Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by TURKI ABUALAIT and Version 2 by Conner Chen.
Epilepsy is a long-term neurological condition that results in recurrent seizures. Approximately 30% of patients with epilepsy have drug-resistant epilepsy (DRE). The ketogenic diet (KD) is considered an effective alternative treatment for epileptic patients.
- epilepsy
- drug-resistant epilepsy
- biomarkers
- parameters
1. Epilepsy
Epilepsy is bimodally distributed with two peaks at both extremes of life: it is highest in the first year, then incidence drops to adult levels by the age of 10, before incidence rises again in people over the age of 85 years [1][10]. Incidence is higher in low-income countries, and usually above 80–100 per 100,000 persons per year for unknown reasons, but sub-standard health-delivery system, poor hygiene, lack of basic sanitation, and a higher risk of infections and traumatic brain injury may contribute [2][3][11,12].
Epilepsy is defined as: (1) two unprovoked seizures occurring more than 24 h 24 apart; or (2) a single unprovoked seizure if recurrence risk is high (i.e., >60% over the next 10 years) or (3) a diagnosis of an epilepsy syndrome [3][12]. Figure 1 shows the etiologies of epilepsy at various ages [4][13].
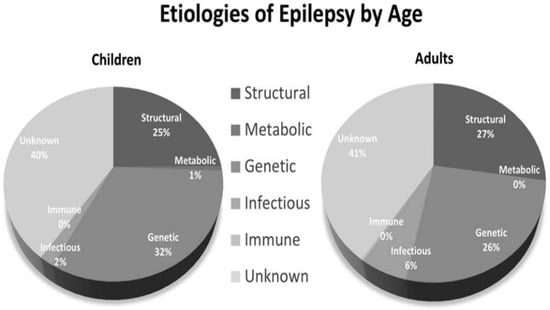
Figure 1.
Etiologies of epilepsy by age.
The process converting a non-epileptic brain into one capable of generating spontaneous, recurrent seizures is known as “Epileptogenesis”. The process is conceptualized to result from an imbalance between excitatory and inhibitory activity within a neuronal network, becomes more disposed to fire in an excessive, hypersynchronous, oscillatory manner which when sustained, disrupts normal neuronal processing, and is capable of recruiting other neuronal networks [1][2][10,11].
During the past several decades, neuroimaging, genomics, and molecular biology have substantially improved our knowledge of the pathophysiology of seizures and epilepsy [5][6][14,15]. Seizure is the main incident indicator found in epilepsy that is related with high persistence pulse, emitted from a set of neurons [7][16]. Seizures can present in various forms; seizures can present with motor symptoms or behavioral changes. Seizures can also happen with the patient aware or unaware [8][17].
2. Prevalence
Epilepsy affects 1–2% of people worldwide [9][10][11][18,19,20]. It is estimated that 23 million Asians suffer from epilepsy, while only 3.3 million Africans are affected by this disease [1][10]. Epilepsy affects both males and females of all ages. Focal seizures are common in children and adults [1][10].
3. Diagnosis
After carful history taking and examination, techniques such as neuroimaging, neurophysiological studies, and lab tests are used in diagnosing epileptic seizures and related disorders [12][21].
4. Mortality
Epilepsy death rates are relatively high in the USA and UK [13][22]. One study has linked epilepsy to 15% of deaths, and it remains unclear how to lessen this risk, while other studies reported 87.5% mortalities due to non-epileptic reasons [14][23]. Age, generalized seizures, and other independent variables raise the risk of death among epileptic patients [14][15][23,24].
5. Epilepsy Therapy
Epilepsy patients must be managed in the aim to become seizure-free. The type of epileptic syndrome determines treatment, as does the patient’s age, gender, and acceptance [16][25].
6. Medicines
Anti-epileptic drugs (AEDs) work by boosting neurotransmitters or decreasing excitatory processes [17][26]. In the US, phenytoin is considered a frequently used AED. Unfortunately, its metabolic role in the liver and random pharmacokinetics is not fruitful for older individuals [17][26].
