Wound management remains a challenging issue around the world, although a lot of wound dressing materials have been produced for the treatment of chronic and acute wounds. Wound healing is a highly dynamic and complex regulatory process that involves four principal integrated phases, including hemostasis, inflammation, proliferation, and remodeling. Chronic non-healing wounds are wounds that heal significantly more slowly, fail to progress to all the phases of the normal wound healing process, and are usually stalled at the inflammatory phase. These wounds cause a lot of challenges to patients, such as severe emotional and physical stress and generate a considerable financial burden on patients and the general public healthcare system. It has been reported that about 1–2% of the global population suffers from chronic non-healing wounds during their lifetime in developed nations. Traditional wound dressings are dry, and therefore cannot provide moist environment for wound healing and do not possess antibacterial properties. Wound dressings that are currently used consist of bandages, films, foams, patches and hydrogels. Currently, hydrogels are gaining much attention as a result of their water-holding capacity, providing a moist wound-healing milieu.
- chitosan
- cellulose nanocrystals
- hydrogels
1. Introduction
2. Types of Wounds
Wounds are of different types, which are caused as a result of physical, chemical, and thermal damages. Depending on the nature of the healing process, wounds can be divided into two main types, namely acute and chronic wounds [6,20][6][19]. Acute wound: an acute wound is an injury to the skin that takes place immediately rather than over time. Acute wounds in a normal healthy person will heal fast at the rate of the normal wound healing process because of a balance of growth factors, cytokines, and matrix metalloproteinase (MMPs) [29][20]. Basically, acute wounds can occur on any part of the body, which can range from superficial bruises to deep wounds causing damage to blood vessels, nerves, and muscles. Acute wounds may last up to 2 to 3 months followed by infection, pain, and necrosis [30][21]. Some examples of acute wound include (i) surgical wounds: Surgical wounds are incisions made intentionally by a medical professional and are cut precisely, creating clean edges around the wound. Surgical wounds may be closed (with stitches, staples or adhesive) or left open to heal by primary intention, (ii) traumatic wounds: These are unplanned injuries that can range from minor injuries such as a skinned knee, to severe injuries such as a gunshot wound. Examples of traumatic wounds consist of abrasions, skin tears, bites, and penetrating trauma wounds, (iii) burns: A burn is a type of injury to skin or other tissues caused by heat, cold, electricity, chemical, friction or radiation. Chronic wound: it is a wound that fails to heal in a well-ordered set of stages and in an expected period of time of normal wound healing process. Wounds that take a long time (that is more than 90 days) to heal are generally considered chronic. Chronic wounds sometimes do not proceed to one or more of the wound healing phases. For example, chronic wounds are often