Climate change and the urgent need to reduce greenhouse gas (GHG) emission from agriculture has resulted in significant pressure on the livestock industry for advanced practices that are environmentally more sustainable. Livestock is responsible for more than 15% of anthropogenic methane (CH4) emission via enteric fermentation and improved strategies for mitigating enteric CH4 production therefore represents a promising target to reduce the overall GHG contribution from agriculture. Ruminal CH4 is produced by methanogenic archaea, combining carbon dioxide (CO2) and hydrogen (H2). Removal of H2 is essential, as its accumulation inhibits many biological functions that are essential for maintaining a healthy rumen ecosystem.
1. Introduction
The rumen harbors a highly diverse and complex mixture of microorganisms, including archaea (10
8−10
9/mL), bacteria (10
10−10
11/mL), ciliate protozoa (10
6/mL), and fungi (10
6/mL), which facilitate the degradation of complex plant carbohydrates into small molecules
[1] and ultimately provide metabolites that can be used by the ruminant animal
[2][3][4][5][2,3,4,5]. Livestock are mainly fed with agricultural crops, which via microbial activity are converted to metabolic intermediates (i.e., volatile fatty acids (VFAs), such as acetate, butyrate and propionate, and hydrogen (H
2) and gaseous end products such as carbon dioxide (CO
2) and methane (CH
4)
[6]. Increased microbial H
2 production and its subsequent accumulation, which can be promoted by a high-starch diet, have several detrimental effects on the rumen ecosystem and that can be attributed to a decrease in rumen pH triggered by starch fermentation. These effects include the deactivation of specific biomass-degrading enzymes from some of the most efficient fiber degraders of the rumen microbiome but also system-level responses, such as the reduction of feed conversion within the rumen
[7][8][7,8]. Methanogens, a group of microbes belonging to the phylogenetic group of the archaea, combine molecular H
2 with CO
2 to produce CH
4 during methanogenesis, enabling the removal of H
2 from the system
[9][10][9,10]. Although this removal of H
2 is important for maintaining a healthy rumen ecosystem, from the viewpoint of nutrient expenditure methanogenesis is a costly process, accounting for a gross energy intake loss of 2–12% in ruminants
[11][12][13][14][11,12,13,14]. Since the annual production of enteric CH
4 accounts for ~15% of total anthropogenic CH
4 emissions
[11][15][11,15], with CH
4 having a global warming potential 23-fold higher than that of CO
2, there is also a real and severe environmental cost associated with the energy of the enteric CH
4 that is released into the atmosphere.
Strategies and factors for CH
4 abatement have been reviewed in the past
[1][9][12][16][17][18][19][20][21][22][23][24][25][1,9,12,16,17,18,19,20,21,22,23,24,25] and many of the strategies used to mitigate CH
4 from ruminants involve the use of antibiotics, ionophores
[26], halogenated CH
4 analogues
[27][28][29][27,28,29], heavy metals
[30], lipid-rich materials such as coconut oil
[31][32][33][31,32,33], probiotics
[27], bacteriocin
[34], and numerous chemicals
[35][36][35,36]. Immunization against methanogens
[37][38][37,38], elimination of ciliate protozoa (defaunation) both in in vivo and in vitro
[39] and addition of acetogenic bacteria to rumen fluid
[40][41][42][40,41,42] in in vitro experiments have also been tested. Use of toxic chemicals and antibiotics as inhibitors, although considered an option in the past, are no longer accepted due to rising concerns regarding their impact on the environment, the animal, and potentially on the consumer of the animal products
[43]. Interventions using phage therapy, altering methanogenic diversity and chemogenomic approaches
[6] are some of the more recent technologies, but the extent to which these processes remove and eliminate the produced H
2 still remains to be investigated. Therefore, a critical step for a successful CH
4 reduction strategy may be one that uses natural processes within the rumen. One such approach relies on establishing a non-methanogenic sink for H
2 produced during fermentation.
