Normal cardiac automaticity is dependent on the pacemaker cells of the sinoatrial node (SAN). Insufficient cardiac pacemaking leads to the development of sick sinus syndrome (SSS). The development of more effective treatments for SSS, including biological pacemakers, requires further understanding of these genes and signaling pathways. Compared with genetic models and bulk RNA sequencing, single-cell RNA sequencing (scRNA-seq) technology promises to advance our understanding of cellular phenotype heterogeneity and molecular regulation during SAN development.
Normal cardiac automaticity is dependent on the pacemaker cells of the sinoatrial node (SAN). Insufficient cardiac pacemaking leads to the development of sick sinus syndrome (SSS). The development of more effective treatments for SSS, including biological pacemakers, requires further understanding of these genes and signaling pathways. Compared with genetic models and bulk RNA sequencing, single-cell RNA sequencing (scRNA-seq) technology promises to advance the understanding of cellular phenotype heterogeneity and molecular regulation during SAN development.
- sinoatrial node
- single-cell RNA sequencing
- transcription factors
1. Introduction
2. SAN Structure
The human SAN is a compact, slightly elongated, banana-shaped structure located at the junction of the lateral superior vena cava (SVC) and right atrium (RA). Functional and structural mapping of the human SAN has revealed that it can be distinguished into head, center, and tail compartments. The head is usually closer to the epicardium, and the tail is typically tilted toward the endocardium [15,16,17,18,19][13][14][15][16][17]. Different regions of the SAN pacemaker clusters exhibit diverse functional and molecular features, which are activated through distinct cellular mechanisms [20,21,22][18][19][20] (Figure 1A). In mice, the SAN artery, autonomic nerve fibers, telocytes, monocytes, macrophages, adipocytes, and fibroblasts were observed via electron microscopy [23][21]. High populations of fibroblasts in the SAN maintain the source–sink balance and ensure normal electrical conduction [3]. The heterogeneous structure of the SAN makes studying its function challenging. Development of the scRNA-seq technology has aided in examining these heterogeneous and rare cells.2.1. Three-Dimensional Structure of the SAN
