. In the case of an animal that ingests feed contaminated with AFB1, a percentage between 0.5% and 5% of the toxin ingested is biotransformed in the liver into AFM1. Milk, cheese, and other dairy products contain residues of AFM1 and AFM2 that should not exceed the limit of 50 ng per kg in Europe, 500 ng per kg in the USA, and 100 ng per kg in Iran [20][23][30][44][45][46][47] for human consumption.
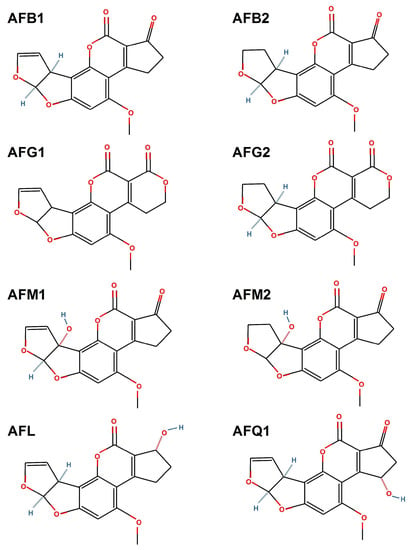
Figure 2. Chemical structures of aflatoxin B1 (AFB1), aflatoxin B2 (AFB2), aflatoxin G1 (AFG1), aflatoxin G2 (AFG2), aflatoxin M1 (AFM1), aflatoxin M2 (AFM2), aflatoxicol (AFL) and aflatoxin Q1 (AFQ1). This image was made in OpenOffice Draw software, v 4.1.9.
2.4. Aflatoxicol
The first report on natural contamination of food with aflatoxicol (AFL) appeared in 1984 [48]. AFL is one of the metabolites of AFB1, formed by the selective reduction of cyclopentanone carbonyl of AFB1, and has two stereoisomers (AFL1 /AFL-A /Ro and AFL2 or AFL-B) which differ by the orientation of the hydroxyl group in the cyclopentene ring. Both AFL forms are produced by the biological reduction catalyzed by enzymes present in fungi, such as: Tetrahymena pyriformis, Trichoderma viride, Dactylium dendroides, Streptococcus lactis, Absidia repens, Mucor griseocyanus, Aspergillus niger, Mucor ambiguus, Tetrahymena pyriformis and Rhizopus spp. Although AFL is eighteen times less toxic than AFB1, it was shown that AFL is carcinogenic and a potent frameshift mutagen [32][49][50][51][52].
2.5. Aflatoxin Q1
Aflatoxin Q1 (AFQ1) is a monohydroxylated derivative of AFB1, being one of the major AFB1 metabolites which appear after incubation of microsomal fraction from the mammalian liver with AFB1. The microsomal fraction is rich in CYP3A4 and other CYP450 enzymes which are responsible for the activation of AFB1 into the epoxide form, and for conversion into a less toxic detoxification metabolite, AFQ1. Initially it was found in the urine of rhesus monkeys orally exposed to AFB1. On the other hand, Yourtee et al. [53] showed that AFQ1 might be a major metabolite in the detoxification pathway of the native mycotoxin. AFQ1 is approximately eighteen times less toxic and approximately eighty-three times less mutagenic than AFB1 [30][53][54][55].
3. Aflatoxins’ Metabolism: Biochemical, Molecular and Cell Signaling Aspects
After ingestion of contaminated food, aflatoxins are absorbed in the intestine; following their distribution, metabolism and excretion, the liver is the first and main organ affected (Figure 3). They also accumulate in muscle. P450 cytochromes play an important role in phase I biotransformation of xenobiotics, especially those belonging to families 1 and 3 [56]. In mammals, the enzymes with the highest levels of protein expression, and involved in the conversion of aflatoxins, are CYP1A2 and CYP3A4. The metabolite resulting from the oxidation reaction can bind to DNA, causing genotoxicity, and proteins generating cytotoxicity. For example, AFB1 binds to guanine residues of nucleic acids, resulting in AFB1 adducts that can lead to transversion of guanine–cytosine (GC) to thymine–adenine (TA) and implicitly to irreversible DNA damage. Binding of AFB1 to proteins is irreversible, the most well-known adduct being ADB1-lysine in albumin. In the first stage of metabolic oxidation in the liver, an epoxy reactive intermediate (e.g., AFB1-8,9-epoxide) is formed or this is hydrolyzed to a less toxic form, AFM1 [57][58].
