Inspired by natural photosynthesis, the photocatalytic CO2 reduction reaction (CO2RR) stands as a viable strategy to produce solar fuels and mitigate the high dependence on highly polluting fossil fuels, as well as to decrease the CO2 concentration in the atmosphere. The design of efficient photocatalytic materials is crucial to ensure the long-term application of the CO2RR process. So far, perovskite materials have shown high efficiencies in CO2RR to generate different solar fuels, specially lead halide perovskites (LHP), which exhibit valuable features for the obtention of high production yields (e.g., narrow band gaps, adequate potentials for CO2RR, good charge transport properties, etc.). Nonetheless, the presence of lead involves an important environmental impact that cannot be negligible in the design of industrial-scale photocatalytic processes. Hence, the search for efficient Lead-free Halide Perovskites (LFHP) remains a high-priority task in the researchtudy of functional materials for CO2RR, since LFHPs could maintain the properties of LHPs, while keeping low environmental impacts and accessible costs of production. As an alternative, bismuth-based LFHPs have gained much attention due to their higher absorption coefficients, their more efficient charge transfer (compared to oxide perovskites), and their required thermodynamic potential for CO2RR. However, despite all the remarkable advantages of bismuth halide perovskites, their use has been limited, owing to instability concerns. TIn this article, the performance of bismuth-based LFHPs are discussed, as well as stability strategies from intrinsic and extrinsic standpoints.
- bismuth halide perovskites
- CO2RR
- fuels
1. Introduction
2. Metal Halide Perovskites for CO2RR
(i) Ease of synthesis. These materials are distinguished by their low enthalpy of formation, which often grants them facile synthesis processes at normal temperatures (~25 °C) and pressures (~1 atm). Also, it allows for the formation of different morphologies with a high number of active sites [41][42].
(ii) Structure modulation. It has been demonstrated that metal halide perovskites can modulate their crystalline structures and energy bands by incorporating anions or cations from different groups [43][44][45][46]. In addition, the polarization refinement structure can induce an intensive internal electric field, which facilitates charge migration to the surface, improving the efficiency of CO2RR [47]. On the other hand, the structure modulation can be carried out by variation of the morphology (e.g., bulk, nanoplates, nanorods, quantum dots, etc.) [48][49][50].
(iii) Light harvesting. The modulation of the composition of a halide perovskite can significantly alter its band gap, which can make it an excellent light harvester compared with other semiconductors. At an atomic level, the most common strategies for light-harvesting enhancement include extending the absorption range via band gap engineering, applying the plasmonic resonance enhancement effect, and the arrangement of light absorption using tandem absorber devices [51]. Recently, Jian et al. have proposed the formation of CsPbBr
- (i)
-
Ease of synthesis. These materials are distinguished by their low enthalpy of formation, which often grants them facile synthesis processes at normal temperatures (~25 °C) and pressures (~1 atm). Also, it allows for the formation of different morphologies with a high number of active sites [53,54].
- (ii)
-
Structure modulation. It has been demonstrated that metal halide perovskites can modulate their crystalline structures and energy bands by incorporating anions or cations from different groups [55,56,57,58]. In addition, the polarization refinement structure can induce an intensive internal electric field, which facilitates charge migration to the surface, improving the efficiency of CO2RR [59]. On the other hand, the structure modulation can be carried out by variation of the morphology (e.g., bulk, nanoplates, nanorods, quantum dots, etc.) [60,61,62].
- (iii)
-
Light harvesting. The modulation of the composition of a halide perovskite can significantly alter its band gap, which can make it an excellent light harvester compared with other semiconductors. At an atomic level, the most common strategies for light-harvesting enhancement include extending the absorption range via band gap engineering, applying the plasmonic resonance enhancement effect, and the arrangement of light absorption using tandem absorber devices [63]. Recently, Jian et al. have proposed the formation of CsPbBr
3
nanocrystals with O-defective WO3 composites as a solution for stable and highly efficient materials that can harvest the broadest solar spectrum possible [52]. This strategy increases CO2RR efficiency up to 7-fold compared to pristine materials.