In adult mice, the SAN head/center, which is densely packed with clusters of PCs, is the leading pacemaker region, extends superiorly, and wraps the right SVC [24][22]. The SAN also extends inferiorly toward the inferior vena cava (IVC), forming the tail [25,26][23][24]. In mice, the SAN can be genetically divided into a TBX18+/NKX2-5− head domain and a TBX18−/NKX2-5+ tail domain [7] (Figure 1B,C). TBX18 deletion in the SAN head of mice has been shown to result in a normal heart rate [7], whereas SHOX2 ablation in the SAN tail has led to severe SSS [27][25]. These findings highlight the different roles of different parts of the SAN in pacemaking and conduction. Understanding the heterogeneity of the SAN complex is necessary to fully characterize its pacing and conduction functions. To further explore the complex 3D anatomy of the SAN, Goodyer et al. used scRNA-seq in E16.5 mice [10]. The cells underwent unsupervised clustering via t-distributed stochastic neighbor embedding (t-SNE). Cluster 9 showed significant enrichment of nodal markers. Further experiments identified two distinct subclusters within cluster 9, i.e., compact SAN (cSAN) and transitional cells (Tz). cSAN showed high levels of established SAN markers with low or no known atrial myocardium (AM) marker expression [10]. Further investigation of the cellular heterogeneity of the cSAN by Goodyer et al. revealed two distinct clusters in the cSAN, consistent with the previously recognized head and tail subdomains [10]. Both clusters showed high expressions of the nodal markers HCN4 and SHOX2. The head cluster showed increased expression of TBX18 and decreased expression of NKX2-5, whereas the tail cluster showed decreased expression of TBX18 and increased expression of NKX2-5 [7], consistent with previous reports [28][26]. A comparison of these two clusters revealed differentially expressed genes not previously reported. IGFBP5, PDE1A, and VSNL1 were abundant in the head, whereas SMPX, ALDH1B1, and SLC22A1 were abundant in the tail. These findings may enable the examination of different functional characteristics of the head and tail [10]. To further explore the different functional and molecular bases of the SAN head and tail, Li et al. (2019) examined the action potentials (APs) of the SAN’s head and tail separately using a patch clamp and found that the SAN tail has unique APs that mediate the SAN head and A. By