Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Amina Yu and Version 1 by Marit Knoop.
Research on oxytocin (OT) was pioneered in the 1920s by German anatomist Ernst Scharrer, after he identified unusual, large-shaped “glandule-like” cells in the hypothalamus of fish. A full anatomical, morphological and functional assessment would follow in the next 50 years, complemented by the Nobel-prize awarded for the synthesis of OT to Vincent du Vigneaud in 1955.
- oxytocin
- developing brain
- neuroprotection
- neuroinflammation
- amygdala
- microglia
1. Oxytocin Synthesis and Release to the Oxytocin Receptor
The human oxytocin (OT) gene is located on chromosome 20p. It consists of 3 exons and encodes for Pre-Pro-OT-Neurophysin I. This OT prohormone is cleaved successively by different enzymes into intermediate OT forms, and finally the mature, amidated OT form. OT-prohormone (at E14.5) and intermediate OT forms (at E16.5) are already detected in the embryonic phase, but mature OT is only detected after birth [38,39][1][2]. Although immature OT forms are gaining support to play a function (yet unspecified) in disorders such as ASD, the main form of functional OT is mature OT.
In the brain, the neuropeptide OT is mainly produced in the hypothalamus. It is principally produced in magnocellular and parvocellular neurons of the periventricular nucleus (PVN) and supraoptic nucleus (SON), with some additional OT-producing neurons in the nucleus circularis and rostral supraoptic nucleus [40][3]. OT is spread through the central nervous system in multiple ways. First, via axonal release, which classically takes place at the synapse but also includes “en passant” release from axonal varicosities [41][4]. In this type of OT release, action potentials trigger OT release from axonal synapses or boutons that directly project to synapses in various brain regions [42][5]. Where synaptic OT release is quick, en passant release is slow and diffusion-based, causing a 60–90 s delay in response [43][6]. Secondly, OT neuropeptide is spread through the brain via somatodendritic release, where OT is stored and released locally in the PVN in large dense-core vesicles via exocytosis from dendrites or the cell soma [44,45,46][7][8][9]. This way, OT can exert autocrine effects on its own cell, thereby creating a strong self-regulatory mechanism. Somatodendritic OT release can also affect surrounding neurons and glia cells. This type of OT release is not targeted for synaptic terminals, but involves volume transmission, in which OT can travel long distances in the brain via passive diffusion, or by bulk flow via the extracellular fluid or cerebral spinal fluid (CSF) that is accessed through the third ventricle located near the PVN [44,47][7][10]. Volume OT transmission is made possible by the long half-life of central OT (about 20 min in CSF). Of note, the difference in release mechanisms make OT neurons capable of managing axonal and dendritic release independently from each other [48][11]. In parallel, OT can be released into the bloodstream via the posterior pituitary gland [49][12] and act as a hormone. These two pathways are independent. Indeed, plasma OT concentrations show no relationship with OT levels in the CSF [28][13]. However, some studies have identified simultaneous OT projections to regions of the forebrain as well as the posterior pituitary [30][14], showing that certain situations such as stress, can invoke an increase in both central and peripheral OT release [50][15].
The effects of OT are implemented via its binding to the oxytocin receptor (OTR), which is a seven-transmembrane G-protein-coupled receptor. Expression of OTR has been found on excitatory and inhibitory neurons throughout the brain (Human protein atlas: https://www.proteinatlas.org/ENSG00000180914-OXTR/brain), but also on astrocytes and its presence on microglia is debated (see Section 5). OTR is not exclusively bound by OT, as it can also be bound (yet with a much lower affinity) by vasopressin (AVP), a nonapeptide that is only 2 out of 9 amino-acids different from OT, and located on the same chromosomal locus [28,51][13][16]. Likewise, OT can bind to AVP receptor subtypes V1a, V1b and V2 [52][17]. However, OT binds to OTR with a much higher affinity than to AVP receptors: the receptor affinity (Ki) of OT is 1.0 nм for OTR and 71 nм, 294 nм and 89 nм for V1a, V1b and V2, respectively [53][18]. Therefore, in this review, we will considererein, the actions of OT as acting exclusively via the OT receptor will be considered . For excellent papers on AVP and OT interaction, wit will be referred to [40,44,54][3][7][19].
2. Development of the Oxytocin System from the Embryonic to Juvenile Age
OTR autoradiography assays, mRNA assessments and immunohistochemistry have been used to map the central OT system, which includes the main targets of OT projections, and the areas with high OTR expression [55,56,57,58,59][20][21][22][23][24]. The OT system shows a large spatial and temporal plasticity of OTR expression during development. Moreover, the development of the OTR system shows different trajectories between humans and rodents, but also between mice and rats [28,55,60][13][20][25]. To improve translational opportunities between OTR experiments in rodents to eventual human patients, it is needed to highlight the similarities and differences of the developmental OTR system. Figure 1 summarizes the development of OTR expression from embryo to juvenile stages in rats, mice and humans. For more information on the specific proteins involved in the OTR network, we refer to Chatterjee and colleagues [61][26] who created an extensive map of the OTR pathway based on data from 1803 screened articles were referred to.