Although there are several therapies, the treatment of epilepsy is based mainly on drugs, which, depending on the year of coming onto the market are classified as first, second, or third generation. The new-generation (third generation) AEDs may offer better tolerability, milder adverse effects, less drug interactions and improved pharmacokinetic characteristics compared to the conventional AEDs. For this reason, the New-generation AEDs may be used earlier in epileptic patients. Further head-to-head comparisons are needed to determine the exact position of New-generation AEDs relative to conventional AEDs, because, despite advancements and the development of New-generation AEDs, a third of patients with epilepsy remain refractory to pharmacotherapy [18][27].
Nanomaterials or nanomedicine, especially biosensor-based methods, can facilitate the analysis of these agents with unique advantages such as rapid analysis, sensitivity, selectivity, and low cost. Additionally, various chemical and biological modifiers to improve the sensitivity and selectivity of the sensor have been also been categorized [19][28]. These new molecules have been developed in order to provide a pharmaceutical profile and tolerance superior to the previously available drugs, and it is forecast that as their use increases, their true potential and profile will be widen. Furthermore, for the first time in Paediatric Epileptology, the extrapolation of the efficacy data in adults have been also been used (together with specific safety and pharmacokinetic studies in the paediatric population), in order to speed up their approval for use in the child population in upcoming years [20][29].
7. Surgery
Epileptic surgery is a potentially curative treatment for children with refractory seizures. Early epileptic surgery has been emphasized to treat medically intractable epilepsy in children. Seizure reduction results in remarkable developmental and cognitive improvements. Prolonged invasive extraoperative electroencephalography (EEG) or stereoEEG monitoring with depth electrodes and/or subdural grids are usually used for patients with nonlesional MRI or discordant EEG epileptogenic zones [21][31]. Epileptic surgery is among the most successful methods to achieve a seizure-free status [22][23][32,33]. Approximately 50%–80% of patients became seizure-free after surgery [24][34].
8. Dietary Treatment
In drug-resistant epilepsy, diet alteration is an alternative non-pharmacological option to treat epileptic seizures and is widely used to treat glucose transporter type 1 deficiency syndrome (GLUT1 DS), pyruvate dehydrogenase deficiency [25][35]. The ketogenic diet (KD) KD has long been used to treat epileptic seizures [26][36]. The potential of the KD to control epileptic seizures has been known about for a century in medical and research institutes. Additionally, efforts are being made to recognize the KD’s therapeutic role in treating acute and severe metabolic disorders [27][37].
9. Ketogenic Diet
KD is defined as a diet containing a high amount of fat, low in carbohydrates, and with adequate protein content. It was first designed in the 1920s to treat seizures and supplies energy through ketone bodies (KBs) to the brain when the glucose level is lower in the body [28][38]. There are three main KBs: β-hydroxybutyrate (BHB), acetoacetate (ACA), and acetone. KBs act as fuel elements and are mainly formed from fatty acids by the liver during starvation and exercise [29][39]. The medium-chain triglycerides diet (MCTD) consists of high fat content with low glycemic index (LGI) [30][40]. Ketogenesis is a metabolic process that provides the body with an alternative form of energy through the production of KBs [31][41]. In ketogenesis, acetyl-CoA derived from β-oxidation of fatty acids is converted into KBs in the mitochondrial matrix of liver cells and then these ketone bodies are carried to the extrahepatic tissues for alternative energy sources. Adenosine has long been linked to metabolic and neural activity, and studies have proven that a ketogenic diet suppresses seizures by increasing inhibitory effects mediated through adenosine A1 receptors [32][33][8,42].
