Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Mohamed F. Zayed and Version 2 by Lindsay Dong.
Quinazoline is an essential scaffold, known to be linked with various biological activities. Some of the prominent biological activities of this system are analgesic, anti-inflammatory, anti-hypertensive, anti-bacterial, anti-diabetic, anti-malarial, sedative–hypnotic, anti-histaminic, anti-cancer, anti-convulsant, anti-tubercular, and anti-viral activities. This diversity in the pharmacological response of the quinazoline system has encouraged medicinal chemists to study and discover this system and its multitude of potential against several biological activities.
- quinazoline
- design
- synthesis
1. Introduction
Quinazoline is a double-ring heterocyclic system with two nitrogen heteroatoms in the six-membered aromatic ring fused to the benzene ring [1][2][3][4][1,2,3,4]. Quinazoline is formed from the pyrimidine ring fused to the benzene ring at two adjacent carbon atoms (Figure 1). It is classified as phenyl pyrimidine [5][6][7][5,6,7]. The first quinazoline derivative was synthesized by Griess et al. in 1869 through a condensation reaction [8]. It was also prepared from 2-carboxylate derivatives by a decarboxylation reaction [9][10][11][12][9,10,11,12]. Several quinazoline derivatives were synthesized and studied for their physical and chemical properties in 1903 by Gabriel and Colman [8]. The quinazoline system may contain an oxo group (=O) at C-2 to form the carbonyl group (C=O), named quinazoline-2(1H)-one (2-quinazolinone) 1 (Figure 1), or at C-4 and named quinazoline-4(3H)-one (4-quinazolinone) 2 (Figure 1). Otherwise, it may contain two oxo groups at C-2 and C-4 to form quinazoline-2,4-dione 3 (quinazolinedione) (Figure 1) [13][14][15][16][13,14,15,16]. Quinazolines are a group of versatile derivatives with a wide range of pharmacological activities [17][18][19][20][17,18,19,20]. They are used as analgesic, anti-hypertensive, anti-inflammatory, anti-bacterial, anti-diabetic, sedative–hypnotic, anti-histaminic, anti-cancer, anti-convulsant, anti-tubercular, and anti-viral agents, as well as having many other uses [21][22][23][24][25][21,22,23,24,25].
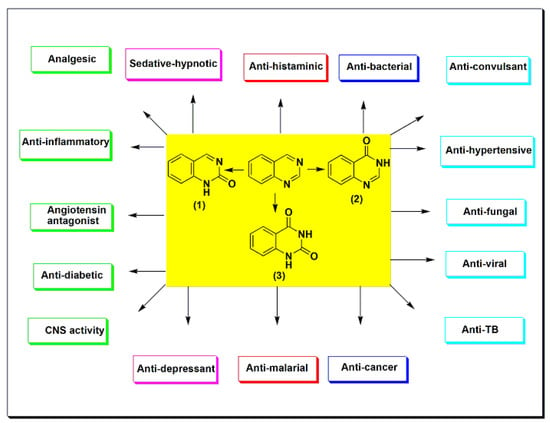
Figure 1.
The quinazolines and some of their pharmacological activities.
2. Physicochemical Characters of Quinazolines
Figure 2 shows the molecular structures of quinazoline, 4-quinazolinone, 2-quinazolinone, and 2,4-quinazolinedione, represented by ball-and-line mode [26]. Table 1 shows the physicochemical characters of quinazoline, 2-quinazolinone, 4-quinazolinone, and 2,4-quinazolinedione [27]. Figure 3 and Figure 4 show the lipophilic and the electrostatic potentials of these quinazolines [26].