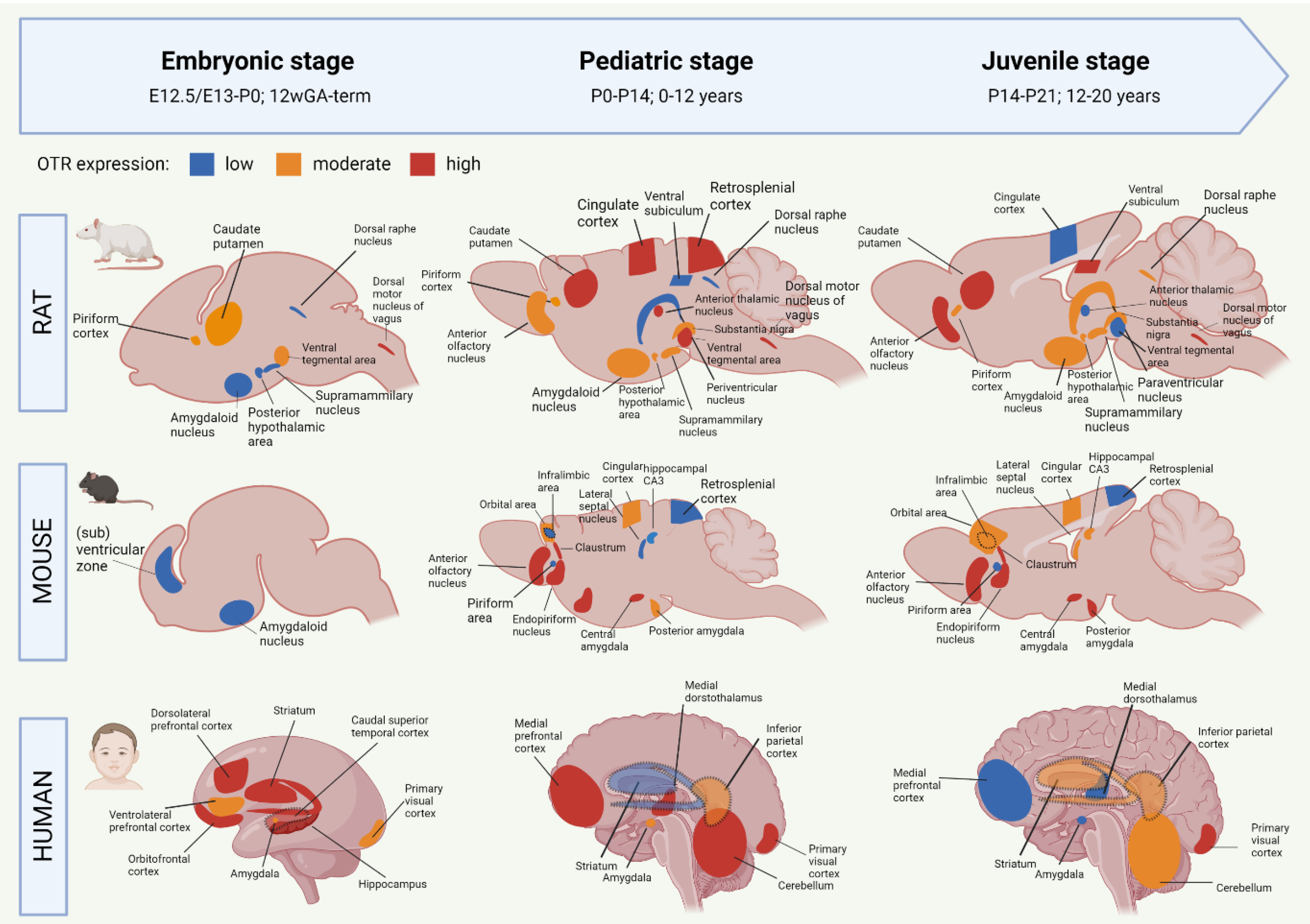
Figure 1. Early life development of the oxytocin receptor system in rat, mouse and human. Data based on OTR-binding autoradiography and OTR immunohistochemistry studies. The degree of OTR expression is visualized as low (blue), intermediate (orange) or high (red). Dashed regions that overlap other regions indicate a more lateral localization on the sagittal plane. Data based on [28,38,55,56,57,58,59][1][13][20][21][22][23][24]. GA = gestational age. Created with BioRender.com.