(iv) Exciton generation. The efficient generation of electron–hole pairs under excitation can enhance the photoconversion efficiency in CO2RR and other applications, such as photovoltaics and photon detection [53]. This phenomenon is mainly seen in narrow-band perovskites, which can promote multiple exciton generation (MEG). Perovskite materials can exhibit a variety of exciton species under different excitation conditions, including biexciton and triple exciton [54]. The surface defects of perovskites are the main source of the production of charged excitons. Under weak light excitation, this is mostly generated by single exciton recombination; meanwhile, at medium excitation intensity, biexciton recombination is favored. On the other hand, by using strong light excitation, Auger recombination dominates the generation of charged excitons, and perovskite materials are mainly characterized by their recombination of these excitons. Lin et al. demonstrated that the decay time of single excitons and biexcitons in Mn–CsPbBr
composites as a solution for stable and highly efficient materials that can harvest the broadest solar spectrum possible [64]. This strategy increases CO2RR efficiency up to 7-fold compared to pristine materials.- (iv)
-
Exciton generation. The efficient generation of electron–hole pairs under excitation can enhance the photoconversion efficiency in CO2RR and other applications, such as photovoltaics and photon detection [65]. This phenomenon is mainly seen in narrow-band perovskites, which can promote multiple exciton generation (MEG). Perovskite materials can exhibit a variety of exciton species under different excitation conditions, including biexciton and triple exciton [66]. The surface defects of perovskites are the main source of the production of charged excitons. Under weak light excitation, this is mostly generated by single exciton recombination; meanwhile, at medium excitation intensity, biexciton recombination is favored. On the other hand, by using strong light excitation, Auger recombination dominates the generation of charged excitons, and perovskite materials are mainly characterized by their recombination of these excitons. Lin et al. demonstrated that the decay time of single excitons and biexcitons in Mn–CsPbBr
3
perovskites can be modified by applying an external magnetic field (300 mT), suppressing the recombination of the photogenerated charges and subsequently improving the efficiency of CO and CH4 generation from CO2RR [55].
generation from CO2RR [67].- (v)
-
Long carrier diffusion lengths. Long carrier lifetime makes halide perovskite materials high-performance photovoltaic materials. Chen et al. proposed that the band-edge carrier lifetime increases when the system transitions from a lower rotational entropy to another phase with higher entropy [68]. These results suggest that the recombination of the photogenerated pair is inhibited by screening, leading to the formation of polarons and thereby extending their lifetime.
-
(v) Long carrier diffusion lengths. Long carrier lifetime makes halide perovskite materials high-performance photovoltaic materials. Chen et al. proposed that the band-edge carrier lifetime increases when the system transitions from a lower rotational entropy to another phase with higher entropy [56]. These results suggest that the recombination of the photogenerated pair is inhibited by screening, leading to the formation of polarons and thereby extending their lifetime.
-
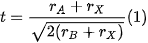
3. Bismuth Halide Perovskites
In the case of LFHPs, bismuth has a similar ionic radius to lead, as well as comparable properties to substitute this atom. Thus, the tolerance factor rule is satisfied, and the stability of the Bi-based perovskite materials can theoretically be enhanced when this substitution takes place. Bismuth provides an attractive option due to its nontoxic nature and chemical stability [74][98]. Its trivalent (3+) oxidation state causes materials to form an A3Bi2X9 structure, where A can be K+, Rb+, Cs+, or methylammonium MA+, and X can be I, Br, or Cl. Moreover, it was found that Bi-based perovskite materials possess a higher absorption coefficient, which renders them an efficient light-absorbing material for solar cell applications and, recently, for photocatalytic applications [73][75][76][77][78][79][92,93,94,95,96,97]. There are also reports on the use of Cs3Bi2I6Br3 films as solar cells, for which the highest power conversion efficiency (PCE) reported so far was 1.15% [75][93]. In addition, bismuth halides have been demonstrated to be good candidates for oxidizing a variety of organic compounds, such as thiamazole [76][94], vanillyl alcohol [77][95], rhodamine B [78][96], phenol [79][97], etc.