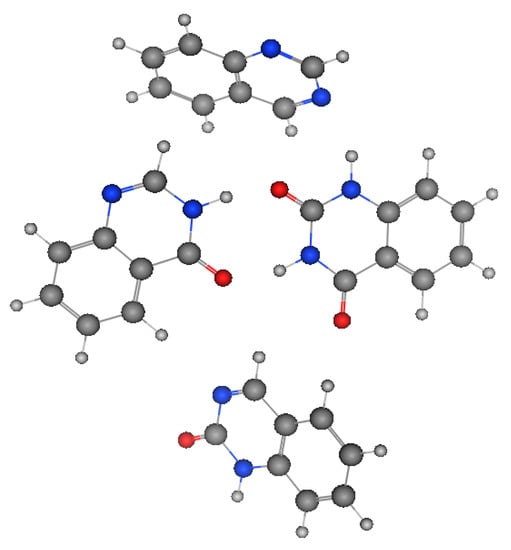
Figure 2. Molecular structure of quinazoline, 2-quinazolinone, 4-quinazolinone, and 2,4-quinazolinedione, represented by ball-and-line mode.
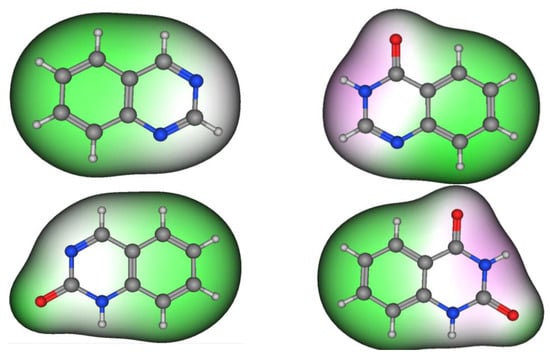
Figure 3. Lipophilic potential of quinazoline, 4-quinazolinone, 2-quinazolinone, and 2,4-quinazolinedione. Green color represents lipophilic area, white color represents neutral area, and pink color represents hydrophilic area.
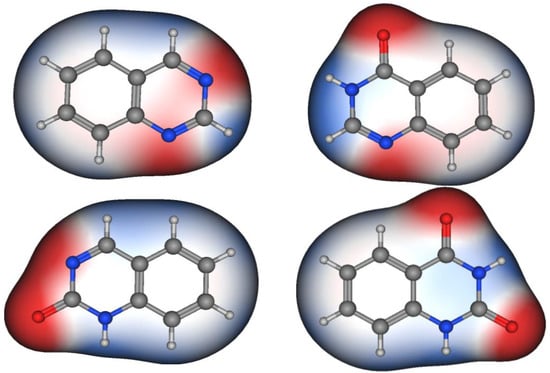
Figure 4. Electrostatic potential of quinazoline, 4-quinazolinone, 2-quinazolinone, and 2,4-quinazolinedione. Red color represents high polarity, white color represents neutral polarity, and blue color represents mild polarity.
Table 1.
The physicochemical characters of quinazoline, 2-quinazolinone, 4-quinazolinone, and 2,4-quinazolinedione.
| Character | 2-Quinazolinone | 4-Quinazolinone | 2,4-Quinazolinedione | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular formula | C | 8 | H | 6 | N | 2 | C | 8 | H | 6 | N | 2 | O | C | 8 | H | 6 | N | 2 | O | 2 |
| Molecular weight | 130.15 g/mol | 146.15 g/mol | 162.15 g/mol | ||||||||||||||||||
| Number of heavy atoms | 10 | 11 | 12 | ||||||||||||||||||
| Number of aromatic heavy atoms | 10 | 10 | 10 | ||||||||||||||||||
| Fraction Csp3 | 0 | 0 | 0 | ||||||||||||||||||
| Number of rotatable bonds | 0 | 0 | 0 | ||||||||||||||||||
| Number of H-bond acceptors | 2 | 2 | 2 | ||||||||||||||||||
| Number of H-bond donors | 0 | 1 | 2 | ||||||||||||||||||
| Molar refractivity | 39.54 | 42.36 | 45.19 | ||||||||||||||||||
| Tropological polar surface area | 25.78 A2 | 45.75 A2 | 65.72 | ||||||||||||||||||
| Lipophilicity | 1.46 | 1.14 | 1.04 | ||||||||||||||||||
| Water solubility | Soluble | Soluble | Soluble | ||||||||||||||||||
| GI absorption | High | High | High | ||||||||||||||||||
| BBB permeation | Yes | Yes | No | ||||||||||||||||||
| Bioavailability score | 0.55 | 0.55 | 0.55 | ||||||||||||||||||
| Lipinski | Yes | Yes | Yes | ||||||||||||||||||
| Synthetic accessibility | Very easy | Very easy | Very easy |
3. Methods of Preparation of Quinazolines
- 1.
