Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Conner Chen and Version 1 by Mamata Singhvi.
Biohydrogen fermentation using lignocellulosic biomass (LCB) materials as feedstocks is better alternative to petroleum-based fuels due to its ecofriendly nature since there is no greenhouse gas (GHG) emissions during combustion. The recalcitrant nature of LCB waste materials is mainly attributed to the rigid cell wall structure, crystalline cellular machinery, and lignin component, which makes lignocellulosic materials resistant toward chemical and biological actions. Hence, the pretreatment process is an obligatory step to make LCB materials accessible for the generation of sugar fractions after disintegration of biomass.
- lignocellulosic biomass
- pretreatment
- biohydrogen
- nanotechnology
1. Pretreatment of Lignocellulosic Biomass
The recalcitrant nature of lignocellulosic biomass (LCB) waste materials is mainly attributed to the rigid cell wall structure, crystalline cellular machinery, and lignin component, which makes lignocellulosic materials resistant toward chemical and biological actions. Hence, the pretreatment process is an obligatory step to make LCB materials accessible for the generation of sugar fractions after disintegration of biomass. Prior to the downstream processing of disintegrated complex LCB materials, appropriate pretreatment techniques can be used to decrease crystallinity and solubilization of hemicellulose moiety. Hence, the recalcitrant LCB materials become more accessible toward enzymes/microbial attack, which also enhances the activity of enzymes on the biomass surface [15][1]. Pretreatment processes mainly propose the disintegration of cellulose, hemicellulose, and lignin moieties of lignocellulosic materials, which results in diminution in the size of the particles of LCB materials. Hence, there is a mode to augment surface areas for proficient enzyme action, which can bring about effective degradation of the complex LCB polysaccharides into simple sugars. These fermentable sugars can be further utilized by microbes to generate biohydrogen [15,16][1][2]. Several pretreatment processes have been used for divergent LCB materials but each with some pros and cons. Several kinds of pretreatment techniques are available (Figure 1), including physical, chemical, physico-chemical, biological, and nanotechnology-based processes [17,18][3][4]. Every pretreatment process trails its own specific experimental settings to disrupt complicated LCB structure to generate value-added products including various chemicals and biofuels.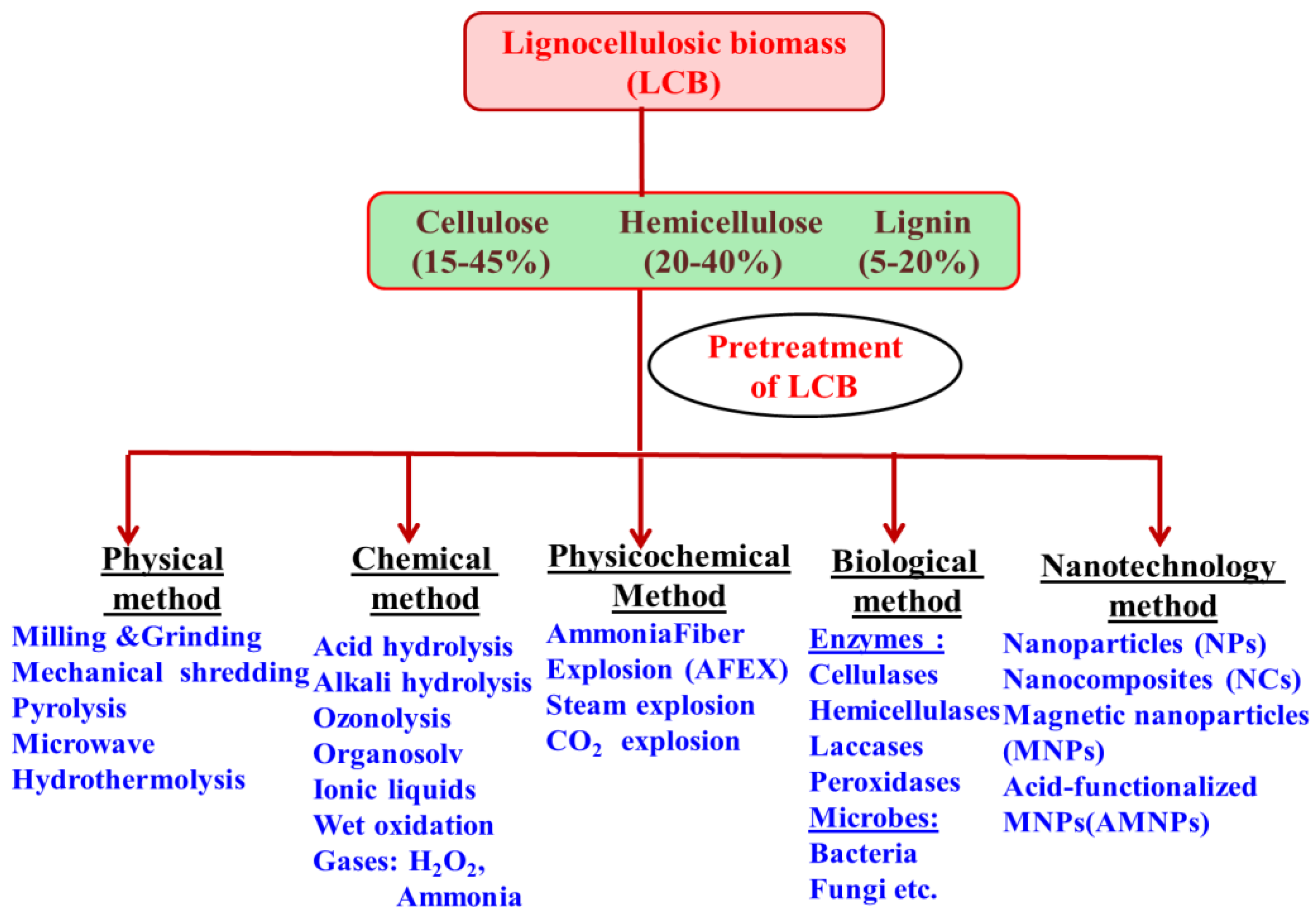
Figure 1.
Different pretreatment methods for disintegrating lignocellulosic biomass materials.
2. LCB Pretreatment by Physical Process
LCB pretreatment by physical methods augments hydrolytic efficiency and decomposition of LCB materials into biofuels and value-added products [19][5]. Various physical methods for treating LCB materials mainly include mechanical milling, steam explosion, ammonia fiber expansion (AFEX), pyrolysis, microwave irradiation method, etc. Mechanical processes include shredding, grinding, milling of LCB materials, which can break LCB fibers and thus decreases the required time to utilize LCB materials for subsequent treatments to obtain fermentative bioethanol [20][6]. The shredded materials after fractionation become finer, which makes their hydrolysis effective. This method can offer green pretreatment without generation of any other inhibitory compounds, which can be