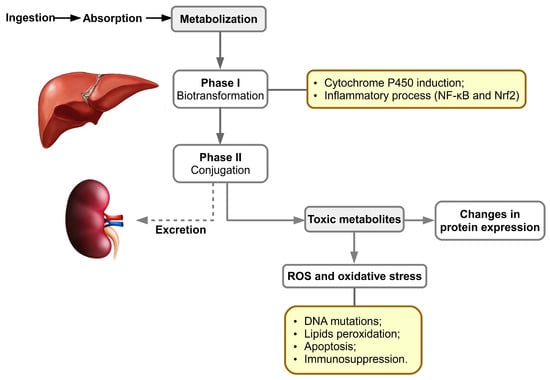
The cytochrome P450 superfamily consists of enzymes involved in xenobiotic metabolism and endogenous compound oxidation; thus, Phase I enzymes catalyze the reactions of hydroxylation, sulphoxidation, epoxidation, N-, O- and S-dealkylation, oxidative aromatic hydroxylation, desulfuration, denitrosation, and dehalogenation aiming for the addition of functional polar group(s). In porcine hepatic tissue, the CYP450 proteins expressed are represented by CYP2A19 (34%), CYP2D25 (25,5%), CYP2C49 (11.2%), CYP2E1 (8.1%), CYP3A39 (8,1%), CYP3A29 (5,8%), CYP2C33 (5%) and CYP1A2 (2.3% of the total liver CYPs, respectively) [62][63][64][65][66][67][68].
Phase II of metabolism implicates conjugation reactions of metabolites previously formed [69] with glucuronic acid and sulfate especially. Subsequently, the epoxide metabolite generated in phase I may be detoxified in phase II by glutathione conjugation, through hydrolysis by an epoxide hydrolase to AFB1-8,9-dihydrodiol, or by reduction to a less toxic metabolite such as AFM1 or AFQ1 [43][70][71][72][73]. The resulting metabolites are excreted through the biliary pathway, followed by the urinary pathway.
By RNA-seq technology it was proved that in vitro exposure of bovine fetal hepatocyte cell line (BFH12) to AFB1 affected the cells` transcriptome. Gap junction protein beta 2 and Follistatin genes—the latter being involved in proliferation and colony expansion of progenitor populations of hepatocytes—as well as those of ornithine decarboxylase and A-Raf proto-oncogene have been upregulated. Instead, genes that codify for tumor suppressors, such as those of collagen type XVIII alpha 1 chain (COL18A1), collagen type 1 alpha 2 chain (COL1A2), as well as that for natriuretic peptide receptor 3 have been downregulated. The treatment with this mycotoxin also upregulated the following CYP isoforms: CYP26B1, CYP3A4, CYP27B1 and downregulated CYP1A1, CYP1B1, CYP19A1, CYP36A1, CYP4B1 [74].
The same study from Pauletto et al. [74] revealed that all analyzed glutathione-S-transferase genes, except those for omega 1 and pi1 isoforms, have been downregulated. The gene sets for TNF-α signaling via NF-kB, oxidative phosphorylation, DNA repair, inflammatory response, KRAS signaling, p53 pathway, PI3K-Akt-mTOR signaling, apoptosis and hypoxia have been upregulated by AFB1 treatment of BFH12 cells. In the same conditions, other gene sets for epithelial–mesenchymal transition, bile acid metabolism, estrogen response and heme metabolism have been downregulated.
Recently, based on transcriptomic data and post translational analyses, it was postulated that Toll-Like Receptor (TLR2) activation is involved in AFB1-induced inflammation and oxidative stress in BFH12 cells [75]. Moreover, in a chicken hepatocarcinoma cell line (LMH) exposed to AFB1 differentially, expression analysis revealed that 1006 genes have been upregulated and 791 downregulated, compared with the control treatment. The mRNA expression of CYP27A1, CYP1A4, FABP2, PPARα and GSTT1 were significantly decreased by this mycotoxin treatment, whereas genes responsible for focal adhesion and MAPK pathways were upregulated compared with control ones [76].