The ketone bodies, which are derived from fatty acid oxidation and usually produced in fasting state or on high-fat diets, have broad neuroprotective effects [34][43]. It is also suggested that the insulin sensitivity increased during a Ketogenic meal [35][44]. Furthermore, the neuroprotection and homeostasis also promotes the activation of inhibitory adenosine A1 receptors (A1Rs) by dephosphorylating extracellular ATP to adenosine [36][45]. Also, it activates GIRKs, which are G protein-coupled inwardly rectifying K+ channels. KATP channels activation may also be linked to A1R activation by a KD [37][46]. Another molecular relationship exists between the KD and γ-aminobutyric acid (GABA) levels and KATP channel activation through GABAB receptors. KATP channels activation has also been reported by other stimulants such as xanthine, diazoxide, etc. KATP channels play basic roles in nerve, muscle, epithelial, and endocrine tissue physiology and their direct activation regulates pancreatic islet β-cell membrane potential, calcium influx, and insulin secretion, and rectifies drug targets for metabolic disorders of glucose homeostasis [38][47]. Enhanced PIP3 signaling in pro-opiomelanocortin (POMC) neurons causes a KATP channel activation that leads to diet-sensitive obesity. In a mice study, a POMC neurons showed a marked hyperpolarization and a reduction in basal firing rate due to increased ATP-sensitive potassium (KATP) channel activity as well. The KATP blocker (e.g., tolbutamide) restored electrical activity and leptin-evoked firing of POMC neurons in mice. These data indicate that PIP3-mediated signals are critical regulators of the melanocortin system via modulation of KATP channels [39][48]. In another study it was well documented that KATP channel blockers control glucagon secretion by distinct mechanisms i.e., a direct stimulation of α-cells involving a [Ca2+]c rise and an indirect inhibition mediated by somatostatin. By closing α-cell KATP channels, sulfonylureas depolarize α-cells, increase [Ca2+]c, and stimulate glucagon secretion. However, their effects also involve an indirect inhibitory effect via somatostatin (SST) secreted by δ-cells on the glucose concentration [40][41][49,50].
Reactive oxygen species (ROS) may be reduced by metabolic modifications, improving seizure resistance (ROS). Fructose 1,6-bisphosphate administered to rats shifts glucose consumption to the pentose phosphate pathway [42][51].
10. Types of KD
KD is widely used to treat patients with refractory epilepsy or those individuals unfit for surgical management [43][52]. There are four types of KD [44][53].
Classic KD: In the classic KD, the ratio of fat and carbohydrates is 4:1. This ratio can be altered to 3:1 for moderate metabolism activity [45][7].
Medium Chain Triglyceride (MCTD): This modified Atkin diet includes high production of KBs than any other class of fats, such as long-chain triglycerides (LCT) [46][1]. It can lower the intake of fatty acids due to its ketogenic properties and greater carbohydrate and protein content due to its ketogenic properties because it contains high fat content (60%) and lower carbohydrate and protein ratio. Moreover, it also leads to marked alterations in brain energy metabolism, with ketone bodies partly replacing glucose as fuel. Though the phenomena is still not completely understood, it is reported that the ketone body acetone has anticonvulsant activity and could play a role in the seizure protection afforded by the diet [44][53]. In addition to acute seizure protection, the ketogenic diet provides protection against the development of spontaneous recurrent seizures in models of chronic epilepsy, and it has neuroprotective properties in diverse models of neurodegenerative disease [47][48][54,55]. The MCTD diet is more flexible for children than other KD because it increases the growth rate, decreases the requirement for other micronutrients, and has a lower cholesterol ratio [44][53].
Low glycemic index treatment (LGIT): LGIT is a non-restrictive treatment that consists of a diet with an increased amount of fat (60%), a high amount of protein (20 to 30%), and 10% carbohydrates [45][7]. It comprises foods with a low glycemic index (i.e., mutton, few fruits, dairy food) [48][55]. The fat:carbohydrate:protein ratio is about 1:6:0. There are no restrictions on diet and calories intake. Although LGIT represent fewer KBs than another KD, it is a better-tolerated diet.
Modified Atkins Diet (MAD): MAD constitutes 65% fat content, 25% protein, and a low carbohydrate intake (10%). The fat ratio is high in MAD and is considered the most savory form of KD and acceptable for adults or individuals suffering from behavioral issues [49][56]. There is no specific amount restriction of liquid or protein intake, but the carbohydrates amount is fixed as 10 to 20 g/day in infants and 15 to 20 g/day in youngsters. It is also recommended to take an appropriate calcium supplementation and a KD [50][51][57,58] because sufficient vitamins and minerals are normally found in a well-balanced diet. However, due to the limited quantities of fruits, vegetables, enriched grains, and foods containing calcium in the KD, supplementation is essential, especially vitamins B and C. Previous study also suggests that there is little vitamin D and calcium in KD and evidence for decreased Vitamin D levels in children with epilepsy, and therefore both vitamin D and calcium should be supplemented [52][59].