performing scRNA-seq assays on SAN regions highlighted by GFP isolated from E13.5 SHOX2Cre/+ in R26RmTmG mice, they also verified that NKX2-5 plays an important role in the development and function of the SAN tail. They found four cardiomyocyte subpopulations, one of which expressed both SAN- and AM-specific genes, consistent with the properties of the SAN tail. They also revealed that the SAN tail has unique electrophysiological properties and transcriptomic features [29][27]. According to Li et al.’s integrated NIOM-3D structural study, the human SAN is functionally insulated/discontinued from the atria by fibrotic tissue, fat, and myofibers, with the exception of 2–5 discrete sinoatrial conduction pathways (SACPs) [19,30][17][28]. SACPs transmit electrical impulses to the RA [31,32][29][30]. SACP structure includes tracts of myofibers with transitional cells on the SAN border that merge with atrial myobundles [33][31]. The human fetal SAN contains predominantly PCs and limited collagen and transitional cells. From approximately 2 weeks after birth, Tz gradually increase in number and intertwine with the collagen framework [34][32]. Importantly, Tz have been implicated in SSS [33][31]. However, they remain poorly understood due to identification and isolation challenges. Goodyer et al. provided transcriptomic data for Tz [10]. In their study, Tz expressed lower levels of nodal markers and were enriched for AM markers GJA5 and SCN5A [10]. However, no new markers were specific to Tz, and it remains uncertain as to how Tz enter the SAN architecture.2.2. SAN Microenvironment
The unique microenvironment of the SAN maintains its functional characteristics. However, the formation and function of the SAN microenvironment remain poorly understood. To determine the cellular processes that contribute to the formation of the SAN microenvironment, Bressan et al. performed RNA-seq on chicken embryos earlier than E3.5 and found that factors associated with epithelial-to-mesenchymal transition and fibrosis were upregulated in the pacemaker region [35][33]. Whole-mount in situ hybridization and microsurgery demonstrated that proepicardium-derived mesenchymal cells (including fibroblasts) contributed to the formation of the SAN microenvironment and that the integration of mesenchymal cells is essential to protecting PCs from the electrical “load” of the adjacent AM [35][33]. However, how mesenchymal cells interact with PCs and regulate physiological functions in the SAN requires further investigation. Recently, Chou et al. compared TBX18-induced PCs and ventricular myocardium via RNA-seq and showed the predominance of glucose metabolism and glycolysis in the GO analysis [36][34]. TBX18-induced PCs co-culturing with fibroblasts activated the integrin-dependent mitogen-activated protein kinase-E2F1 signal through cell–cell contact and induced Aldoc expression in PCs [36][34]. Their results provided new insights into the link between fibroblasts and PCs and a new way to mediate SSS therapy by regulating Aldoc. The human SAN comprises 35%–55% fibrotic tissue [37][35]; cardiac diseases may further increase fibrosis within the SAN, potentially impairing automaticity and the conduction of electricity to the atria. Recently, Li et al. described the transcriptional characteristics of fibroblasts in the SANs of patients with heart failure but did not further elaborate on the connection between fibroblasts and PCs [38][36]. In addition to fibroblasts, many other cell types exist in the SAN [23][21]. The interactions among these cells and their roles in maintaining sinus rhythm have not been well elucidated. Single-cell transcriptomics is a promising tool for investigating cell–cell interaction networks [39][37], which is also the direction of ourthe research group in the future.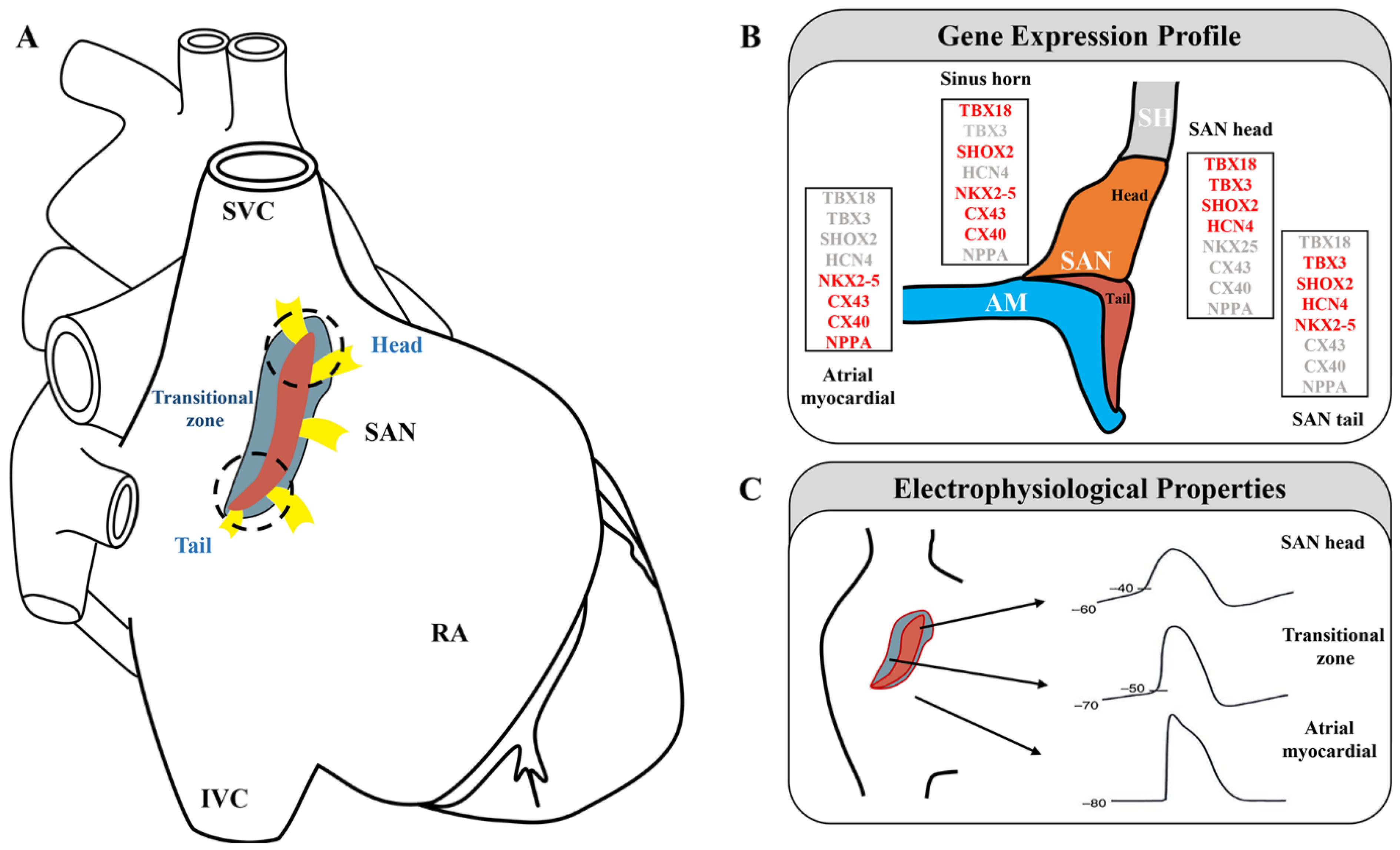
3. Transcriptional Regulation and Specific Molecular Markers of the SAN
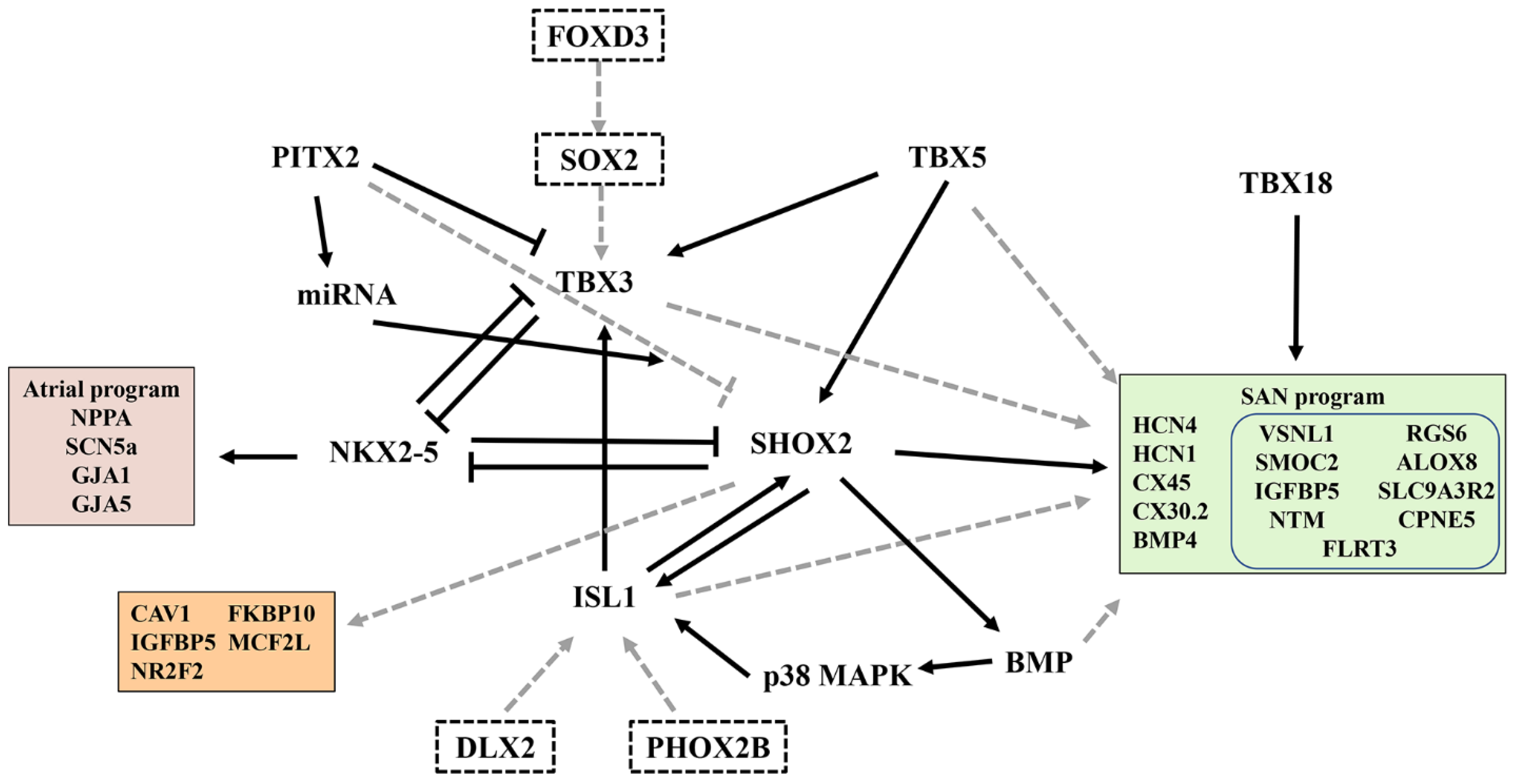
3.1. Transcription Factors
3.2. Specific Molecular Markers of the SAN
References
- Wallace, M.J.; El Refaey, M.; Mesirca, P.; Hund, T.J.; Mangoni, M.E.; Mohler, P.J. Genetic Complexity of Sinoatrial Node Dysfunction. Front. Genet. 2021, 12, 654925.
- Weiss, J.N.; Qu, Z. The Sinus Node: Still Mysterious After All These Years. JACC Clin. Electrophysiol. 2020, 6, 1841–1843.
- Csepe, T.A.; Kalyanasundaram, A.; Hansen, B.J.; Zhao, J.; Fedorov, V.V. Fibrosis: A structural modulator of sinoatrial node physiology and dysfunction. Front Physiol 2015, 6, 37.
- Mori, A.D.; Zhu, Y.; Vahora, I.; Nieman, B.; Koshiba-Takeuchi, K.; Davidson, L.; Pizard, A.; Seidman, J.G.; Seidman, C.E.; Chen, X.J.; et al. Tbx5-dependent rheostatic control of cardiac gene expression and morphogenesis. Dev. Biol. 2006, 297, 566–586.