Previously it was noticed that in HepG2 cells treated with AFB1, increases in the expressions of miR-34A and miR-33a-5p led to an important decrease of β-catenin, c-myc and cyclin D1 levels in the Wnt signaling pathway, generating an important risk of hepatocellular carcinoma [77][78]. The exposure to this mycotoxin also inhibits protein synthesis and due to this, enzymes` levels of different metabolic pathways are affected [79].
Recently, it was proved that AFB1 exposure of cells affects the respiratory chain, generating reactive oxygen species (ROS). If these are not counteracted by the antioxidant enzymatic and non-enzymatic systems, oxidative stress occurs [80]. The excess of ROS attacks polyunsaturated fatty acids from glycerophospholipids, generating end products of lipid peroxidation, as well as DNA and proteins. Lipid peroxidation and oxidative damage to DNA play a major role in the toxicity of aflatoxins.
References
- Arroyo-Manzanares, N.; Huertas-Pérez, J.F.; García-Campaña, A.M.; Gámiz-Gracia, L. Aflatoxins in animal feeds: A straightforward and cost-effective analytical method. Food Control 2015, 54, 74–78.
- Pinotti, L.; Ottoboni, M.; Giromini, C.; Dell’Orto, V.; Cheli, F. Mycotoxin contamination in the EU feed supply chain: A focus on Cereal Byproducts. Toxins 2016, 8, 45.
- Senthilkumar, T.; Jayas, D.S.; White, N.D.G.; Fields, P.G.; Gräfenhan, T. Near-Infrared (NIR) hyperspectral imaging: Theory and applications to detect fungal infection and mycotoxin contamination in food products. Indian J. Entomol. 2016, 78, 91.
- Sobral, M.M.C.; Faria, M.A.; Cunha, S.C.; Ferreira, I.M.P.L.V.O. Toxicological interactions between mycotoxins from ubiquitous fungi: Impact on hepatic and intestinal human epithelial cells. Chemosphere 2018, 202, 538–548.
- Tola, M.; Kebede, B. Occurrence, importance and control of mycotoxins: A review. Cogent Food Agric. 2016, 2, 1191103.
- Alshannaq, A.; Yu, J.H. Occurrence, toxicity, and analysis of major mycotoxins in food. Int. J. Environ. Res. Public Health 2017, 14, 632.
- Pleadin, J.; Kovačević, D.; Perković, I. Impact of casing damaging on aflatoxin B 1 concentration during the ripening of dry-fermented meat sausages. J. Immunoass. Immunochem. 2015, 36, 655–666.
- Rheeder, J.P.; Marasas, W.F.O.; Vismer, H.F. Production of Fumonisin Analogs by. Society 2002, 68, 2101–2105.
- Ehrlich, K.C.; Kobbeman, K.; Montalbano, B.G.; Cotty, P.J. Aflatoxin-producing Aspergillus species from Thailand. Int. J. Food Microbiol. 2007, 114, 153–159.
- McCormick, S.P.; Stanley, A.M.; Stover, N.A.; Alexander, N.J. Trichothecenes: From simple to complex mycotoxins. Toxins 2011, 3, 802–814.
- Fakruddin, M.; Chowdhury, A.; Hossain, M.N.; Ahmed, M.M. Characterization of aflatoxin producing Aspergillus flavus from food and feed samples. Springerplus 2015, 4, 1–6.
- Wang, Y.; Wang, L.; Liu, F.; Wang, Q.; Selvaraj, J.N.; Xing, F.; Zhao, Y.; Liu, Y. Ochratoxin A producing fungi, biosynthetic pathway and regulatory mechanisms. Toxins 2016, 8, 83.
- Wentzel, J.F.; Lombard, M.J.; Du Plessis, L.H.; Zandberg, L. Evaluation of the cytotoxic properties, gene expression profiles and secondary signalling responses of cultured cells exposed to fumonisin B1, deoxynivalenol and zearalenone mycotoxins. Arch. Toxicol. 2017, 91, 2265–2282.
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajšlová, J.; Mayar, S.; Krska, R. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’ of 25%. Crit. Rev. Food Sci. Nutr. 2020, 60, 2773–2789.
- Gagiu, V.; Mateescu, E.; Armeanu, I.; Dobre, A.A.; Smeu, I.; Cucu, M.E.; Oprea, O.A.; Iorga, E.; Belc, N. Post-harvest contamination with mycotoxins in the context of the geographic and agroclimatic conditions in Romania. Toxins 2018, 10, 533.