2.1. Oxytocin Receptor System of the Developing Rat Brain
Most of the studies on the OTR system have been performed in rats. Mature OT is produced from E21 onwards, but OT receptors are developed already before [62][27]. The earliest sighting of OTR mRNA in rats was found at E13/E15, in the posterior portion of the neuronal tube that will become the vagal motor nucleus [58][23]. At E20, OTR expression has spread to the tenia tecta (a component of the olfactory cortex), piriform cortex, caudate putamen, ventral tegmental area and several nuclei of the brainstem (Figure 1). It is only after P1 that OTR mRNA is starting to be expressed in the thalamic nuclei [58][23]. After birth, the distribution of OTR in rats becomes more localized and OTR expression starts to appear in new locations as well (Figure 1). The expression of OTR mRNA in the PVN peaks at P7 and remains stable throughout adulthood [28][13]. Around P10, a ‘pediatric’ pattern of OTR expression is found throughout the rat brain (Figure 1). In rats, mice, and mammals in general, no central OT projections are found during the embryonic and early postnatal phase, which indicates that early life OT signaling happens predominantly through dendritic release and volume transmission [60][25]. Axonal OT projections start emerging in the pediatric period. As such, axonal OT offers addressed modulation to the development of detailed skills that the young rodent undergoes in this period [60][25]. The distribution of OTR expression is highly transient during development, and only certain regions show OTR expression both in early life and in adulthood [63,64][28][29]. One of these regions is the amygdala, which shows clear OTR expression in all stages of rat development (Figure 1). Areas that show a surge in OTR expression that consequentially disappears after the postnatal period include the parietal and cingulate cortices, the caudate putamen and the PVN [38][1]. There are two periods in rat development that show a particular strong spatial shift in OTR expression. They are the third postnatal week (finalized at P18) and the juvenile age [28][13]. In juvenile rats, decreased expression of OTR can be found for the cingulate cortex, anterior thalamic nuclei, and ventral tegmental area (Figure 1). This suggests that these regions are involved in the juvenile-characteristic changes in social behavior such as an increase in risk-taking behavior and the self-regulatory system [65][30]. The rat adult OTR pattern is reached by P60.
2.2. Oxytocin Receptor System of the Developing Mouse Brain
Initial OT mRNA expression in mice is found at E15.5, and by P0, OT neurons are found in almost all hypothalamic nuclei [38,66][1][31]. Unlike rats, the production of mature OT in mice does not start until after birth [67][32]. Mouse OTR mRNA can be detected as early as E12.5, and from E16.5 onwards, OTR expression is shown in ventricular, subventricular zones as well as in amygdala (Figure 1) [68][33]. There appears to be a sex-effect, in that females show much higher levels of OTR transcriptome in the embryonic and postnatal period compared to males [68][33]. OTR expression increases generally during the first two postnatal weeks, and pediatric OTR patterns are reached between P7 and P14. At this age, OTR expression is found throughout the brain, including the olfactory bulbs, neocortex, hypothalamus, hippocampus and amygdala (Figure 1) [59][24]. Between the pediatric and the juvenile age, the general mouse OTR expression patterns are replaced by more region-specific up-or downregulation of OTR expression [59][24]. Namely, OTR expression remains high until P21 in the lateral septum and dorsal CA3, but OTR expression in the neocortex peaks at P14. Similar to the rat, this developmental peak of receptor expression in the neocortex of the mouse coincides with major developments in synaptic pruning and wiring that are taking place, which suggests that OT is involved in these synaptic processes [59][24]. The juvenile OTR pattern in the mouse is similar to the adult pattern. However, the juvenile age shows a characteristically higher OTR expression in the dorsal/intermediate lateral septum, the cingulate cortex and the posterior PVN, compared to adult-mice [69][34]. Moreover, OTR expression at the juvenile age is increased in the ventromedial hypothalamus, a region that is involved in social behaviors that develop after puberty (sexual and aggressive behaviors) [70][35]. The pattern of increased OTR expression in the juvenile age is seen in mice and rats alike. Some differences between these species are that juvenile mice show an abundant yet transient distribution of OTR in the cingulate cortex and hippocampal regions, which is much lower, but chronic in juvenile rats [69][34]. The cingulate cortex is important for reward-based behavior, which suggests that the different OTR expression patterns in this region between rats and mice could attribute to the species-specific differences in social behavior [71,72][36][37]. For further visualization of postnatal OTR mapping in mouse coupes between P7 and P56, we refer to tthe online tool developed by the Kim lab (https://kimlab.io/brain-map/OTR/) were referred to.
2.3. Oxytocin Receptor System of the Developing Human Brain
Human brain development is different from rodents in that many key processes happen earlier on the developmental timeline, including in the prenatal phase [73,74][38][39] (see [75][40] for an extensive overview of temporal differences in brain development between rodents and humans). As such, the neurodevelopmental equivalent of the human brain at birth corresponds to the rat and mouse brain at P10 [76][41]. Moreover, the human OTR system already reaches its infant-like spatial pattern before birth, while in rats and mice, this does not happen before P10 and P7–P14, respectively [38][1].