- Hoogaars, W.M.; Engel, A.; Brons, J.F.; Verkerk, A.O.; de Lange, F.J.; Wong, L.Y.; Bakker, M.L.; Clout, D.E.; Wakker, V.; Barnett, P.; et al. Tbx3 controls the sinoatrial node gene program and imposes pacemaker function on the atria. Genes Dev. 2007, 21, 1098–1112.
- Espinoza-Lewis, R.A.; Yu, L.; He, F.; Liu, H.; Tang, R.; Shi, J.; Sun, X.; Martin, J.F.; Wang, D.; Yang, J.; et al. Shox2 is essential for the differentiation of cardiac pacemaker cells by repressing Nkx2-5. Dev. Biol. 2009, 327, 376–385.
- Wiese, C.; Grieskamp, T.; Airik, R.; Mommersteeg, M.T.; Gardiwal, A.; de Gier-de Vries, C.; Schuster-Gossler, K.; Moorman, A.F.; Kispert, A.; Christoffels, V.M. Formation of the sinus node head and differentiation of sinus node myocardium are independently regulated by Tbx18 and Tbx3. Circ. Res. 2009, 104, 388–397.
- Mommersteeg, M.T.; Hoogaars, W.M.; Prall, O.W.; de Gier-de Vries, C.; Wiese, C.; Clout, D.E.; Papaioannou, V.E.; Brown, N.A.; Harvey, R.P.; Moorman, A.F.; et al. Molecular pathway for the localized formation of the sinoatrial node. Circ. Res. 2007, 100, 354–362.
- Mantri, S.; Wu, S.M.; Goodyer, W.R. Molecular Profiling of the Cardiac Conduction System: The Dawn of a New Era. Curr. Cardiol. Rep. 2021, 23, 103.
- Goodyer, W.R.; Beyersdorf, B.M.; Paik, D.T.; Tian, L.; Li, G.; Buikema, J.W.; Chirikian, O.; Choi, S.; Venkatraman, S.; Adams, E.L.; et al. Transcriptomic Profiling of the Developing Cardiac Conduction System at Single-Cell Resolution. Circ. Res. 2019, 125, 379–397.
- Park, D.S.; Fishman, G.I. The cardiac conduction system. Circulation 2011, 123, 904–915.
- Cao, J.; Spielmann, M.; Qiu, X.; Huang, X.; Ibrahim, D.M.; Hill, A.J.; Zhang, F.; Mundlos, S.; Christiansen, L.; Steemers, F.J.; et al. The single-cell transcriptional landscape of mammalian organogenesis. Nature 2019, 566, 496–502.
- Sanchez-Quintana, D.; Cabrera, J.A.; Farre, J.; Climent, V.; Anderson, R.H.; Ho, S.Y. Sinus node revisited in the era of electroanatomical mapping and catheter ablation. Heart 2005, 91, 189–194.
- Keith, A.; Flack, M. The Form and Nature of the Muscular Connections between the Primary Divisions of the Vertebrate Heart. J. Anat. Physiol. 1907, 41, 172–189.
- James, T.N.; Sherf, L.; Fine, G.; Morales, A.R. Comparative ultrastructure of the sinus node in man and dog. Circulation 1966, 34, 139–163.
- Truex, R.C.; Smythe, M.Q.; Taylor, M.J. Reconstruction of the human sinoatrial node. Anat. Rec. 1967, 159, 371–378.
- Kalyanasundaram, A.; Li, N.; Augostini, R.S.; Weiss, R.; Hummel, J.D.; Fedorov, V.V. Three-Dimensional Functional Anatomy of Human Sinoatrial node for Epicardial and Endocardial Mapping and Ablation. Heart Rhythm. 2022, 22, S1547–S5271.
- Lang, D.; Petrov, V.; Lou, Q.; Osipov, G.; Efimov, I.R. Spatiotemporal control of heart rate in a rabbit heart. J. Electrocardiol. 2011, 44, 626–634.