used directly for conversion into simple sugars [21][7]. The only problem is that this is highly energy requiring method and hence it can be used for limited applications. Pyrolysis is another technique for LCB pretreatment [20[6][7],21], which mainly comprises of thermochemical breakdown of LCB materials initiating at approximately 200 °C. Microwave is one of the new options for pretreatment of biomass materials to disintegrate complex 3D structure of LCB materials, which can make them accessible for further sugar generation [20][6]. The microwave irradiation treatment on sugarcane bagasse using phosphoric acid along with glycerol exhibited the release of lignin (5.4%) and xylan (11.3%) fractions. Further, to obtain higher yields, sugarcane bagasse can be treated with microwave irradiation along with enzyme hydrolysis of cellulose/hemicellulose fractions [20][6]. Ultrasonication radiation (of approximately 20–40 kHz) can be applied for biomass pretreatment, which leads to degradation of LCB structures by dint of rupturing and loosening fibrils through breakage of inter-chain hydrogen linkages [22][8], which can be used for biohydrogen production.3. LCB Pretreatment by Chemical Process
Usually, chemical pretreatment methods are used extensively as compared to other biological or physical methods due to their effectiveness toward degradation of complex LCB materials. In the acid pretreatment process, LCB materials can be treated by organic/inorganic acids, such as HNO3, HCl, H2SO4, formic acid, phosphoric acid, etc., to breakdown hydrogen and glycosidic bonds present in cellulose-hemicellulose fractions to liberate simple sugar units [10,23][9][10]. Mostly, during the acid pretreatment process, concentrated acids (30–70% at lower temperature) or dilute acids (0–10% v/v at 120–250 °C) are added to the LCB materials [24][11]. Dilute acid pretreatment can be used for pretreatment of various substrates, such as, poplar, corn stover, corn cob, switch grass, etc. The concentrated acid pretreatment can increase the rate of sugar release, but acids are more toxic in nature. The use of acid during pretreatment of biomass generates a large amount of inhibitory compounds, such as phenolic acids, aldehydes, furfurals, and 5–hydroxymethyl furfural (5-HMF). Further treatment is also required to recover acids after hydrolysis [25][12]. Thus, dilute acid treatment is usually preferred to hydrolyze LCB materials into sugar with the generation of lower amount of inhibitor products, which is an economical and environmentally friendly option [23][10]. The dilute acid, i.e., H2SO4 (0.4%) pretreatment on wild rice grass exhibited the release of 163 mg sugar per gram of substrate [26][13]. Alkaline pretreatment of LCB materials encompasses the application of bases (e.g., NaOH, NH4OH, etc.) resulting into increase in surface area and decline in crystallinity through breakage of ester and other bonds among lignin, hemicellulose and others [10,25][9][12]. Thus, after pretreatment, the obtained cellulose/hemicellulose fractions exhibit higher hydrolysis to generate simple fermentable sugars through enzymes/microbial actions. Usually, LCB sources, including agricultural wastes, hardwood, low lignin containing plants are suitable for alkali treatment. Nonetheless, the extreme use of alkali inhibits the anaerobic methanogenesis processes and it may also lead to soil salination and water pollution [27][14]. The pretreatment of wheat straw with NaOH at 100 °C for 6 h exhibited dissolution efficiency of 