-
The Niemetowski method (Scheme 1) is based on the reaction of anthranilic acid and formamide under high temperature to yield 4-(3H)-quinazolinone [25][28][25,28].
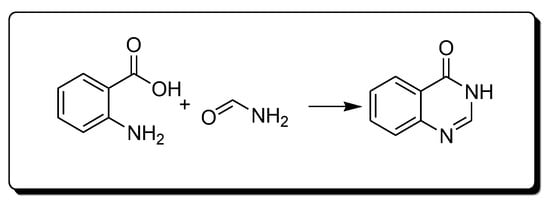 Scheme 1.Synthesis of 4-quinazolinone by Niemetowski method.
Scheme 1.Synthesis of 4-quinazolinone by Niemetowski method.- 2.
-
Morgan’s method for the synthesis of quinazoline (Scheme 2) uses the reaction between 2-acetamidobenzoic acid and an amine in the presence of phosphorous trichloride to yield 2-methyl-3-phenylquinazolin-4(3H)-one [25][28][25,28]
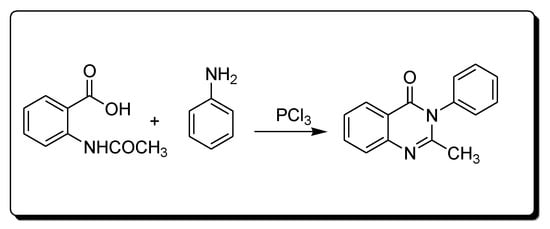 Scheme 2.Synthesis of 4-quinazolineone by Morgan’s method.
Scheme 2.Synthesis of 4-quinazolineone by Morgan’s method.- 3.
-
The reaction between isatoic anhydride and an amine, followed by refluxing with ethyl orthoformate (Scheme 3) produces 4-(3H)-quinazolinone [25][29][25,29].
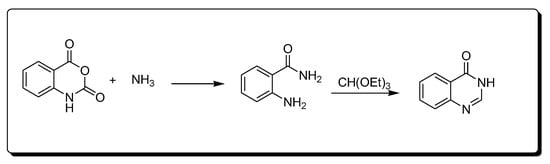 Scheme 3.Synthesis of 4-quinazolinone.
Scheme 3.Synthesis of 4-quinazolinone.- 4.
-
The reaction of amines 2-methyl-4-nitro-bezoxazine-4-one derivatives produces 2-methyl-4-nitro-quinazolin-4-one derivatives (Scheme 4) [28].
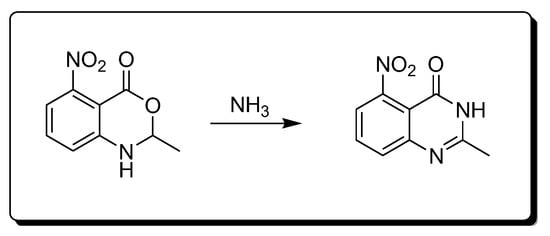 Scheme 4.Synthesis of 4-quinazolinone by amination reaction.
Scheme 4.Synthesis of 4-quinazolinone by amination reaction.- 5.
-
Anthranilic acid and potassium cyanate react together (Scheme 5) to produce 2,4-quinazolinedione derivatives [29][30][29,30].