- Monfredi, O.; Tsutsui, K.; Ziman, B.; Stern, M.D.; Lakatta, E.G.; Maltsev, V.A. Electrophysiological heterogeneity of pacemaker cells in the rabbit intercaval region, including the SA node: Insights from recording multiple ion currents in each cell. Am. J. Physiol. Heart Circ. Physiol. 2018, 314, H403–H414.
- Murphy, C.; Lazzara, R. Current concepts of anatomy and electrophysiology of the sinus node. J. Interv. Card. Electrophysiol. 2016, 46, 9–18.
- Linscheid, N.; Logantha, S.; Poulsen, P.C.; Zhang, S.; Schrolkamp, M.; Egerod, K.L.; Thompson, J.J.; Kitmitto, A.; Galli, G.; Humphries, M.J.; et al. Quantitative proteomics and single-nucleus transcriptomics of the sinus node elucidates the foundation of cardiac pacemaking. Nat. Commun. 2019, 10, 2889.
- Verheijck, E.E.; van Kempen, M.J.; Veereschild, M.; Lurvink, J.; Jongsma, H.J.; Bouman, L.N. Electrophysiological features of the mouse sinoatrial node in relation to connexin distribution. Cardiovasc. Res. 2001, 52, 40–50.
- Dobrzynski, H.; Boyett, M.R.; Anderson, R.H. New insights into pacemaker activity: Promoting understanding of sick sinus syndrome. Circulation 2007, 115, 1921–1932.
- Dobrzynski, H.; Li, J.; Tellez, J.; Greener, I.D.; Nikolski, V.P.; Wright, S.E.; Parson, S.H.; Jones, S.A.; Lancaster, M.K.; Yamamoto, M.; et al. Computer three-dimensional reconstruction of the sinoatrial node. Circulation 2005, 111, 846–854.
- Ye, W.; Wang, J.; Song, Y.; Yu, D.; Sun, C.; Liu, C.; Chen, F.; Zhang, Y.; Wang, F.; Harvey, R.P.; et al. A common Shox2-Nkx2-5 antagonistic mechanism primes the pacemaker cell fate in the pulmonary vein myocardium and sinoatrial node. Development 2015, 142, 2521–2532.
- Christoffels, V.M.; Smits, G.J.; Kispert, A.; Moorman, A.F. Development of the pacemaker tissues of the heart. Circ. Res. 2010, 106, 240–254.
- Li, H.; Li, D.; Wang, Y.; Huang, Z.; Xu, J.; Yang, T.; Wang, L.; Tang, Q.; Cai, C.L.; Huang, H.; et al. Nkx2-5 defines a subpopulation of pacemaker cells and is essential for the physiological function of the sinoatrial node in mice. Development 2019, 146, dev178145.
- Li, N.; Hansen, B.J.; Csepe, T.A.; Zhao, J.; Ignozzi, A.J.; Sul, L.V.; Zakharkin, S.O.; Kalyanasundaram, A.; Davis, J.P.; Biesiadecki, B.J.; et al. Redundant and diverse intranodal pacemakers and conduction pathways protect the human sinoatrial node from failure. Sci. Transl. Med. 2017, 9, eaam5607.
- Boineau, J.P.; Canavan, T.E.; Schuessler, R.B.; Cain, M.E.; Corr, P.B.; Cox, J.L. Demonstration of a widely distributed atrial pacemaker complex in the human heart. Circulation 1988, 77, 1221–1237.
- Fedorov, V.V.; Glukhov, A.V.; Chang, R. Conduction barriers and pathways of the sinoatrial pacemaker complex: Their role in normal rhythm and atrial arrhythmias. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H1773–H1783.
- Csepe, T.A.; Zhao, J.; Hansen, B.J.; Li, N.; Sul, L.V.; Lim, P.; Wang, Y.; Simonetti, O.P.; Kilic, A.; Mohler, P.J.; et al. Human sinoatrial node structure: 3D microanatomy of sinoatrial conduction pathways. Prog. Biophys. Mol. Biol. 2016, 120, 164–178.
- James, T.N. Structure and function of the sinus node, AV node and His bundle of the human heart: Part I-structure. Prog. Cardiovasc. Dis. 2002, 45, 235–267.