86.7% [28][15]. Various studies have been reported for enhanced biofuel production titers, using alkali treated LCB materials, including corn stover, corn cob, wheat straw, etc. [29,30][16][17]. The advantage of using alkali for biomass pretreatment is that it can remove lignin/hemicellulose moieties effectively to enhance biomass surface area and make it more amenable for further hydrolysis. Another promising approach for LCB pretreatment is organosolv method which can be conducted using organic solvents, such as phenol, acetone, alcohols, ethylene glycol, etc., along with the addition of some inorganic acids to stimulate the pretreatment efficiency of LCB materials under specific conditions [10,31][9][18]. This organosolv pretreatment can absolutely remove hemicellulose fractions in LCB, without affecting cellulose moiety, which provides a larger surface area and pore volume of cellulose. Hence, lignin moieties are dissolved in the liquid solvent phase and cellulose can be isolated in solid form [32][19]. The ethanosolv pretreatment along with sulphuric acid of poplar biomass exhibited 78% hydrolysis [33][20]. The organosolv pretreatment method has several advantages over other methods, such as easier solvent recovery, environmentally safer, and obtaining superlative lignin to generate value-added products. Ozonolysis can be considered as a powerful method to pretreat LCB materials using ozone (O3), which degrades hemicellulose and lignin moieties by keeping cellulose intact [22][8]. During ozonolysis, lignin is oxidized into low molecular weight compounds, such as acetic acid, formic acid, etc. This pretreatment exhibited more accessibility toward enzymatic hydrolysis, which improved sugar release significantly using various LCB materials, such as corn stalks, wheat straw, rye straw, etc. [34,35][21][22]. The best thing about using this pretreatment is that there is no generation of toxic inhibitory products, but the overall process is more expensive than others. Ionic liquids (ILs) can be another better option for disintegration of various LCB feedstocks. ILs are thermostable organic salts constituted of cations and anions in liquid form with lower melting point and vapor pressure [23][10]. During LCB pretreatment using ILs, both cations and anions form a strong interaction with surface –OH groups present on carbohydrate components through hydrogen bonding. Consequently, lignin is dissolved in ILs and the extent of cellulose suspension increases in the presence of electron-donating groups of IL cations, which can be obtained in the form of precipitate [36][23]. Several ILs, such as, 1–butyl–3–methylimidazoliumchloride, 1–butyl–3–methylimidazoliummethylsulfate, 1–benzyl– 3–methylimidazoliumchloride, 1,3–dimethylimidazolium groups, etc., have been broadly applied for pretreating different substrates, such as rice straw, rice husk, poplar wood, wheat straw, and pine [22,37][8][24]. Several studies reported hydrolysis and fermentation using LCB materials through different ILs such as choliniumlysinate and ethanolamine acetate produced glucose (25–85%) and xylose (15–80%) [38,39][25][26]. Although all methods described here are effective to a certain extent for biomass disintegration, they have some flaws concerning environmental aspects, generation of inhibitory compounds, and final yields of individual components. Table 1 presents several advantages and disadvantages associated with different chemical methods.Table 1. Advantages and disadvantages of various chemical pretreatment methods.