- Shabeer, S.; Asad, S.; Jamal, A.; Ali, A. Aflatoxin Contamination, Its Impact and Management Strategies: An Updated Review. Toxins 2022, 14, 307.
- Kolawole, O.; Siri-Anusornsak, W.; Petchkongkaw, A.; Meneely, J.; Elliott, C. The Efficacy of Additives for the Mitigation of Aflatoxins in Animal Feed: A Systematic Review and Network Meta-Analysis. Toxins 2022, 14, 707.
- Jallow, A.; Xie, H.; Tang, X.; Qi, Z.; Li, P. Worldwide aflatoxin contamination of agricultural products and foods: From occurrence to control. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2332–2381.
- Filazi, A.; Sireli, U.T. Chapter 7. Occurrence of aflatoxins in food. In Aflatoxins: Recent Advances and Future Prospects; Mehdi, R.-A., Ed.; InTech Open Access: London, UK, 2012; pp. 143–170.
- Feddern, V.; Dors, G.C.; Tavernari, F.; Mazzuco, H.; Cunha, J.A.; Krabbe, E.L.; Scheuermann, G.N. Aflatoxins: Importance on animal nutrition. Aflatoxins Recent Adv. Futur. Prospect. InTech Open Access Croat. 2013, 171–195.
- Seetha, A.; Munthali, W.; Msere, H.W.; Swai, E.; Muzanila, Y.; Sichone, E.; Tsusaka, T.W.; Rathore, A.; Okori, P. Occurrence of aflatoxins and its management in diverse cropping systems of central Tanzania. Mycotoxin Res. 2017, 33, 323–331.
- Ismail, A.; Gonçalves, B.L.; de Neeff, D.V.; Ponzilacqua, B.; Coppa, C.F.S.C.; Hintzsche, H.; Sajid, M.; Cruz, A.G.; Corassin, C.H.; Oliveira, C.A.F. Aflatoxin in foodstuffs: Occurrence and recent advances in decontamination. Food Res. Int. 2018, 113, 74–85.
- Negash, D. A Review of Aflatoxin: Occurrence, Prevention, and Gaps in Both Food and Feed Safety. J. Appl. Microbiol. Res. 2018, 1, 1–35.
- Okoth, S. Improving the Evidence Base on Aflatoxin Contamination and Exposure in Africa; CTA Working Paper; 16/13; CTA: Wageningen, The Netherland, 2016; pp. 1–113. Available online: https://hdl.handle.net/10568/90118 (accessed on 23 September 2022).
- MdQuadri, S.H.; Niranjan, M.; Chaluvaraju, K.; Shantaram, U.; Enamul, H. An Overview on Chemistry, Toxicity, Analysis and Control of Aflatoxins. Int. J. Chem. Life Sci. 2017, 2, 1071–1078.
- Sailaja, O.; Krishnaven, G.; Manoranjani, M. Identification and High-performance Liquid Chromatography Quantification of Aflatoxins in Red Chili. Asian J. Pharm. 2017, 2017, 933–937.
- Akeberegn, D.; Alemneh, T.; Zewudie, D. Effects of Aflatoxin Contamination in Milk: A Review. MRJMBS 2018, 6, 118–128.
- Sedova, I.; Kiseleva, M.; Tutelyan, V. Mycotoxins in Tea: Occurrence, Methods of Determination and Risk Evaluation. Toxins 2018, 10, 444.
- Vijaya Kumar, V. Aflatoxins: Properties, Toxicity and Detoxification. Nutr. Food Sci. Int. J. 2018, 6, 555696.
- Bbosa, G.S.; Kitya, D.; Odda, J.; Ogwal-Okeng, J. Aflatoxins metabolism. Health 2013, 5, 14–34.
- Franco, C.M.; Fente, C.A.; Vázquez, B.I.; Cepeda, A.; Mahuzier, G.; Prognon, P. Interaction between cyclodextrins and aflatoxins Q1, M1 and P1. Fluorescence and chromatographic studies. J. Chromatogr. A 1998, 815, 21–29.
- Carvajal, M.; Rojo, F.; Méndez, I.; Bolños, A. Aflatoxin B1and its interconverting metabolite aflatoxicol in milk: The situation in Mexico. Food Addit. Contam. 2003, 20, 1077–1086.