stalled at the inflammatory phase for too long a period of time. Some of the common types of chronic wounds are diabetic foot ulcers, venous and arterial ulcers, and pressure ulcers [6,31][6][22]. Chronic wounds may take a very long period to heal or may never heal. These wounds cause severe emotional and physical stress, and pain in patients. Many factors are usually responsible for wound impairment. This is as a result of overlapping mechanisms in normal wound healing process that tends to prevent one factor from disrupting the process. However, when the healing process is disrupted and wound healing is impaired, this will lead to the development of chronic wounds. Generally, the main factors affecting chronic non-healing wounds include infection, imbalance in matrix metalloproteinases and matrix metalloproteinases inhibitors, oxidative stress, metabolic conditions, immunosuppression, and radiation. Bacterial infection in wounds is the most often reason of the wound healing process interruption. Bacteria generate inflammatory markers that prevent the inflammatory phase as well as epithelialization phase of wound healing. The presence of bacteria in an infected wound leads to cell death, which causes an increase in inflammation response and persistent inflammatory phase. Necrotic tissues present in wounds disrupts the ingrowth of new tissues. In addition, necrotic tissue also serves as a ground for bacterial growth, leading to a pathologic cycle. When the bacterial burden of a chronic wound is more than 1 × 106 colony forming units per gram of tissue, it is considered as being clinically infected [32][23]. Commonly encountered, chronic wound bacteria include Staphylococcus aureus, Pseudomonas aeruginosa, Enterococcus faecalis, Proteus spp., Streptococcus spp., Escherichia coli, Citrobacter spp., Morganella spp. and Corynebacterium spp. Bacteria form protective biofilms that are not recognized by the host cells. Biofilms severely affect the wound healing process because they disrupt the immune response, prolong epithelialization, and decrease the growth of granulation tissues. Persistent oxidative stress in chronic wounds disrupt inflammatory responses resulting in poor angiogenesis and re-epithelialization is impaired [33][24]. Oxidative stress is as a result of excess reactive oxygen species (ROS) production in the wound. ROS consist of hydrogen peroxide H2O2, superoxide anion O2− or peroxide O22−. They are powerful oxidants and contribute enormously to cell damage, but they also play a vital role in the preparation of the normal healing process. Therefore, a balance between low and high level of ROS is very important. Low levels of reactive oxygen species are essential in the protection of tissues against bacterial infection and promoting wound healing by the production of cell surviving signaling [34][25]. There is no clear cut-off point for reactive oxygen species level in tissues but for normal wounds, the level of hydrogen peroxide (which is the most common oxidant) is in the range 100–250 µM [34][25].3. Hydrogels as Biomaterials