| Pretreatment Process |
Advantages | Limitations and Disadvantages |
References | |
|---|---|---|---|---|
| Ionic liquids (ILs) | Environmentally friendly Nonderivatizing, nonvolatile, thermostable single component solvent for cellulose with potential applications incellulose fractionation and dissolution |
High cost Poor biodegradability Toxic to micro-organisms |
[40] | [27] |
| Ozonolysis | Reduces lignin content Does not produce toxic residues |
Large amount of ozone required Expensive |
[41] | [28] |
| Acid hydrolysis |
Hydrolyzes hemicellulose to xylose and other sugars to alter lignin structure | High cost Equipment corrosion Formation of toxic substances |
[42] | [29] |
| Alkaline hydrolysis | Removes hemicellulose and lignin Increases accessible surface area |
Long residence times required Irrecoverable salts formed and incorporated into biomass |
[43] | [30] |
| Organosolv | Organosolv lignin is sulfur free with high purity and low molecular weight Can be used as fuel to power pretreatment plant or further purified to obtain high quality lignin, which is used as a substitute for polymeric materials Very effective for the pretreatment of high-lignin lignocellulose materials |
Solvents need to be drained from the reactor, evaporated, condensed, and recycled High cost Generation of compounds inhibitory to micro-organisms |
[44] | [31] |
| Pyrolysis | Produces gas and liquid products | High temperature Ash production |
[45] | [32] |
4. LCB Pretreatment by Physico-Chemical Process
Amongst the known physicochemical methods, hot water, steam explosion, and ammonia fiber explosion (AFEX) are promising processes to disintegrate the obstinate structure of LCB materials. Steam explosion and hot water pretreatment processes generate high amounts of degradation products, such as furfural, 5-HMF, phenolic compounds, formic acid, etc. which can be inhibitory to microbial strains [46][33]. In the case of steam explosion, LCB materials are treated at high steam pressure of approximately 0.6–4.9 MPa and a temperature ranging from 160–210 °C for different time. At very high pressure/temperature conditions, both steam explosion and AFEX can certainly breakdown the rigid components of LCB materials to generate fermentable sugars [47][34]. The major flaw of using the steam explosion method is the partial hemicellulose degradation, which thus divert into generation of toxic byproducts. The application of the steam explosion method for corn stover treatment was used to generate 113.5 million liters of butanol annually. The higher release of glucose was achieved using olive tree prunes, which was up to 86% at high temperature, and pressure conditions for 15 min [48][35]. Hot water pretreatment in the presence of chemicals is an ultimate method for LCB substrates, which can result in the effective utilization of biomass. This process is somewhat similar to steam explosion pretreatment method, which does not need chemicals, and hence there is no generation of inhibitory compounds [49][36]. Further, AFEX is an effective and novel process for breaking down LCB components into simple sugars. This method can act effectively on low lignin containing LCB substrates, such as corn stover, miscanthus, switch grass, etc., exhibiting approximately 90% of glucose generation during hydrolysis process.5. LCB Pretreatment by Biological Process
Usually, biological pretreatment methods are better than other chemical and physical methods [40][27] due to several benefits, such as a low energy requirement, no generation of toxic compounds, etc. However, biological processes show a lower hydrolysis rate, which is the major drawback of these processes. In biological pretreatment of LCB substrates, different microbes (e.g., bacteria, fungi, etc.) and enzymes have been employed which plays a significant role in pretreating biomass [50][37]. Usually, fungal strains, such as soft rot, brown, and white fungi have been broadly used to deconstruct LCB materials to be used for fermentation [50][37]. White rot fungus is able to degrade the hard layer of lignin present in biomass, due to the action of lignin degrading enzymes, such as laccase, peroxidase, etc. Brown rot fungi attack specifically cellulose moiety, whereas white and soft rot fungi mainly act on both lignin and cellulose components of LCB. The widely used white rot fungi include Pleurotus ostreatus, Pycnoporus cinnabarinus, Ceriporiopsis subvermispora, Cyathus stercolerus, Cyathus cinnabarinus, etc., which can degrade lignin as these strains can secrete lignin degrading enzymes, such as ligninase, peroxidase, laccases, etc. [46][33]. The pretreatment of bamboo using lignin degrading Punctularia sp. exhibited an increase in the sugar concentration of almost 60% by reducing lignin content [51][38].6. LCB Pretreatment Using Nanotechnology