OT is detected in human embryos as early as 11 weeks of gestational age (GA) [54][19]. The production and migration of OT neurons are completed around 25 weeks GA, but their morphological and electrophysiological maturation does not finalize until the first two weeks after birth [77][42]. Transcriptomic analysis of human brains shows a progressive increase in OTR mRNA expression in the embryonic period [56][21]. This included the amygdala, mediodorsal thalamus, hippocampus, striatum and numerous neocortical areas (Figure 1). In the third trimester, a peak in OTR expression is found for the striatum, hippocampus and orbitofrontal, dorsolateral prefrontal and caudal superior temporal cortices [57][22]. This is followed by OTR increases in the amygdala, hippocampus, primary visual cortex and inferolateral temporal cortex just before birth (Figure 1). The peak level of OTR expression in humans occurs during early childhood (6 years of age), which coincides with increased OTR levels in the mediodorsal nucleus of the thalamus and medial prefrontal cortex [57][22]. Later childhood phases (12 years of age) show an increase in OTR expression in the medial prefrontal and cerebellar cortices, as well as the mediodorsal hypothalamus (Figure 1). Compared to the pediatric phase, the juvenile stage of the human OTR system seems characterized by a strong decrease in mediodorsal thalamus OTR expression, a moderately lower expression in the medial prefrontal cortex, as well as increased OTR expression in the striatum and primary auditory and visual cortices (Figure 1) [57][22]. Similar to rats and mice, some regions in the human OTR system show a peaked expression in the pediatric phase, followed by a decrease during adolescence [55,56,57,58][20][21][22][23]. These regions have key functions for brain development in the pediatric phase. They include the cerebellar cortex and primary motor cortex (linked with the immense development of the motor system during infancy [78][43]), and the primary visual and somatosensory cortices (the somatosensory cortex similarly peaks at the pediatric age in rodents). OTR expression notably decreases in the juvenile age in the striatum and the medial prefrontal cortex, which are two regions important for the control of behavior and social reward processing. The adolescent period is characterized by an increase in risk-taking behavior and negative health outcomes, which is associated with increased reactivity of the striatum in response to rewards [79][44]. The fact that OTR expression is decreased in this region during the adolescent age fits in this line, for it means that the limbic system has decreased control over the striatal response to rewards.
Although there are different OTR patterns across brain development in mice, rats and humans, the development in the amygdala stands out because it is one of the few regions that show OTR expression already prenatally in both mice and rats (Figure 1). Moreover, OTR expression in this limbic region is found in every developmental period, which makes the amygdala a probable important, chronic effector for the functions of OT in the brain.
3. The Functions of Oxytocin in the Brain
The wide-spread nature of the central OT system relates to with the diversity of functions identified for OT in the brain [28,30][13][14]. These include the traditional links with lactation, parturition and social behaviors such as social bonding and maternal behavior and aggression, but also extend to non-social behaviors such as anxiety, fear, decision making and memory [29,30,80,81,82,83][14][45][46][47][48][49]. Most of these functions are directed by central OT release, but some effects of OT, for example pain control, arrive from a combination of central OT projections onto the spinal cord, and hormonal OT circulating in the bloodstream [84][50]. It has further been shown that the OT system can self-adapt by increasing or decreasing the expression of OTR or density of OT fibers in response to environmental stimuli (for example, mother-infant bonding increases OTR expression in areas involved in social behavior [54][19]). However, while the density of OT connections can be variable, the spatial profile of connections appears to remain unchanged upon stimulation [30][14].
There are specific functional ‘subsystems’ within the OT network [85][51]. Recently, input-output wiring diagrams of OT neurons revealed 9 distinct circuit-specific functions for OT in the adult mouse brain [86][52]. These include internal state control, including attention and threat, somatic visceral control including pain and sensory regulation, and cognitive control including learning and value assessment [85,86][51][52]. We refer tFo [86] for an overview of the specific brain regions involved in each of the 9 functional circuits of OT, it will be referred to[52]. Hypothalamic OT neurons show axonal projections to most of the forebrain regions in the adult rat [30][14]. This includes the limbic system, olfactory system, basal ganglia and cortical areas. OT projections were also found in the hippocampus, which is functionally linked to the discrimination of social stimuli [87][53] and long-term social memory [88,89][54][55]. Social behavior and social decision-making are one of the strongest functional attributes known to the OT system [28,90][13][56]. Various OTR-enriched regions have been linked to aggression, sexual behavior, maternal care and pain inhibition (medial amygdala, lateral septum, stria terminalis, hypothalamus and periaqueductal and central gray) and the processing of salient social stimuli (basolateral amygdala, stria terminalis, lateral septum, nucleus accumbens, striatum, ventral pallidum, ventral tegmental area and prefrontal cortex) (reviewed in [60][25]).
In addition to the contributions of OT to functional brain development, OT also plays a role in structural brain development. OT contributes to neurite growth and the formation of neural circuits [91][57]. Moreover, experimental research in OT-knockout mice has shown that OT is required for the promotion of excitatory synaptic transmission during sensory cortical development [47][10]. Additionally, OTR expression in hippocampal CA3 has been associated with the promotion of cell survival and development of newly formed dentate granule cells [92][58]. Another major contribution of OT to normal brain development concerns the GABA switch. The GABA switch refers to the change from excitation to inhibition of the GABA neurotransmitter during the first week of life, which is important for the maturation of neuronal network functioning [93][59]. The large amount of OT that is released during parturition helps facilitate this switch [94,95][60][61]. Neonatal complications that reduce OT release, such as neonatal maternal separation, have shown to delay the GABA-switch, which induces an imbalance to excitation/inhibition, that is characteristic of many neurodevelopmental disorders [96,97][62][63].