- Behfar, A.; Khorasgani, Z.N.; Alemzadeh, Z.; Goudarzi, M.; Ebrahimi, R.; Tarhani, N. Determination of Aflatoxin M1 Levels in Produced Pasteurized Milk in Ahvaz City by Using HPLC. Jundishapur J. Nat. Pharm. Prod. 2012, 7, 80–84.
- Lee, D.; Lee, K.G. Analysis of aflatoxin M1 and M2 in commercial dairy products using high-performance liquid chromatography with a fluorescence detector. Food Control. 2015, 50, 467–471.
- Marai, I.F.M.; Asker, A.A. Aflatoxins in rabbit production: Hazards and control. Trop. Subtrop. Agroecosystems 2008, 8, 1–28.
- Rawal, S.; Kim, J.E.; Coulombe, R. Aflatoxin B1 in poultry: Toxicology, metabolism and prevention. Res. Vet. Sci. 2010, 89, 325–331.
- Omar, M.H.E.-D. (Ed.) Mycotoxins-Induced Oxidative Stress and Disease. In Mycotoxin and Food Safety in Developing Countries; InTech Open Access: London, UK, 2013; pp. 63–92.
- Ishikawa, A.T.; Hirooka, E.Y.; e Silva, P.L.A.; Bracarense, A.P.F.R.L.; Da Costa, K.K.M.; Akagi, C.Y.; Kawamura, O.; Da Costa, M.C.; Itano, E.N. Impact of a single oral acute dose of aflatoxin b1on liver function/cytokines and the lymphoproliferative response in C57BL/6 mice. Toxins 2017, 9, 374.
- Chang, S.B.; Abdel Kader, M.M.; Wick, E.L.; Wogan, G.N. Aflatoxin B2: Chemical identity and biological activity. Science 1963, 142, 1191–1192.
- Yabe, K.; Ando, Y.; Hamasaki, T. Biosynthetic Relationship among aflatoxins B1, B2, G1 and G2. Appl. Environ. Microbiol. 1988, 54, 2101–2106.
- Zhou, G.; Corey, E.J. Short, enantioselective total synthesis of aflatoxin B2 using an asymmetric -cycloaddition step. J. Am. Chem. Soc. 2005, 127, 11958–11959.
- Wong, J.J.; Hsieh, D.P.H. Mutagenicity of aflatoxins related to their metabolism and carcinogenic potential. Proc. Natl. Acad. Sci. USA 1976, 73, 2241–2244.
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516.
- Galvano, F.; Galofaro, V.; Galvano, G. Occurrence and Stability of Aflatoxin M 1 in Milk and Milk Products: A Worldwide Review. J. Food Prot. 1996, 59, 1079–1090.
- Yu, J. Current understanding on aflatoxin biosynthesis and future perspective in reducing aflatoxin contamination. Toxins 2012, 4, 1024–1057.
- Ahlberg, S.; Grace, D.; Kiarie, G.; Kirino, Y.; Lindahl, J. A risk assessment of Aflatoxin M1 exposure in low and mid-income dairy consumers in Kenya. Toxins 2018, 10, 348.
- Puga-Torres, B.; Salazar, D.; Cachiguango, M.; Cisneros, G.; Gómez-Bravo, C. Determination of aflatoxin M1 in raw milk from different provinces of Ecuador. Toxins 2020, 12, 498.
- Righetti, L.; Rolli, E.; Dellafiora, L.; Galaverna, G.; Suman, M.; Bruni, R.; Dall’Asta, C. Thinking Out of the Box: On the Ability of Zea mays L. to Biotrasform Aflatoxin B1 Into Its Modified Forms. Front. Plant Sci. 2021, 11, 1–11.
- Salhab, A.S.; Edwards, G.S. Comparative in Vitro Metabolism of Aflatoxicol by Liver Preparations from Animals and Humans. Cancer Res. 1976, 37, 1016–1021.
- Schoenhard, G.L.; Hendricks, J.D.; Nixon, J.E.; Lee, D.J.; Wales, J.H.; Sinnhuber, R.O.; Pawlowski, N.E. Aflatoxicol-induced hepatocellular carcinoma in Rainbow Trout (Salmogairdneri) and the synergistic effects of cyclopropenoid fatty acids. Cancer Res. 1981, 41, 1011–1014.