Pretreatment of LCB materials using a nanotechnology approach is one of the significant methodologies to generate biofuels. The application of nanoparticles (NPs) can display catalytic actions for LCB processing almost similar to using chemical methods [34][21]. Mostly, magnetic nanoparticles (MNPs) have been used widely for the pretreatment of LCB materials since they can be reused for subsequent cycles which ultimately reduces the overall process cost [19][5]. Due to the nano size of particles, NPs enter the cell wall of LCB materials, thereby interacting with biomass components to generate fermentable sugars [25][12]. Acid-functionalized MNPs possess higher affinity for hydrolyzed LCB materials, which are known as solid acid nanocatalysts. Owing to the strong magnetic nature, the reusability of MNPs has added a beneficial role in various applications. Such acid-functionalized MNPs exhibit better hydrolytic ability for biofuel production [14,52][39][40]. The treatment of wheat straw with perfluoroalkylsufonic and alkylsufonic acid-functionalized NPs liberated almost 31% higher sugar concentration as compared to the control reaction [14][39]. In addition, NPs are required in small quantities and can be reused for the next cycle of the process. Recently, the effect of magnetic iron oxide (Fe3O4) NPs on LCB pretreatment and further biogas production has been studied which exhibited the remarkable improvement in biogas production in the presence of Fe3O4 MNPs [53][41]. Nonetheless, till date there is not much information available on LCB pretreatment using NPs. Thus, more efforts are needed to search for possible ways to make the process viable at commercial scale. In recent years, an inventive improvement has been made in nanotechnology field, where enzyme/biocatalyst can be immobilized on MNPs. The immobilization of enzymes on MNPs is a budding method that can increase the enzyme’s catalytic efficiency [13][42]. Owing to the magnetic nature of MNPs, the immobilized enzymes can be recycled and reused for several cycles of biomass hydrolysis. Enzyme immobilization using NPs is called ‘nanobiocatalyst’, which is a depiction of emerging developments in the field of nanobiotechnology. The different aspects of enzymatic hydrolysis of LCB substrates have already been described by Singhvi et al. [15][1].References
- Singhvi, M.S.; Kim, B.S. Current developments in lignocellulosic biomass conversion into biofuels using nanobiotechology approach. Energies 2020, 13, 5300.
- Asadi, N.; Zilouei, H. Optimization of organosolv pretreatment of rice straw for enhanced biohydrogen production using Enterobacter aerogenes. Bioresour. Technol. 2017, 227, 335–344.
- Wang, P.; Zhang, J.; Feng, J.; Wang, S.; Guo, L.; Wang, Y.; Lee, Y.Y.; Taylor, S.; McDonald, T.; Wang, Y. Enhancement of acid re-assimilation and biosolvent production in Clostridium saccharoperbutylacetonicum through metabolic engineering for efficient biofuel production from lignocellulosic biomass. Bioresour. Technol. 2019, 281, 217–225.
- Dharmaraja, J.; Shobana, S.; Arvindnarayan, S.; Vadivel, M.; Atabani, A.E.; Pugazhendhi, A.; Kumar, G. Biobutanol from lignocellulosic biomass: Bioprocess strategies. In Lignocellulosic Biomass to Liquid Biofuels, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 169–193.
- Chandel, H.; Kumar, P.; Chandel, A.K.; Verma, M.L. Biotechnological advances in biomass pretreatment for bio-renewable production through nanotechnological intervention. Biomass Convers. Biorefinery 2022, 2022, 1–23.
- Singh, H.; Tomar, S.; Qureshi, K.A.; Jaremko, M.; Rai, P.K. Recent advances in biomass pretreatment technologies for biohydrogen production. Energies 2022, 15, 999.
- Karimi, K.; Taherzadeh, M.J. A critical review of analytical methods in pretreatment of lignocelluloses: Composition, imaging, and crystallinity. Bioresour. Technol. 2016, 200, 1008–1018.
- Kucharska, K.; Rybarczyk, P.; Hołowacz, I.; Łukajtis, R.; Glinka, M.; Kamiński, M. Pretreatment of lignocellulosic materials as substrates for fermentation processes. Molecules 2018, 23, 2937.
- Ashokkumar, V.; Venkatkarthick, R.; Jayashree, S.; Chuetor, S.; Dharmaraj, S.; Kumar, G.; Chen, W.-H.; Ngamcharussrivichai, C. Recent advances in lignocellulosic biomass for biofuels and value-added bioproducts—A critical review. Bioresour. Technol. 2012, 344, 126195.