- Bressan, M.; Henley, T.; Louie, J.D.; Liu, G.; Christodoulou, D.; Bai, X.; Taylor, J.; Seidman, C.E.; Seidman, J.G.; Mikawa, T. Dynamic Cellular Integration Drives Functional Assembly of the Heart’s Pacemaker Complex. Cell Rep. 2018, 23, 2283–2291.
- Chou, P.C.; Liu, C.M.; Weng, C.H.; Yang, K.C.; Cheng, M.L.; Lin, Y.C.; Yang, R.B.; Shyu, B.C.; Shyue, S.K.; Liu, J.D.; et al. Fibroblasts Drive Metabolic Reprogramming in Pacemaker Cardiomyocytes. Circ. Res. 2022, 131, 6–20.
- Shiraishi, I.; Takamatsu, T.; Minamikawa, T.; Onouchi, Z.; Fujita, S. Quantitative histological analysis of the human sinoatrial node during growth and aging. Circulation 1992, 85, 2176–2184.
- Kalyanasundaram, A.; Li, N.; Gardner, M.L.; Artiga, E.J.; Hansen, B.J.; Webb, A.; Freitas, M.A.; Pietrzak, M.; Whitson, B.A.; Mokadam, N.A.; et al. Fibroblast-Specific Proteo-Transcriptomes Reveal Distinct Fibrotic Signatures of Human Sinoatrial Node in Non-Failing and Failing Hearts. Circulation 2021, 144, 126–143.
- Zhang, L.; Li, Z.; Skrzypczynska, K.M.; Fang, Q.; Zhang, W.; O’Brien, S.A.; He, Y.; Wang, L.; Zhang, Q.; Kim, A.; et al. Single-Cell Analyses Inform Mechanisms of Myeloid-Targeted Therapies in Colon Cancer. Cell 2020, 181, 442–459.e429.
- Van Eif, V.W.W.; Devalla, H.D.; Boink, G.J.J.; Christoffels, V.M. Transcriptional regulation of the cardiac conduction system. Nat. Rev. Cardiol. 2018, 15, 617–630.
- Chandler, N.J.; Greener, I.D.; Tellez, J.O.; Inada, S.; Musa, H.; Molenaar, P.; Difrancesco, D.; Baruscotti, M.; Longhi, R.; Anderson, R.H.; et al. Molecular architecture of the human sinus node: Insights into the function of the cardiac pacemaker. Circulation 2009, 119, 1562–1575.
- Darche, F.F.; Rivinius, R.; Kollensperger, E.; Leimer, U.; Germann, G.; Seckinger, A.; Hose, D.; Schroter, J.; Bruehl, C.; Draguhn, A.; et al. Pacemaker cell characteristics of differentiated and HCN4-transduced human mesenchymal stem cells. Life Sci. 2019, 232, 116620.
- Kalyanasundaram, A.; Li, N.; Hansen, B.J.; Zhao, J.; Fedorov, V.V. Canine and human sinoatrial node: Differences and similarities in the structure, function, molecular profiles, and arrhythmia. J. Vet. Cardiol. 2019, 22, 2–19.
- Scavone, A.; Capilupo, D.; Mazzocchi, N.; Crespi, A.; Zoia, S.; Campostrini, G.; Bucchi, A.; Milanesi, R.; Baruscotti, M.; Benedetti, S.; et al. Embryonic stem cell-derived CD166+ precursors develop into fully functional sinoatrial-like cells. Circ. Res. 2013, 113, 389–398.
- Liang, X.; Evans, S.M.; Sun, Y. Insights into cardiac conduction system formation provided by HCN4 expression. Trends Cardiovasc. Med. 2015, 25, 1–9.
- Vedantham, V.; Galang, G.; Evangelista, M.; Deo, R.C.; Srivastava, D. RNA sequencing of mouse sinoatrial node reveals an upstream regulatory role for Islet-1 in cardiac pacemaker cells. Circ. Res. 2015, 116, 797–803.
- Liang, X.; Zhang, Q.; Cattaneo, P.; Zhuang, S.; Gong, X.; Spann, N.J.; Jiang, C.; Cao, X.; Zhao, X.; Zhang, X.; et al. Transcription factor ISL1 is essential for pacemaker development and function. J. Clin. Investig. 2015, 125, 3256–3268.