So far, most of the reports related to CO2RR using Bismuth Halide Perovskites employ Cs+ as the A-site cation in the A3Bi2X9 (X= I, Cl, Br, F) configuration. The best result for CO generation for these perovskites was obtained via the design of a Cs3Bi2I9–CeO2 heterostructure (135 μmol g−1 [80][139]), whereas the implementation of three stability strategies such as encapsulation, structure reconfiguration, and heterostructure design allowed for the highest efficiency to produce CH4 (151 μmol g−1 [81][127]). Both efficiencies (higher than other traditional and LHP perovskites) demonstrated the feasibility of using CO2RR to generate clean and renewable solar fuels, and these results were often attributed to a more efficient charge transfer with longer lifetimes, which grants more free charges for CO2RR.
Even with these remarkable findings, it is well-known that the low stability of Bismuth Halide Perovskites in ambient conditions hinders their commercialization and upscaling to the industrial level. Hence the lack of evidence regarding the recycling of these materials in consecutive cycles of evaluation, only reporting 5 h to 21 days of stability in gas–solid reactions. Some of the most common reasons for low stability in perovskites are shown in Figure 2Figure 3.
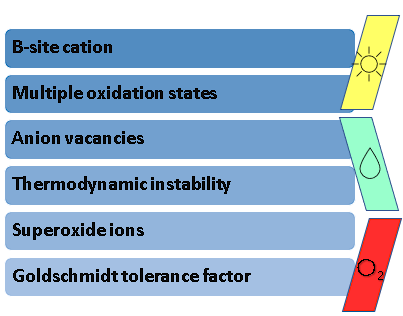
Figure 23. Factors that hinder perovskite stability.
In the researchtudy of stable perovskite photocatalysts, there are concerns regarding several factors that lead to instability, such as: (i) the B-site cation in the perovskite A3B2X9 configuration, as it must possess a good compatibility with this structure; (ii) the oxidation states of the ions that conform said structure, which determine the spontaneity with which these can undergo reduction or oxidation processes and lead to instability; (iii) anion vacancies, as these are inherent defects present in the perovskite structure that cause its degradation; (iv) thermodynamic instability, since the energetic feasibility that allows perovskites to be synthesized at room temperatures can also signify that these materials are prone to dissociate with the same ease; (v) the formation of superoxide ions, which are derived from the excitation of irradiated perovskites in the presence of atmospheric oxygen; and (vi) the aforementioned Goldschmidt tolerance factor, as it is an accurate indicator of the stability of a theoretical perovskite structure. Considering these aspects, several authors have proposed different strategies to increase the stability of halide perovskites.
4. Stability of Bismuth Halide Perovskites
The strategies with which the stability of photocatalysts can be improved are classified into two main approaches: intrinsic and extrinsic, depending on the nature of the employed methodology.
4.1 Intrinsic approach
This approach is widely used to improve the stability of metal halide perovskites through structural modification, which involves the alteration of the composition of cations and anions that are inherent to the perovskite structure [82][83][111,112]. The formation of solid solutions and double perovskites are examples of this approach. Likewise, the obtention of low-dimensional-networked perovskites promotes the formation of more stable crystal structures by selecting appropriate manufacturing techniques. Stoichiometry modification then settles the network in a quasi-zero-dimensional (0D) configuration, where the metal halide octahedra are almost isolated [84][113]. Another successfully intrinsic strategy is passivation [85][114]. This strategy allows the removal of dangling bonds, which act as recombination centers associated with defects, promoting better stability and optical properties. Antisolvent engineering is another strategy that speeds up nucleation during perovskite synthesis through solvent extraction [86][87][88][115,116,117]. This strategy allows the obtention of dense, high-quality films of bismuth halide perovskites with enhanced stability.
4.2 Extrinsic approach
This approach is characterized by the modification of the perovskite’s external properties without altering its internal composition. Among these strategies, encapsulation is one of the most used. It consists of the coverage of the perovskite to prevent exposure to moisture, oxygen, UV light, and temperature, thus protecting the structure. The encapsulation can be done by glass–glass covering of the edges of the substrate with an adhesive, as well as the deposition of a polymer coating. The adhesives can be ethylene methacrylate (EMA), ethylene vinyl acetate (EVA), polyisobutylene (PIB), and epoxy resins activated with UV light [89][118]. Alternatively, encapsulation can be done by depositing polymer coatings, e.g., polyethylene oxide, polyvinyl pyridine. The construction of heterostructures or Z-schemes between perovskites and other semiconductors has contributed to the design of promising stabilities for photocatalytic reactions since electrons are not available to react with the adsorbed oxygen. This avoids the degradation of the perovskite structure [90][91][92][119,120,121]. Another option is the dispersion of the perovskite nanoparticles in porous support for enhanced electron and hole separation, more accessible active sites, and close contact with the reaction species [42][54]. At the same time, adding adequate reactants to the medium can promote good stability of the perovskite structure. For example, it has been proved that the addition of HI during the evaluation of MA3Bi2I9 promotes excellent phase stability and enhanced photocatalytic activity for H2 evolution [93][122].