- Baruah, J.; Nath, B.K.; Sharma, R.; Kumar, S.; Deka, R.C.; Baruah, D.C.; Kalita, E. Recent trends in the pretreatment of lignocellulosic biomass for value-added products. Front. Energy Res. 2018, 6, 141.
- Badiei, M.; Asim, N.; Jahim, J.M.; Sopian, K. Comparison of chemical pretreatment methods for cellulosic biomass. APCBEE Procedia 2014, 9, 170–174.
- Amin, F.R.; Khalid, H.; Zhang, H.; Rahman, S.; Zhang, R.; Liu, G.; Chen, C. Pretreatment methods of lignocellulosic biomass for anaerobic digestion. AMB Express 2017, 7, 72.
- Sahoo, D.; Ummalyma, S.B.; Okram, A.K.; Pandey, A.; Sankar, M.; Sukumaran, R.K. Effect of dilute acid pretreatment of wild rice grass (Zizania latifolia) from Loktak Lake for enzymatic hydrolysis. Bioresour. Technol. 2018, 253, 252–255.
- Kumari, D.; Singh, R. Pretreatment of lignocellulosic wastes for biofuel production: A critical review. Renew. Sustain. Energy Rev. 2018, 90, 877–891.
- Yuan, Z.; Wen, Y.; Li, G. Production of bioethanol and value added compounds from wheat straw through combined alkaline/alkaline-peroxide pretreatment. Bioresour. Technol. 2018, 259, 228–236.
- Shah, T.A.; Tabassum, R. Enhancing biogas production from lime soaked corn cob residue. Int. J. Renew. Energy Res. 2018, 8, 761–766.
- Shen, J.; Zhao, C.; Liu, G.; Chen, C. Enhancing the performance on anaerobic digestion of vinegar residue by sodium hydroxide pretreatment. Waste Biomass Valorization 2017, 8, 1119–1126.
- Khan, M.U.; Usman, M.; Ashraf, M.A.; Dutta, N.; Luo, G.; Zhang, S. A review of recent advancements in pretreatment techniques of lignocellulosic materials for biogas production: Opportunities and imitations. Chem. Eng. J. Adv. 2022, 10, 100263.
- Amiri, H.; Karimi, K.; Zilouei, H. Organosolv pretreatment of rice straw for efficient acetone, butanol, and ethanol production. Bioresour. Technol. 2014, 152, 450–456.
- Chu, Q.; Tong, W.; Chen, J.; Wu, S.; Jin, Y.; Hu, J.; Song, K. Organosolv pretreatment assisted by carbocation scavenger to mitigate surface barrier effect of lignin for improving biomass saccharification and utilization. Biotechnol. Biofuels 2021, 14, 136.
- Silverstein, R.A.; Chen, Y.; Sharma-Shivappa, R.R.; Boyette, M.D.; Osborne, J. A comparison of chemical pretreatment methods for improving saccharification of cotton stalks. Bioresour. Technol. 2007, 98, 3000–3011.
- García-Cubero, M.T.; González-Benito, G.; Indacoechea, I.; Coca, M.; Bolado, S. Effect of ozonolysis pretreatment on enzymatic digestibility of wheat and rye straw. Bioresour. Technol. 2009, 100, 1608–1613.
- Chen, H.; Liu, J.; Chang, X.; Chen, D.; Xue, Y.; Liu, P.; Lin, H.; Han, S. A review on the pretreatment of lignocellulose for high-value chemicals. Fuel Process. Technol. 2017, 160, 196–206.
- Shirkavand, E.; Baroutian, S.; Gapes, D.J.; Young, B.R. Combination of fungal and physicochemical processes for lignocellulosic biomass pretreatment—A review. Renew. Sustain. Energy Rev. 2016, 54, 217–234.
- Das, L.; Achinivu, E.C.; Barcelos, C.A.; Sundstrom, E.; Amer, B.; Baidoo, E.E.K.; Simmons, B.A.; Sun, N.; Gladden, J.M. Deconstruction of woody biomass via protic and aprotic ionic liquid pretreatment for ethanol production. ACS Sustain. Chem. Eng. 2021, 9, 4422–4432.