Biomaterial is any material (synthetic or natural) used as a complete or as part of a biological system which has been impaired or to interact with living systems for medical purposes [35,36][26][27]. Biomaterial should be compatible and biodegradable. Biomaterials should not possess any kind of unfavorable or side reaction from the living tissue and vice versa. The biomedical applications of biomaterials include hip joints, drug carrier devices, bone plates, contact lenses, wound dressings [35][26]. Biomaterials such as gelatin, alginate, hyaluronic acid, dextran, elastin, collagen, cellulose nanocrystals, chitosan have gained great interest and are widely used for wound dressings and as drug delivery systems. Wound dressings are primarily produced from natural and synthetic polymers. In this revisew, wearch, the researchers focused on chitosan and cellulose nanocrystals as biomaterials for the development of hydrogels for wound management.3.1. Gelatin
3.1. Gelatin
3.2. Cellulose
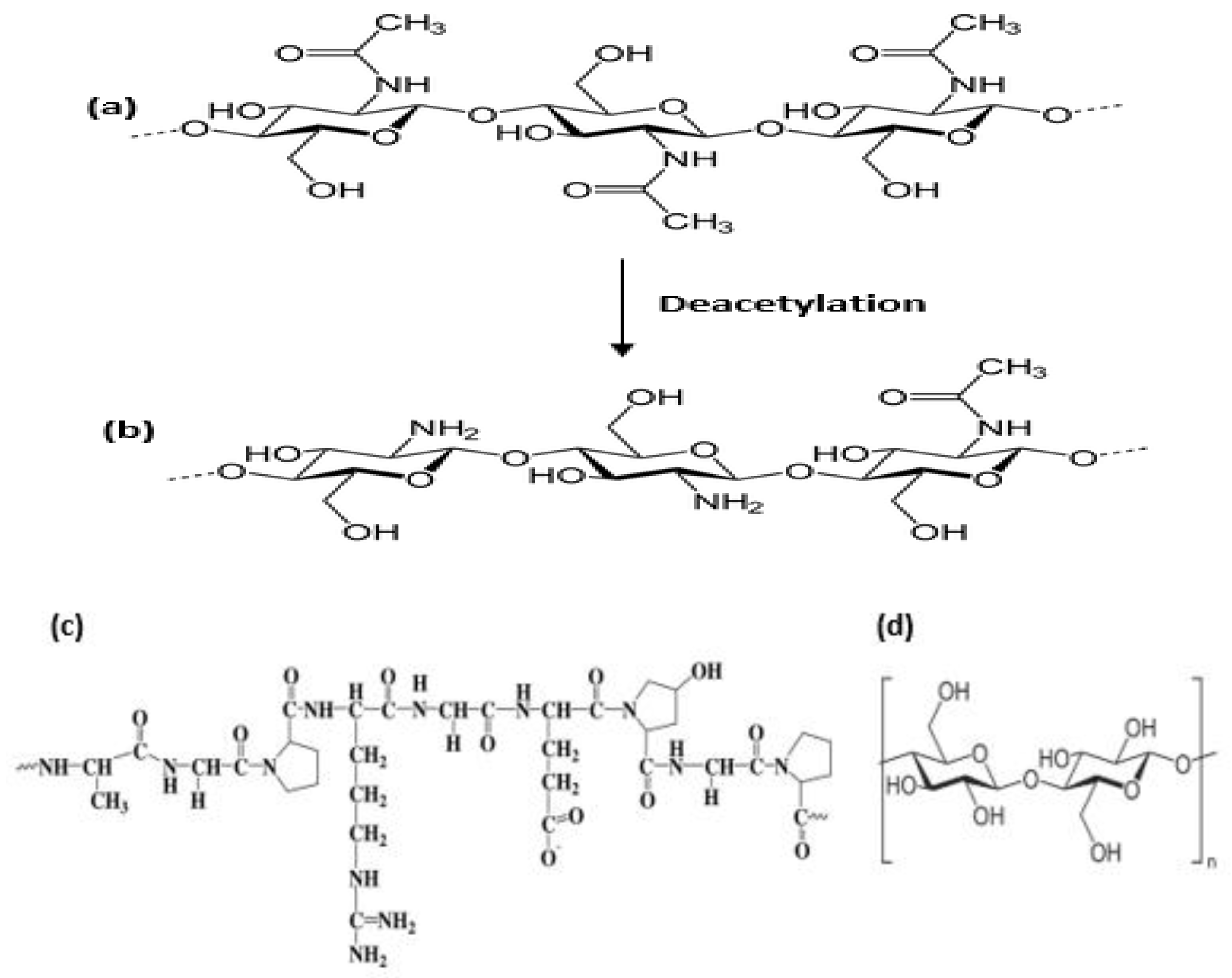
3.3. Chitosan
| Modification | Properties |
|---|---|
| Carboxymethyl chitosan |
Improved solubility in water. The commonly explored derivative of chitosan; it is amphoteric in nature and its solubility depends on pH, when the pH is >7 it is water soluble. |
| Thiolated urea derivatives |
Thiourea chitosan boost the antibacterial properties. |
| Carbohydrate branched chitosan |
Water soluble. Carbohydrate can be grafted on the chitosan backbone at the C2 position by reductive alkylation. They could be used for wound dressing and drug targeting. |
| Sugar derivatives | N-Succinyl chitosan is an amphoteric polymer consisting of amine, hydroxyl, and carbonyl groups. It has excellent physical, chemical, and biological properties as required in biomedical applications. |
| Alkylation chitosan | It is an essential amphiphilic polymer based on polysaccharides. Improves the stability of the interfacial films, promotes its solubility. |
References
- Kamoun, E.A.; Kenawy, E.-R.S.; Chen, X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 2017, 8, 217–233.
- Robson, M.; Steed, D.; Franz, M. Wound healing: Biological features and approaches to maximize healing trajectories. Curr. Probl. Surg. 2001, 38, 72–140.
- Dhivya, S.; Padma, V.V.; Santhini, E. Wound dressings—A review. BioMedicine 2015, 5, 22–28.
- Su, J.; Li, J.; Liang, J.; Zhang, K.; Li, J. Hydrogel Preparation Methods and Biomaterials for Wound Dressing. Life 2021, 11, 1016.
- Wang, W.; Wang, A. Nanocomposite of carboxymethyl cellulose and attapulgite as a novel pH-sensitive superabsorbent: Synthesis, characterization and properties. Carbohydr. Polym. 2010, 82, 83–91.
- Ooi, S.Y.; Ahmad, I.; Amin, M.C.I.M. Cellulose nanocrystals extracted from rice husks as a reinforcing material in gelatin hydrogels for use in controlled drug delivery systems. Ind. Crops Prod. 2016, 93, 227–234.
- Dufresne, A. Interfacial phenomena in nanocomposites based on polysaccharide nanocrystals. Compos. Interfaces 2003, 10, 369–387.
- Iqbal, M.S.; Akbar, J.; Saghir, S.; Karim, A.; Koschella, A.; Heinze, T.; Sher, M. Thermal studies of plant carbohydrate polymer hydrogels. Carbohydr. Polym. 2011, 86, 1775–1783.
- Fang, X.; Wang, C.; Zhou, S.; Cui, P.; Hu, H.; Ni, X.; Jiang, P.; Wang, J. Hydrogels for Antitumor and Antibacterial Therapy. Gels 2022, 8, 315.