Some of these strategies have been implemented in bismuth halide perovskites to achieve better stabilities and efficiencies in photocatalytic CO2RR, mainly for Cs3Bi2X9 perovskites. So far, the stability of the Cs3Bi2X9 perovskite has been boosted by implementing two intrinsic strategies: solid solutions [94][124] and double perovskites [95][128]; as well as two extrinsic methodologies: the formation of heterojunctions with other semiconductors [96][137] and the encapsulation of the perovskites in porous supports [97][141]. The strategies implemented for the stable performance of bismuth halide perovskite photocatalysts are summarized in Figure 3 Figure 4.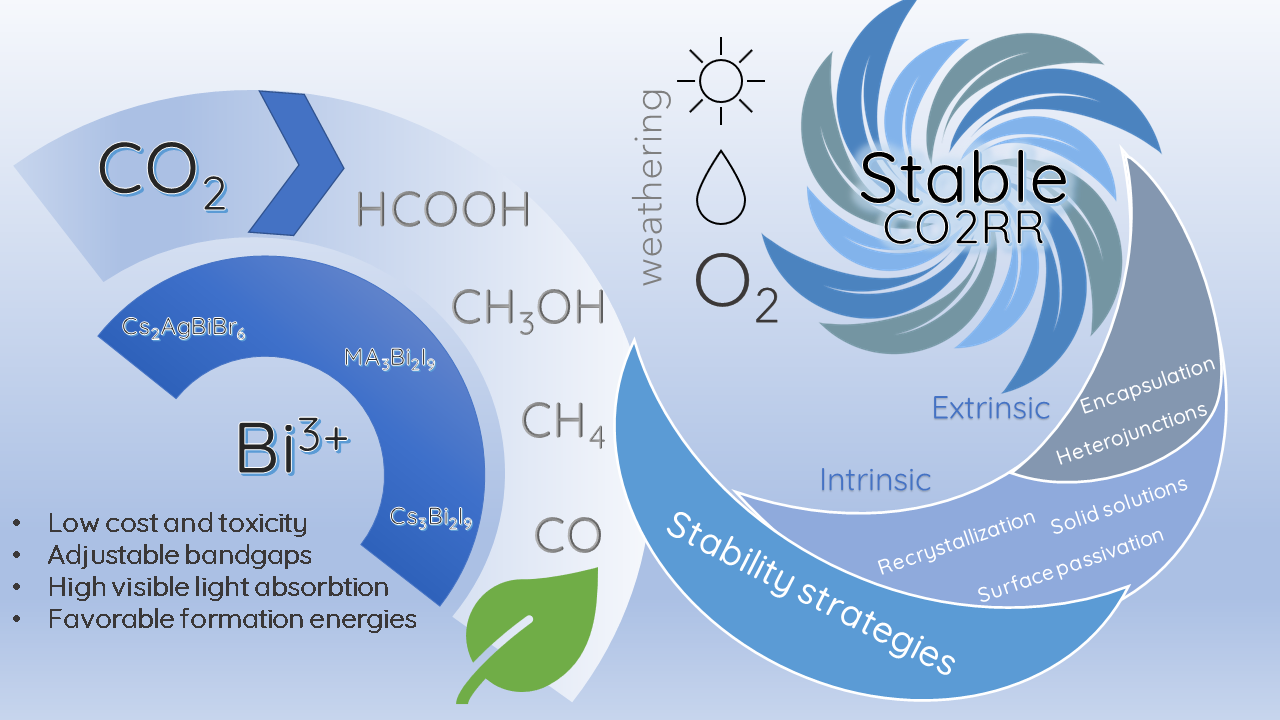
Figure 34. Strategies in bismuth halide perovskite for a stable CO2RR.