Major Functional Part of the Oxytocin Network: The Amygdala
One area that has been extensively linked to OT functioning is the amygdala. Indeed, the study by Knobloch and colleagues [30][14] found a high number of OT fibers in the central and medial regions of the adult rat amygdala. This has been shown in humans as well [56][21], including on the transcriptome level, where amygdala-associated cognitive terms “anxiety”, “fear” and “emotional” showed a 97.5% stronger correlation with OTR mRNA expression than any other genes in a 20.737 gene dataset [90][56]. The amygdala plays a major role in social decision making [98,99][64][65]. Amygdala damage impairs social behavior, and OT injection to the basolateral amygdala stimulates prosocial behavior in primates [98][64]. Moreover, intranasal OT administration in humans increases social cognitive processing and emotional empathy, which is amygdala-dependent [100,101][66][67]. On a network level, the increased sociability following OT administration was associated with decreased amygdala reactivity to negative stimuli, but increased coupling between the right amygdala with the posterior cingulate cortex and insula for positive stimuli [100][66].
In line with this function of social decision making, there has been much support underlining the role of OT in modulating anxiety and fear via its effect on the central amygdala [30,31,80][14][46][68]. Optogenetic activation of OT neurons has been shown to decrease freezing responses in rats that were previously fear-conditioned, which was associated with enhanced glutamate co-release by the projecting OT terminals in the amygdala [29,30][14][45]. The OT-induced anxiolytic effect is further emphasized by studies that showed a reduction in fear memory retrieval after OT was infused into the central amygdala before the fear acquisition phase [31][68]. Regional specificity within the central amygdala has further identified “Fear-OFF” neurons in the lateral division, which are protein kinase C-δ-expressing GABAergic neurons that decrease firing upon fearful events [102][69]. Notably, OTR expression has been found in more than half of this neuronal population [102,103][69][70]. Moreover, it appears that astrocytes play a role in the OT-induced anxiolytic effect in the central amygdala. Gain- and loss-of-function paradigms demonstrated that a particular local astrocyte subtype positively reinforces the effects of OT on the central amygdala, which challenges the long-held idea that OT acts exclusively on neurons to modulate emotional states [32][71].
These data show that the contribution of OT to brain development is both functional and structural, because it helps form functional pathways between regions involved in important behaviors, but it also contributes to the growth and maturation of individual neurons. Given this importance of OT for brain development, it is likely that OT functioning is affected when perinatal complications cause the brain to develop abnormally.
References
- Grinevich, V.; Desarmã©Nien, M.G.; Chini, B.; Tauber, M.; Muscatelli, F. Ontogenesis of oxytocin pathways in the mammalian brain: Late maturation and psychosocial disorders. Front. Neuroanat. 2015, 8, 164.
- Muscatelli, F.; Desarménien, M.G.; Matarazzo, V.; Grinevich, V. Oxytocin Signaling in the Early Life of Mammals: Link to Neurodevelopmental Disorders Associated with ASD. Curr. Top Behav. Neurosci. 2018, 35, 239–268.
- Matsunaga, W.; Miyata, S.; Takamata, A.; Bun, H.; Nakashima, T.; Kiyohara, T. LPS-induced Fos expression in oxytocin and vasopressin neurons of the rat hypothalamus. Brain Res. 2000, 858, 9–18.
- Grinevich, V.; Ludwig, M. The multiple faces of the oxytocin and vasopressin systems in the brain. J. Neuroendocr. 2021, 33, e13004.
- Bakos, J.; Srancikova, A.; Havranek, T.; Bacova, Z. Molecular Mechanisms of Oxytocin Signaling at the Synaptic Connection. Neural Plast. 2018, 2018, 1–9.
- Chini, B.; Verhage, M.; Grinevich, V. The Action Radius of Oxytocin Release in the Mammalian CNS: From Single Vesicles to Behavior. Trends Pharmacol. Sci. 2017, 38, 982–991.
- Ludwig, M.; Apps, D.; Menzies, J.; Patel, J.C.; Rice, M.E. Dendritic Release of Neurotransmitters. Compr. Physiol. 2016, 7, 235–252.
- Ludwig, M.; Leng, G. Dendritic peptide release and peptide-dependent behaviours. Nat. Rev. Neurosci. 2006, 7, 126–136.
- Ludwig, M.; Stern, J. Multiple signalling modalities mediated by dendritic exocytosis of oxytocin and vasopressin. Philos Trans R Soc Lond B Biol Sci. 2015, 370, 20140182.
- Zheng, J.-J.; Li, S.-J.; Zhang, X.-D.; Miao, W.-Y.; Zhang, D.; Yao, H.; Yu, X. Oxytocin mediates early experience–dependent cross-modal plasticity in the sensory cortices. Nat. Neurosci. 2014, 17, 391–399.
- Goaillard, J.-M.; Moubarak, E.; Tapia, M.; Tell, F. Diversity of Axonal and Dendritic Contributions to Neuronal Output. Front. Cell. Neurosci. 2020, 13.
- Grinevich, V.; Hurlemann, R. (Eds.) Behavioral Pharmacology of Neuropeptides: Oxytocin, 1st ed.; Current Topics in Behavioral Neurosciences; Springer International Publishing: Cham, Switzerland, 2018; p. 1.
- Gimpl, G.; Fahrenholz, F. The Oxytocin Receptor System: Structure, Function, and Regulation. Physiol. Rev. 2001, 81, 629–683.