This review will focus on these H2 elimination pathways.2. Hydrogen: A Key Player in Rumen Fermentation
H
2 concentration plays a major role in the regulation of microbial fermentation in the rumen
[44][45][46][47][44,45,46,47]. The partial H
2 pressure is a key regulator of H
2 metabolism and the fate of ruminal H
2 disposal with dissolved H
2 gas and H
2 ion determining the redox potential of the rumen liquor. The efficient elimination of H
2 enhances fermentation by reducing its inhibitory effect on microbial growth and microbial degradation of plant material
[48][49][48,49]. Destiny of H
2 liberation is associated with favorable thermodynamic changes and an inverse correlation between Gibbs free energy (ΔG
0) and the minimum partial H
2-pressure that is required for a reaction to continue: a reaction is considered to be thermodynamically more competitive when its requirement of H
2 partial pressure is low
[50]. Due to this central regulatory role in rumen fermentation, H
2 can be considered to be the currency of ruminal fermentation
[51]. Removal of the major fraction of the rumen H
2 occurs via the methanogenic archaea to CH
4, during which four moles of H
2 are consumed and converted into one mole of CH
4, which is then released into the atmosphere though eructation. During hydrogenotrophic methanogenesis, methanogens use CO
2 as carbon source and terminal electron acceptor and H
2 as electron donor. Other non-methanogenic rumen microbes, using CO
2 and other electron acceptors such as sulfate, nitrate, and fumarate, compete with methanogens for H
2, but they play a less dominant role in the removal of H
2 from the rumen ecosystem
[52][53][52,53]. Non-methanogenic bacteria that use H
2 as electron donor include acetogens that reduce CO
2 to form acetate by the Wood-Ljungdahl pathway
[54], sulfate-reducing bacteria (SRB) that reduce sulfate to hydrogen sulfide
[55], nitrate-reducing bacteria (NRB) that reduce nitrate (NO
3) to ammonia (NH
4) and fumarate-reducing bacteria that use H
2 to form succinate
[56][57][56,57]. Succinate can subsequentially be decarboxylated to propionate, which is a valuable nutrient for the ruminant animal
[58], either by the succinate producer itself or it can be transferred to succinate users as an intercellular electron carrier
[45].
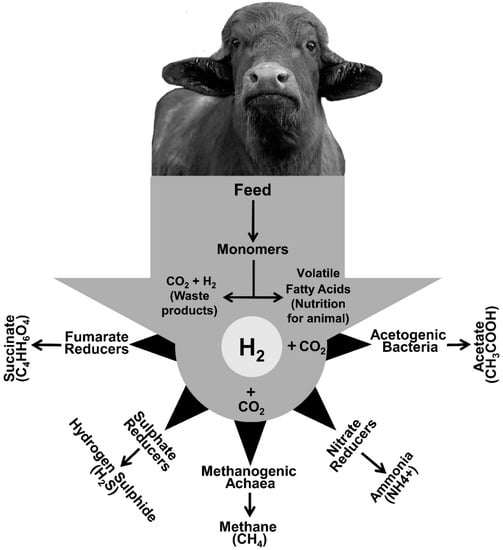
Figure 1 summarizes the microbial pathways for H
2 removal from the rumen.
Figure 1.
Major and minor H
2
and CO
2
sequestering pathways in rumen.
Ruminal methanogenesis, contributing CH4 to the atmosphere, is directly and inversely linked to the animal productivity. The ability to control CH4 emission especially reduce methanogenesis from agriculture has enormous environmental and socioeconomic implication, but it also requires a detailed understanding of the microorganisms and microbial processes that are involved. Although a complete understanding of these highly interwoven microbial and metabolic networks has still not been achieved and most likely will not be feasible in the immediate future, there are some aspects that are reasonably well understood. These aspects represent a promising starting point for targeted CH4 reduction from ruminants. One of the promising key intermediates that has been recognized as such and that has received significant attention for targeted CH4 mitigation is metabolic H2 and the metabolic pathways, microbes and enzymes involved its production and consumption.
Since H2 is an immediate precursor for the archaeal reduction of CO2 into CH4, biological approaches that redirect H2 away from archaeal methanogenesis and into alternate metabolic pathways seem to be the most promising approaches to convert feed carbon into metabolic energy for the ruminant instead of releasing it into the atmosphere. Redirecting H2 through reductive acetogenesis and propionogenesis has advantages over other pathways due to production of valuable metabolic end products that can be used by the host animal as nutrients and can be converted into animal proteins for human consumption. Although the understanding of how to redirect metabolic H2 into more favorable pathways facilitates the production of value-added metabolic intermediates and therefore redirects otherwise lost feed energy, several issues related to the fine tuning of this redirection, such as the co-factor requirements, toxicity of metabolic intermediates, as well as thermodynamics of competing metabolic processes, need to be investigated in greater detail. A further aspect that will have to be investigated in future and that will have direct implications for the translational value of findings on the area of rumen nutrition and function is the link and dependence of the rumen microbiome and its function in dietary conversion.