- Nakazato, M.; Morozumi, S.; Saito, K.; Fujinuma, K.; Nishima, T.; Kasai, N. Interconversion of aflatoxin B1 and aflatoxicol by several fungi. Appl. Environ. Microbiol. 1990, 56, 1465–1470.
- Karabulut, S.; Paytakov, G.; Leszczynski, J. Reduction of aflatoxin B1 to aflatoxicol: A comprehensive DFT study provides clues to its toxicity. J. Sci. Food Agric. 2014, 94, 3134–3140.
- Yourtee, D.M.; Bean, T.A.; Kirk-Yourtee, C.L. Human aflatoxin B1metabolism: An investigation of the importance of aflatoxin Q1as a metabolite of hepatic post-mitochondrial fraction. Toxicol. Lett. 1987, 38, 213–224.
- Hendricks, J.D.; Sinnhuber, R.O.; Nixon, J.E.; Wales, J.H.; Masri, M.S.; Hsieh, D.P. Carcinogenic response of rainbow trout (Salmo gairdneri) to aflatoxin Q1 and synergistic effect of cyclopropenoid fatty acids. J. Natl. Cancer Inst. 1980, 64, 523–528.
- Fan, T.S.L.; Zhang, G.S.; Chu, F.S. Production and characterization of antibody against aflatoxin Q1. Appl. Environ. Microbiol. 1986, 47, 526–532.
- Antonissen, G.; Devreese, M.; De Baere, S.; Martel, A.; Van Immerseel, F.; Croubels, S. Impact of Fusarium mycotoxins on hepatic and intestinal mRNA expression of cytochrome P450 enzymes and drug transporters, and on the pharmacokinetics of oral enrofloxacin in broiler chickens. Food Chem. Toxicol. 2017, 101, 75–83.
- Allocati, N.; Masulli, M.; Di Ilio, C.; Federici, L. Glutathione transferases: Substrates, inihibitors and pro-drugs in cancer and neurodegenerative diseases. Oncogenesis 2018, 7, 1–15.
- Carvajal-Moreno, M. Metabolic Changes of Aflatoxin B1 to become an Active Carcinogen and the Control of this Toxin. Immunome Res. 2015, 11, 1.
- Mulero, J.; Martínez, G.; Oliva, J.; Cermeño, S.; Cayuela, J.M.; Zafrilla, P.; Martínez-Cachá, A.; Barba, A. Phenolic compounds and antioxidant activity of red wine made from grapes treated with different fungicides. Food Chem. 2015, 180, 25–31.
- Jarolim, K.; Del Favero, G.; Pahlke, G.; Dostal, V.; Zimmermann, K.; Heiss, E.; Ellmer, D.; Stark, T.D.; Hofmann, T.; Marko, D. Activation of the Nrf2-ARE pathway by the Alternaria alternata mycotoxins altertoxin I and II. Arch. Toxicol. 2017, 91, 203–216.
- Wen, J.; Mu, P.; Deng, Y. Mycotoxins: Cytotoxicity and biotransformation in animal cells. Toxicol. Res. 2016, 5, 377–387.
- Burkina, V.; Rasmussen, M.K.; Oliinychenko, Y.; Zamaratskaia, G. Porcine cytochrome 2A19 and 2E1. Basic Clin. Pharmacol. Toxicol. 2019, 124, 32–39.
- Nannelli, A.; Chirulli, V.; Longo, V.; Gervasi, P.G. Expression and induction by rifampicin of CAR- and PXR-regulated CYP2B and CYP3A in liver, kidney and airways of pig. Toxicology 2008, 252, 105–112.
- Yao, M.; Dai, M.; Liu, Z.; Huang, L.; Chen, D.; Wang, Y.; Peng, D.; Wang, X.; Liu, Z.; Yuan, Z. Comparison of the substrate kinetics of pig CYP3A29 with pig liver microsomes and human CYP3A4. Biosci. Rep. 2011, 31, 211–220.
- Puccinelli, E.; Gervasi, P.G.; La Marca, M.; Beffy, P.; Longo, V. Expression and inducibility by phenobarbital of CYP2C33, CYP2C42, CYP2C49, CYP2B22, and CYP3As in porcine liver, kidney, small intestine, and nasal tissues. Xenobiotica 2010, 40, 525–535.