- Knobloch, H.S.; Charlet, A.; Hoffmann, L.C.; Eliava, M.; Khrulev, S.; Cetin, A.H.; Osten, P.; Schwarz, M.K.; Seeburg, P.H.; Stoop, R.; et al. Evoked Axonal Oxytocin Release in the Central Amygdala Attenuates Fear Response. Neuron 2012, 73, 553–566.
- Neumann, I. Stimuli and consequences of dendritic release of oxytocin within the brain. Biochem. Soc. Trans. 2007, 35, 1252–1257.
- Carter, C.S. Sex differences in oxytocin and vasopressin: Implications for autism spectrum disorders? Behav Brain Res. 2007, 176, 170–186.
- Busnelli, M.; Chini, B. Molecular Basis of Oxytocin Receptor Signalling in the Brain: What We Know and What We Need to Know. Curr. Top Behav. Neurosci. 2017, 35, 3–29.
- Manning, M.; Misicka, A.; Olma, A.; Bankowski, K.; Stoev, S.; Chini, B.; Durroux, T.; Mouillac, B.; Corbani, M.; Guillon, G. Oxytocin and Vasopressin Agonists and Antagonists as Research Tools and Potential Therapeutics. J. Neuroendocr. 2012, 24, 609–628.
- Hammock, E.A.D. Developmental Perspectives on Oxytocin and Vasopressin. Neuropsychopharmacology 2014, 40, 24–42.
- Newmaster, K.T.; Nolan, Z.T.; Chon, U.; Vanselow, D.J.; Weit, A.R.; Tabbaa, M.; Hidema, S.; Nishimori, K.; Hammock, E.A.D.; Kim, Y. Quantitative cellular-resolution map of the oxytocin receptor in postnatally developing mouse brains. Nat. Commun. 2020, 11, 1–12.
- Kang, H.J.; Kawasawa, Y.I.; Cheng, F.; Zhu, Y.; Xu, X.; Li, M.; Sousa, A.M.M.; Pletikos, M.; Meyer, K.A.; Sedmak, G.; et al. Spatio-temporal transcriptome of the human brain. Nature 2011, 478, 483–489.
- Rokicki, J.; Kaufmann, T.; de Lange, A.-M.G.; van der Meer, D.; Bahrami, S.; Sartorius, A.M.; Haukvik, U.K.; Steen, N.E.; Schwarz, E.; Stein, D.J.; et al. Oxytocin receptor expression patterns in the human brain across development. Neuropsychopharmacology 2022, 47, 1550–1560.
- Yoshimura, R.; Kimura, T.; Watanabe, D.; Kiyama, H. Differential expression of oxytocin receptor mRNA in the developing rat brain. Neurosci. Res. 1996, 24, 291–304.
- Hammock, E.A.D.; Levitt, P. Oxytocin receptor ligand binding in embryonic tissue and postnatal brain development of the C57BL/6J mouse. Front. Behav. Neurosci. 2013, 7.
- Grinevich, V.; Knobloch-Bollmann, H.S.; Eliava, M.; Busnelli, M.; Chini, B. Assembling the Puzzle: Pathways of Oxytocin Signaling in the Brain. Biol. Psychiatry 2015, 79, 155–164.
- Chatterjee, O.; Patil, K.; Sahu, A.; Gopalakrishnan, L.; Mol, P.; Advani, J.; Mukherjee, S.; Christopher, R.; Prasad, T.S.K. An overview of the oxytocin-oxytocin receptor signaling network. J. Cell Commun. Signal. 2016, 10, 355–360.
- Mitre, M.; Minder, J.; Morina, E.X.; Chao, M.V.; Froemke, R.C. Oxytocin Modulation of Neural Circuits. Curr. Top Behav. Neurosci. 2017, 35, 31–53.
- Kimura, T. Differential expression of oxytocin receptor mRNA in the developing rat brain. Neuroscience Research . 1 January 1996. Available online: https://www.academia.edu/27604227/Differential_expression_of_oxytocin_receptor_mRNA_in_the_developing_rat_brain (accessed on 3 November 2022).
- Tribollet, E.; Dubois-Dauphin, M.; Dreifuss, J.J.; Barberis, C.; Jard, S. Oxytocin Receptors in the Central Nervous System. Ann. New York Acad. Sci. 1992, 652, 29–38.
- McCutcheon, J.E.; Conrad, K.L.; Carr, S.B.; Ford, K.A.; McGehee, D.S.; Marinelli, M. Dopamine neurons in the ventral tegmental area fire faster in adolescent rats than in adults. J. Neurophysiol. 2012, 108, 1620–1630.
- Madrigal, M.P.; Jurado, S. Specification of oxytocinergic and vasopressinergic circuits in the developing mouse brain. Commun Biol. 2021, 4, 1–16.
- Schaller, F.; Watrin, F.; Sturny, R.; Massacrier, A.; Szepetowski, P.; Muscatelli, F. A single postnatal injection of oxytocin rescues the lethal feeding behaviour in mouse newborns deficient for the imprinted Magel2 gene. Hum. Mol. Genet. 2010, 19, 4895–4905.
- Tamborski, S.; Mintz, E.M.; Caldwell, H.K. Sex Differences in the Embryonic Development of the Central Oxytocin System in Mice. J. Neuroendocr. 2016, 28.