- Lima, L.P.T.; Passos, M.F. Skin wounds, the healing process, and hydrogel-based wound dressings: A short review. J. Biomater. Sci. Polym. Ed. 2021, 32, 1910–1925.
- Vashist, A.; Vashist, A.; Gupta, Y.K.; Ahmad, S. Recent Advances in Hydrogel Based Drug Delivery Systems for the Human Body. J. Mater. Chem. B 2014, 2, 147–166.
- Xu, Q.; Ji, Y.; Sun, Q.; Fu, Y.; Xu, Y.; Jin, L. Fabrication of Cellulose Nanocrystal/Chitosan Hydrogel for Controlled Drug Release. Nanomaterials 2019, 9, 253.
- Alizadehgiashi, M.; Nemr, R.C.; Chekini, M.; Ramos, P.D.; Mittal, N.; Ahmed, U.S.; Khuu, N.; Kelley, O.S.; Kumacheva, E. Multifunctional 3D-Printed Wound Dressings. ACS Nano 2021, 15, 12375–12387.
- Gojgini, S.; Tokatlian, T.; Segura, T. Utilizing cell–matrix interactions to modulate gene transfer to stem cells inside hyaluronic acid hydrogels. Mol. Pharm. 2011, 8, 1582–1591.
- Youngblood, L.R.; Truong, F.N.; Segura, T.; Shea, D.L. Review: It’s All in the Delivery: Designing Hydrogels for Cell and Non-viral Gene Therapies. Mol. Ther. 2018, 26, 2087–2106.
- Spicer, C.D. Review: Hydrogel scaffolds for tissue engineering: The importance of polymer choice. Polym. Chem. 2020, 11, 184–219.
- Hou, Y.; Schoener, C.A.; Regan, K.R.; Munoz-Pinto, D.; Hahn, M.S.; Grunlan, M.A. Photo-cross-linked PDMSstar-PEG hydrogels: Synthesis, characterization, and potential application for tissue engineering scaffolds. Biomacromolecules 2010, 11, 648–656.
- Tavakoli, J.; Tang, Y. Review: Hydrogel Based Sensors for Biomedical Applications. Polymers 2017, 9, 364.
- Tavakoli, S.; Klar, S.A. Review: Advanced Hydrogels as Wound Dressings. Biomolecules 2020, 10, 1169.
- Metelmann, H.R.; Woedtke, T.V.; Weltmann, K.D. Comprehensive Clinical Plasma Medicine, Cold Physical Plasma for Medical Application; Springer: Berlin/Heidelberg, Germany, 2018; ISBN 978-3-319-67627-2.
- Tsegay, F.; Elsherif, M.; Butt, H. Smart 3D Printed Hydrogel Skin Wound Bandages: A Review. Polymers 2022, 14, 1012.
- Agale, S.V. Chronic leg ulcers: Epidemiology, aetiopathogenesis, and management. Ulcers 2013, 2013, 1–9.
- Misic, A.M.; Gardner, S.E.; Grice, E.A. The wound microbiome: Modern approached to examining the role of microorganisms in impaired chronic wound healing. Adv. Wound Care 2014, 3, 502–510.
- Comino-Sanz, I.M.; López-Franco, M.D.; Castro, B.; Pancorbo-Hidalgo, P.L. The Role of Antioxidants on Wound Healing: A Review of the Current Evidence. J. Clin. Med. 2021, 10, 3558.
- Cano, S.M.; Lancel, S.; Boulanger, E.; Neviere, R. Targeting Oxidative Stress and Mitochondrial Dysfunction in the Treatment of Impaired Wound Healing: A Systematic Review. Antioxidants 2018, 7, 98.
- Mehtani, D.; Seth, A.; Sharma, P.; Maheshwari, N.; Kapoor, D.; Shrivastava, K.S.; Tekade, K.R. Chapter 4: Biomaterials for Sustained and Controlled Delivery of Small Drug Molecules. In Biomaterials and Bionanotechnology Advances in Pharmaceutical Product Development and Research; Academic Press: Cambridge, MA, USA, 2019; pp. 89–152.