- Shang, W.; Nuffer, J.H.; Muñiz-Papandrea, V.A.; Colón, W.; Siegel, R.W.; Dordick, J.S. Cytochrome c on silica nanoparticles: Influence of nanoparticle size on protein structure, stability, and activity. Small 2009, 5, 470–476.
- Kojima, M.; Morozumi, T. Cloning of six full-length cDNAs encoding pig cytochrome P450 enzymes and gene expression of these enzymes in the liver and kidney. J. Health Sci. 2004, 50, 518–529.
- Achour, B.; Barber, J.; Rostami-Hodjegan, A. Correction to “Cytochrome P450 pig liver pie: Determination of individual cytochrome P450 isoform contents in microsomes from two pig livers using liquid chromatography in conjunction with mass spectroscopy” (Drug Metabolism and Disposition (2011) 39, (2130-2134)). Drug Metab. Dispos. 2012, 40, 227.
- Lehman-McKeeman, L.D.; Ruepp, S.U. Biochemical and Molecular Basis of Toxicity, 3rd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128098424.
- Gallagher, E.P.; Kunze, K.L.; Stapleton, P.L.; Eaton, D.L. The kinetics of aflatoxin B1oxidation by human cDNA-expressed and human liver microsomal cytochromes P450 1A2 and 3A4. Toxicol. Appl. Pharmacol. 1996, 141, 595–606.
- Devreese, M.; De Backer, P.; Croubels, S. Overview of the most important mycotoxins for the pig and poultry husbandry Overzicht van de meest belangrijke mycotoxines voor de varkens-en pluimveehouderij. Vlaams Diergeneeskd. Tijdschr. 2013, 82, 171–180.
- Dhama, K.; Singh, K.P. Aflatoxins- Hazard to Livestock and Poultry Production: A Review. Vet. Immunol. Immunopathol. 2007, 9, 1–15.
- Diaz, G.J.; Murcia, H.W.; Cepeda, S.M.; Boermans, H.J. The role of selected cytochrome P450 enzymes on the bioactivation of aflatoxin B1 by duck liver microsomes. Avian Pathol. 2010, 39, 279–285.
- Pauletto, M.; Tolosi, R.; Giantin, M.; Guerra, G.; Barbarossa, A.; Zaghini, A.; Dacasto, M. Insights into Aflatoxin B1 Toxicity in Cattle: An in vitro whole-transcriptomic approach. Toxins 2020, 12, 429.
- Iori, S.; Pauletto, M.; Bassan, I.; Bonsembiante, F.; Gelain, M.E.; Bardhi, A.; Barbarossa, A.; Zaghini, A.; Dacasto, M.; Giantin, M. Deepening the Whole Transcriptomics of Bovine Liver Cells Exposed to AFB1: A Spotlight on Toll-like Receptor 2. Toxins 2022, 14, 504.
- Choi, S.Y.; Kim, T.H.; Hong, M.W.; Park, T.S.; Lee, H.; Lee, S.J. Transcriptomic alterations induced by aflatoxin B1 and ochratoxin A in LMH cell line. Poult. Sci. 2020, 99, 5265–5274.
- Fang, Y.; Feng, Y.; Wu, T.; Srinivas, S.; Yang, W.; Fan, J.; Yang, C.; Wang, S. Aflatoxin B1 Negatively Regulates Wnt/β-Catenin Signaling Pathway through Activating miR-33a. PLoS ONE 2013, 8, 1–12.
- Zhu, L.; Gao, J.; Huang, K.; Luo, Y.; Zhang, B.; Xu, W. miR-34a screened by miRNA profiling negatively regulates Wnt/β-catenin signaling pathway in Aflatoxin B1 induced hepatotoxicity. Sci. Rep. 2015, 5, 1–13.
- Caloni, F.; Cortinovis, C. Toxicological effects of aflatoxins in horses. Vet. J. 2011, 188, 270–273.
- Ma, J.; Liu, Y.; Guo, Y.; Ma, Q.; Ji, C.; Zhao, L. Transcriptional profiling of aflatoxin b1-induced oxidative stress and inflammatory response in macrophages. Toxins 2021, 13, 401.
More