- Hoffmann, S.; Schmitteckert, S.; Raedecke, K.; Rheinert, D.; Diebold, S.; Roeth, R.; Weiss, B.; Granzow, M.; Niesler, B.; Griesbeck, A.; et al. Network-driven discovery yields new insight into Shox2-dependent cardiac rhythm control. Biochim. Biophys. Acta Gene Regul. Mech. 2021, 1864, 194702.
- Galli, D.; Dominguez, J.N.; Zaffran, S.; Munk, A.; Brown, N.A.; Buckingham, M.E. Atrial myocardium derives from the posterior region of the second heart field, which acquires left-right identity as Pitx2c is expressed. Development 2008, 135, 1157–1167.
- Ammirabile, G.; Tessari, A.; Pignataro, V.; Szumska, D.; Sutera Sardo, F.; Benes, J., Jr.; Balistreri, M.; Bhattacharya, S.; Sedmera, D.; Campione, M. Pitx2 confers left morphological, molecular, and functional identity to the sinus venosus myocardium. Cardiovasc. Res. 2012, 93, 291–301.
- Wang, J.; Klysik, E.; Sood, S.; Johnson, R.L.; Wehrens, X.H.; Martin, J.F. Pitx2 prevents susceptibility to atrial arrhythmias by inhibiting left-sided pacemaker specification. Proc. Natl. Acad. Sci. USA 2010, 107, 9753–9758.
- Hill, M.C.; Kadow, Z.A.; Li, L.; Tran, T.T.; Wythe, J.D.; Martin, J.F. A cellular atlas of Pitx2-dependent cardiac development. Development 2019, 146, dev180398.
- Aminu, A.J.; Petkova, M.; Atkinson, A.J.; Yanni, J.; Morris, A.D.; Simms, R.T.; Chen, W.; Yin, Z.; Kuniewicz, M.; Holda, M.K.; et al. Further insights into the molecular complexity of the human sinus node—The role of ‘novel’ transcription factors and microRNAs. Prog. Biophys. Mol. Biol. 2021, 166, 86–104.
- Raghunathan, S.; Islas, J.F.; Mistretta, B.; Iyer, D.; Shi, L.; Gunaratne, P.H.; Ko, G.; Schwartz, R.J.; McConnell, B.K. Conversion of human cardiac progenitor cells into cardiac pacemaker-like cells. J. Mol. Cell. Cardiol. 2020, 138, 12–22.
- Van Eif, V.W.W.; Stefanovic, S.; van Duijvenboden, K.; Bakker, M.; Wakker, V.; de Gier-de Vries, C.; Zaffran, S.; Verkerk, A.O.; Boukens, B.J.; Christoffels, V.M. Transcriptome analysis of mouse and human sinoatrial node cells reveals a conserved genetic program. Development 2019, 146, dev173161.
- Yang, J.; Huang, J.; Maity, B.; Gao, Z.; Lorca, R.A.; Gudmundsson, H.; Li, J.; Stewart, A.; Swaminathan, P.D.; Ibeawuchi, S.R.; et al. RGS6, a modulator of parasympathetic activation in heart. Circ. Res. 2010, 107, 1345–1349.
- Thomas, K.; Henley, T.; Rossi, S.; Costello, M.J.; Polacheck, W.; Griffith, B.E.; Bressan, M. Adherens junction engagement regulates functional patterning of the cardiac pacemaker cell lineage. Dev. Cell 2021, 56, 1498–1511.e7.
- Liang, D.; Xue, J.; Geng, L.; Zhou, L.; Lv, B.; Zeng, Q.; Xiong, K.; Zhou, H.; Xie, D.; Zhang, F.; et al. Cellular and molecular landscape of mammalian sinoatrial node revealed by single-cell RNA sequencing. Nat. Commun. 2021, 12, 287.
- Ola, R.; Lefebvre, S.; Braunewell, K.H.; Sainio, K.; Sariola, H. The expression of Visinin-like 1 during mouse embryonic development. Gene Expr. Patterns 2012, 12, 53–62.