- Olazábal, D.E.; Alsina-Llanes, M. Are age and sex differences in brain oxytocin receptors related to maternal and infanticidal behavior in naïve mice? Horm. Behav. 2016, 77, 132–140.
- Onaka, T.; Takayanagi, Y. The oxytocin system and early-life experience-dependent plastic changes. J. Neuroendocrinol. 2021, 33, e13049.
- Netser, S.; Meyer, A.; Magalnik, H.; Zylbertal, A.; De La Zerda, S.H.; Briller, M.; Bizer, A.; Grinevich, V.; Wagner, S. Distinct dynamics of social motivation drive differential social behavior in laboratory rat and mouse strains. Nat. Commun. 2020, 11, 5908.
- Sachuriga; Nishimaru, H.; Takamura, Y.; Matsumoto, J.; Ferreira Pereira de Araújo, M.; Ono, T.; Nishijo, H. Neuronal Representation of Locomotion During Motivated Behavior in the Mouse Anterior Cingulate Cortex. Front. Syst. Neurosci. 2021, 15, 655110.
- Craig, A.; Luo, N.L.; Beardsley, D.J.; Wingate-Pearse, N.; Walker, D.W.; Hohimer, A.; A Back, S. Quantitative analysis of perinatal rodent oligodendrocyte lineage progression and its correlation with human. Exp. Neurol. 2003, 181, 231–240.
- Dobbing, J.; Sands, J. Comparative aspects of the brain growth spurt. Early Hum. Dev. 1979, 3, 79–83.
- Semple, B.D.; Blomgren, K.; Gimlin, K.; Ferriero, D.M.; Noble-Haeusslein, L.J. Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog. Neurobiol. 2013, 106-107, 1–16.
- Workman, A.D.; Charvet, C.J.; Clancy, B.; Darlington, R.B.; Finlay, B.L. Modeling Transformations of Neurodevelopmental Sequences across Mammalian Species. J. Neurosci. 2013, 33, 7368–7383.
- Swaab, D. Development of the human hypothalamus. Neurochem. Res. 1995, 20, 509–519.
- Cratty, B.J. Perceptual and Motor Development in Infants and Children, 2nd ed.; 1979.
- Peters, S.; Crone, E.A. Increased striatal activity in adolescence benefits learning. Nat. Commun. 2017, 8, 1–9.
- Hasan, M.T.; Althammer, F.; da Gouveia, M.S.; Goyon, S.; Eliava, M.; Lefevre, A.; Kerspern, D.; Schimmer, J.; Raftogianni, A.; Wahis, J.; et al. A Fear Memory Engram and Its Plasticity in the Hypothalamic Oxytocin System. Neuron 2019, 103, 133–146.e8.
- Bosch, O.; Meddle, S.L.; Beiderbeck, D.I.; Douglas, A.J.; Neumann, I.D. Brain Oxytocin Correlates with Maternal Aggression: Link to Anxiety. J. Neurosci. 2005, 25, 6807–6815.
- Lukas, M.; Toth, I.; Veenema, A.H.; Neumann, I.D. Oxytocin mediates rodent social memory within the lateral septum and the medial amygdala depending on the relevance of the social stimulus: Male juvenile versus female adult conspecifics. Psychoneuroendocrinology 2012, 38, 916–926.
- Pedersen, C.A.; Prange, A.J. Induction of maternal behavior in virgin rats after intracerebroventricular administration of oxytocin. Proc. Natl. Acad. Sci. USA 1979, 76, 6661–6665.
- Tapp, D.N.; Singstock, M.D.; Gottliebson, M.S.; McMurray, M.S. Central but not peripheral oxytocin administration reduces risk-based decision-making in male rats. Horm. Behav. 2020, 125, 104840.
- Eliava, M.; Melchior, M.; Knobloch-Bollmann, H.S.; Wahis, J.; Gouveia, M.D.S.; Tang, Y.; Ciobanu, A.C.; del Rio, R.T.; Roth, L.C.; Althammer, F.; et al. A New Population of Parvocellular Oxytocin Neurons Controlling Magnocellular Neuron Activity and Inflammatory Pain Processing. Neuron 2016, 89, 1291–1304.
- Manjila, S.B.; Betty, R.; Kim, Y. Missing pieces in decoding the brain oxytocin puzzle: Functional insights from mouse brain wiring diagrams. Front. Neurosci. 2020, 16, 1044736.
- Son, S.; Manjila, S.B.; Newmaster, K.T.; Wu, Y.-T.; Vanselow, D.J.; Ciarletta, M.; Anthony, T.E.; Cheng, K.C.; Kim, Y. Whole-Brain Wiring Diagram of Oxytocin System in Adult Mice. J. Neurosci. 2022, 42, 5021–5033.
- Raam, T.; McAvoy, K.M.; Besnard, A.; Veenema, A.H.; Sahay, A. Hippocampal oxytocin receptors are necessary for discrimination of social stimuli. Nat. Commun. 2017, 8, 2001.
- Lin, Y.T.; Hsieh, T.Y.; Tsai, T.C.; Chen, C.C.; Huang, C.C.; Hsu, K.S. Conditional Deletion of Hippocampal CA2/CA3a Oxytocin Receptors Impairs the Persistence of Long-Term Social Recognition Memory in Mice. J. Neurosci. 2018, 38, 1218–1231.