- Rahim, A.H.A.; Man, Z.; Sarwono, A.; Muhammad, N.; Khan, A.S.; Hamzah, W.S.W.; Yunus, N.M.; Elsheikh, Y.A. Probe sonication assisted ionic liquid pretreatment for rapid dissolution of lignocellulosic biomass. Cellulose 2020, 27, 2135–2148.
- Zhang, Y.; Lynd, L.R. A functionally based model for hydrolysis of cellulose by fungal cellulase. Biotechnol. Bioeng. 2006, 694, 888–898.
- Quesada, J.; Rubio, M.; Gómez, D. Ozonation of lignin rich solid fractions from corn stalks. J. Wood Chem. Technol. 1999, 19, 115–137.
- Mosier, N.S.; Hendrickson, R.; Brewer, M.; Ho, N.; Sedlak, M.; Dreshel, R.; Ladisch, M.R. Industrial scale-up of pH-controlled liquid hot water pretreatment of corn fiber for fuel ethanol production. Appl. Biochem. Biotechnol. 2005, 125, 77–97.
- Wyman, C.E.; Dale, B.E.; Elander, R.T.; Holtzapple, M.; Ladish, M.R.; Lee, Y.Y. Coordinated development of leading biomass pretreatment technologies. Bioresour. Technol. 2005, 96, 1959–1966.
- Zhang, Y.H.P. Reviving the carbohydrate economy via multi-product lignocellulose biorefineries. J. Ind. Microbiol. Biotechnol. 2008, 35, 367–375.
- Zwart, R.W.; Boerrigter, H.; van der Drift, A. The impact of biomass pretreatment on the feasibility of overseas biomass conversion to Fischer-Tropsch products. Energy Fuels 2006, 20, 2192–2197.
- Anu, K.A.; Rapoport, A.; Kunze, G.; Kumar, S.; Singh, D.; Singh, B. Multifarious pretreatment strategies for the lignocellulosic substrates for the generation of renewable and sustainable biofuels: A review. Renew. Energy 2020, 160, 1228–1252.
- Banoth, C.; Sunkar, B.; Tondamanati, P.R.; Bhukya, B. Improved physicochemical pretreatment and enzymatic hydrolysis of rice straw for bioethanol production by yeast fermentation. 3 Biotech 2017, 7, 334.
- Barbanera, M.; Buratti, C.; Cotana, F.; Foschini, D.; Lascaro, E. Effect of steam explosion pretreatment on sugar production by enzymatic hydrolysis of olive tree pruning. Energy Procedia 2015, 81, 146–154.
- Ravindran, R.; Jaiswal, A.K. A comprehensive review on pre-treatment strategy for lignocellulosic food industry waste: Challenges and opportunities. Bioresour. Technol. 2016, 199, 92–102.
- Dey, N.; Kumar, G.; Vickram, A.S.; Mohan, M.; Singhania, R.R.; Patel, A.K.; Dong, C.-D.; Anbarasu, K.; Thanigaivel, S.; Ponnusamy, V.K. Nanotechnology-assisted production of value-added biopotent energy-yielding products from lignocellulosic biomass refinery—A review. Bioresour. Technol. 2022, 344, 126171.
- Suhara, H.; Kodama, S.; Kamei, I.; Maekawa, N.; Meguro, S. Screening of selective lignin-degrading basidiomycetes and biological pretreatment for enzymatic hydrolysis of bamboo culms. Int. Biodeterior. Biodegrad. 2012, 75, 176–180.
- Peña, L.; Ikenberry, M.; Hohn, K.L.; Wang, D. Acid-functionalized nanoparticles for pre-treatment of wheat straw. J. Biomater. Nanobiotechnol. 2012, 3, 342.
- Wang, J.; Qian, Y.; Li, L.; Qiu, X. Atomic force microscopy and molecular dynamics simulations for study of lignin solution self-assembly mechanisms in organic–aqueous solvent mixtures. Chem. Sus. Chem. 2020, 13, 4420–4427.
- Khalid, M.J.; Waqas, A.; Nawaz, I. Synergistic effect of alkaline pretreatment and magnetite nanoparticle application on biogas production from rice straw. Bioresour. Technol. 2015, 275, 288–296.
- Rai, M.; Ingle, A.P.; Gaikwad, S.; Dussán, K.J.; da Silva, S.S. Role of nanoparticles in enzymatic hydrolysis of lignocellulose in ethanol. In Nanotechnology for Bioenergy and Biofuel Production, 2nd ed.; Springer: Cham, Switzerland; Berlin, Germany, 2017; pp. 153–171.
More