- Naomi, R.; Bahari, H.; Ridzuan, P.M.; Othman, F. Natural-Based Biomaterial for Skin Wound Healing (Gelatin vs. Collagen): Expert Review. Polymers 2021, 13, 2319.
- Neves, N.M.; Reis, R.L. (Eds.) Biomaterials from Nature for Advanced Devices and Therapies. In Chapter 3: Gelatin-Based Biomaterials for Tissue Engineering and Stem Cell Bioengineering; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 37–62.
- Dash, R.; Foston, M.; Ragauskas, A.J. Improving the mechanical and thermal properties of gelatin hydrogels cross-linked by cellulose nanowhiskers. Carbohydr. Polym. 2013, 91, 638–645.
- Kim, S.; Park, K. Tailor-Made Hydrogels for Tumor Delivery, Drug Delivery in Oncology; Wiley-VCH Verlag GmbH & Co. KGaA.: Weinheim, Germany, 2011; pp. 1071–1097.
- Shojaeiarania, J.; Bajwaa, D.; Shirzadifar, A. A review on cellulose nanocrystals as promising biocompounds for the synthesis of nanocomposite hydrogels. Carbohydr. Polym. 2019, 216, 247–259.
- Liu, S.; Qamar, A.S.; Qamar, M.; Basharat, K.; Bilal, M. Review: Engineered nanocellulose-based hydrogels for smart drug delivery applications. Int. J. Biol. Macromol. 2021, 181, 275–290.
- Liu, Y.; Song, S.; Liu, S.; Zhu, X.; Wang, P. Application of Nanomaterial in Hydrogels Related to Wound Healing. A review. J. Nanomater. 2022, 2022, 4656037.
- Zhou, Y.; Saito, T.; Bergström, L.; Isogai, A. Acid-Free Preparation of Cellulose Nanocrystals by TEMPO Oxidation and Subsequent Cavitation. Biomacromolecules 2018, 19, 633–639.
- Wegrzynowska-Drzymalska, K.; Mlynarczyk, D.T.; Chelminiak-Dudkiewicz, D.; Kaczmarek, H.; Goslinski, T.; Ziegler-Borowska, M. Chitosan-Gelatin Films Cross-Linked with Dialdehyde Cellulose Nanocrystals as Potential Materials for Wound Dressings. Int. J. Mol. Sci. 2022, 23, 9700.
- Lu, F.-F.; Yu, H.-Y.; Zhou, Y.; Yao, J.-M. Spherical and rod-like dialdehyde cellulose nanocrystals by sodium periodate oxidation: Optimization with double response surface model and templates for silver nanoparticles. Express. Polym. Lett. 2016, 10, 965–976.
- Zhang, P.; Chen, L.; Zhang, Q.S.; Hong, F.F. Using in situ dynamic cultures to rapidly biofabricate fabric-reinforced composites of chitosan/bacterial nanocellulose for antibacterial wound dressings. Front. Microbiol. 2016, 7, 260.
- Güiza-Argüello, V.R.; Solarte-David, V.A.; Pinzón-Mora, A.V.; Ávila-Quiroga, J.E.; Becerra-Bayona, S.M. Current Advances in the Development of Hydrogel-Based Wound Dressings for Diabetic Foot Ulcer Treatment. Polymers 2022, 14, 2764.
- Gupta, A.; Kowalczuk, M.; Heaselgrave, W.; Britland, S.T.; Martin, C.; Radecka, I. The Production and Application of Hydrogels for Wound Management: A Review. Eur. Polym. J. 2019, 111, 134–151.
- Alven, S.; Aderibigbe, B.A. Chitosan and Cellulose-Based Hydrogels for Wound Management. Int. J. Mol. Sci. 2020, 21, 9656.
- Hamedi, H.; Moradi, S.; Hudson, S.M.; Tonelli, A.E. Chitosan based hydrogels and their applications for drug delivery in wound dressings: A review. Carbohydr. Polym. 2018, 199, 445–460.
- Liu, H.; Wang, C.; Li, C.; Qin, Y.; Wang, Z.; Yang, F.; Li, Z.; Wang, J. A functional chitosan-based hydrogel as a wound dressing and drug delivery system in the treatment of wound healing. RSC Adv. 2018, 8, 7533–7549.