- Tsai, T.-C.; Fang, Y.-S.; Hung, Y.-C.; Hung, L.-C.; Hsu, K.-S. A dorsal CA2 to ventral CA1 circuit contributes to oxytocinergic modulation of long-term social recognition memory. J. Biomed. Sci. 2022, 29, 1–20.
- Quintana, D.S.; Rokicki, J.; van der Meer, D.; Alnæs, D.; Kaufmann, T.; Córdova-Palomera, A.; Dieset, I.; Andreassen, O.A.; Westlye, L.T. Oxytocin pathway gene networks in the human brain. Nat. Commun. 2019, 10, 1–12.
- Bakos, J.; Zatkova, M.; Bacova, Z.; Ostatnikova, D. The Role of Hypothalamic Neuropeptides in Neurogenesis and Neuritogenesis. Neural Plast. 2016, 2016, 1–10.
- Lin, Y.-T.; Chen, C.-C.; Huang, C.-C.; Nishimori, K.; Hsu, K.-S. Oxytocin stimulates hippocampal neurogenesis via oxytocin receptor expressed in CA3 pyramidal neurons. Nat. Commun. 2017, 8, 1–16.
- Han, B.; Bellemer, A.; Koelle, M.R. An Evolutionarily Conserved Switch in Response to GABA Affects Development and Behavior of the Locomotor Circuit of Caenorhabditis elegans. Genetics 2015, 199, 1159–1172.
- Leonzino, M.; Busnelli, M.; Antonucci, F.; Verderio, C.; Mazzanti, M.; Chini, B. The Timing of the Excitatory-to-Inhibitory GABA Switch Is Regulated by the Oxytocin Receptor via KCC2. Cell Rep. 2016, 15, 96–103.
- Tyzio, R.; Cossart, R.; Khalilov, I.; Minlebaev, M.; Hübner, C.A.; Represa, A.; Ben-Ari, Y.; Khazipov, R. Maternal Oxytocin Triggers a Transient Inhibitory Switch in GABA Signaling in the Fetal Brain During Delivery. Science 2006, 314, 1788–1792.
- Ben-Ari, Y. Oxytocin and Vasopressin, and the GABA Developmental Shift During Labor and Birth: Friends or Foes? Front. Cell Neurosci. 2018, 12, 254.
- Furukawa, M.; Tsukahara, T.; Tomita, K.; Iwai, H.; Sonomura, T.; Miyawaki, S.; Sato, T. Neonatal maternal separation delays the GABA excitatory-to-inhibitory functional switch by inhibiting KCC2 expression. Biochem. Biophys. Res. Commun. 2017, 493, 1243–1249.
- Chang, S.W.C.; Fagan, N.A.; Toda, K.; Utevsky, A.V.; Pearson, J.M.; Platt, M.L. Neural mechanisms of social decision-making in the primate amygdala. Proc. Natl. Acad. Sci. USA 2015, 112, 16012–16017.
- Petrovic, P.; Kalisch, R.; Singer, T.; Dolan, R.J. Oxytocin Attenuates Affective Evaluations of Conditioned Faces and Amygdala Activity. J Neurosci. 2008, 28, 6607–6615.
- Geng, Y.; Zhao, W.; Zhou, F.; Ma, X.; Yao, S.; Hurlemann, R.; Becker, B.; Kendrick, K.M. Oxytocin Enhancement of Emotional Empathy: Generalization Across Cultures and Effects on Amygdala Activity. Front. Neurosci. 2018, 12, 512.
- Hurlemann, R.; Patin, A.; Onur, O.A.; Cohen, M.X.; Baumgartner, T.; Metzler, S.; Dziobek, I.; Gallinat, J.; Wagner, M.; Maier, W.; et al. Oxytocin Enhances Amygdala-Dependent, Socially Reinforced Learning and Emotional Empathy in Humans. J. Neurosci. 2010, 30, 4999–5007.
- Viviani, D.; Charlet, A.; Burg, E.V.D.; Robinet, C.; Hurni, N.; Abatis, M.; Magara, F.; Stoop, R. Oxytocin Selectively Gates Fear Responses Through Distinct Outputs from the Central Amygdala. Science 2011, 333, 104–107.
- Haubensak, W.; Kunwar, P.S.; Cai, H.; Ciocchi, S.; Wall, N.R.; Ponnusamy, R.; Biag, J.; Dong, H.-W.; Deisseroth, K.; Callaway, E.M.; et al. Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature 2010, 468, 270–276.
- Huber, D.; Veinante, P.; Stoop, R. Vasopressin and Oxytocin Excite Distinct Neuronal Populations in the Central Amygdala. Science 2005, 308, 245–248.
- Wahis, J.; Baudon, A.; Althammer, F.; Kerspern, D.; Goyon, S.; Hagiwara, D.; Lefevre, A.; Barteczko, L.; Boury-Jamot, B.; Bellanger, B.; et al. Astrocytes mediate the effect of oxytocin in the central amygdala on neuronal activity and affective states in rodents. Nat. Neurosci. 2021, 24, 529–541.
More