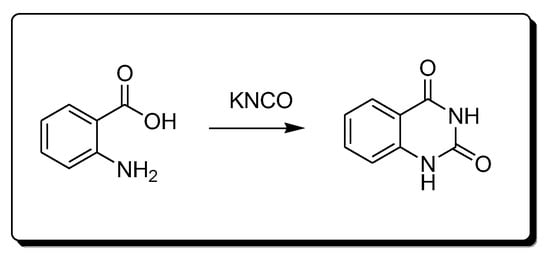 Scheme 5.Synthesis of 2,4-quinazolindione.
Scheme 5.Synthesis of 2,4-quinazolindione.- 6.
-
The reaction of 2-aminobenzamide and styrene in the presence of Di-tertiary-butyl peroxide (DTBP) and P-toluene sulfonic acid (p-TsOH) produces 2-phenylquinazoline-4(3H)-one derivatives (Scheme 6) [29][30][29,30].
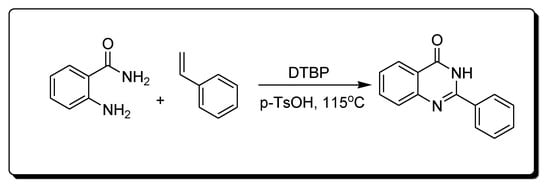 Scheme 6.Synthesis of 4-quinazolinone.
Scheme 6.Synthesis of 4-quinazolinone.- 7.
-
Transition metals are catalyzed by the synthesis of quinazoline (Scheme 7) [30]. This method is based on the catalytic reduction in the nitro benzamide derivative using palladium chloride (PdCl2) and iron pentacarbonyl Fe(CO)5.
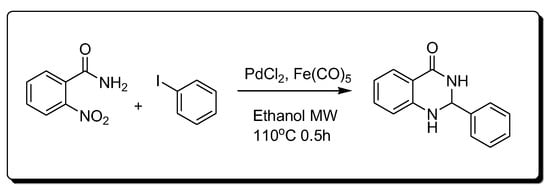 Scheme 7.Synthesis of 4-quinazolinone by transition-metal-catalyzed method.
Scheme 7.Synthesis of 4-quinazolinone by transition-metal-catalyzed method.4. Analgesic Activity
Many quinazolines have been synthesized and evaluated for their analgesic and anti-inflammatory activities [31][32][33][34][35][36][37][38][31,32,33,34,35,36,37,38]. 2-Phenyl quinazolinone 4 (Figure 5) was synthesized by Alagarsamy et al. in 2002 [39]. It was biologically evaluated as an analgesic agent. The structure–activity relationship study explained that the highest activity was obtained by the compound with diethyl substitution, while aromatic and alicyclic amine substitution decreased analgesic activity. This activity was 58 ± 0.45% at 2 h and at 20 mg/kg compared to that of standard diclofenac sodium, which is 53 ± 0.35% at 2 h at 20 mg/kg. The modification of compound 4 to thiourea-substituted 2-methyl quinazolinone derivatives 5 produced more active compounds. The most active one was the compound which had the pyrrolidine ring at C-3 (Figure 5) [40]. The activity of this compound was 65 ± 0.79% at 2 h at 20 mg/kg. The activity of standard diclofenac during this experiment was 60 ± 0.54% at 1 h at 20 mg/kg. Further modification to thiourea-substituted 2-methylthio quinazolinone 6 yielded higher activity, i.e., 67 ± 1.18% at 2 h at 20 mg/kg [41]. Increasing lipophilicity at C-2 by placing the butyl group instead of the methyl group yielded a more active compound 7 with 73 ± 1.49% analgesic activity at 2 h at 20 mg/kg. Standard diclofenac produced 62 ± 1.49% analgesic activity at 2 h at 20 mg/kg. Placing the benzylamino group at C-2 produced active compound 8 with 55 ± 0.36% analgesic activity at 2 h at 20 mg/kg [42]. This activity was the same as standard diclofenac. Methylamino substituted 2-phenylquinazolinones 9 were synthesized and evaluated for analgesic activity [43]. They yielded 43 ± 0.51 to 61 ± 1.08% analgesic activity at 2 h at 20 mg/kg.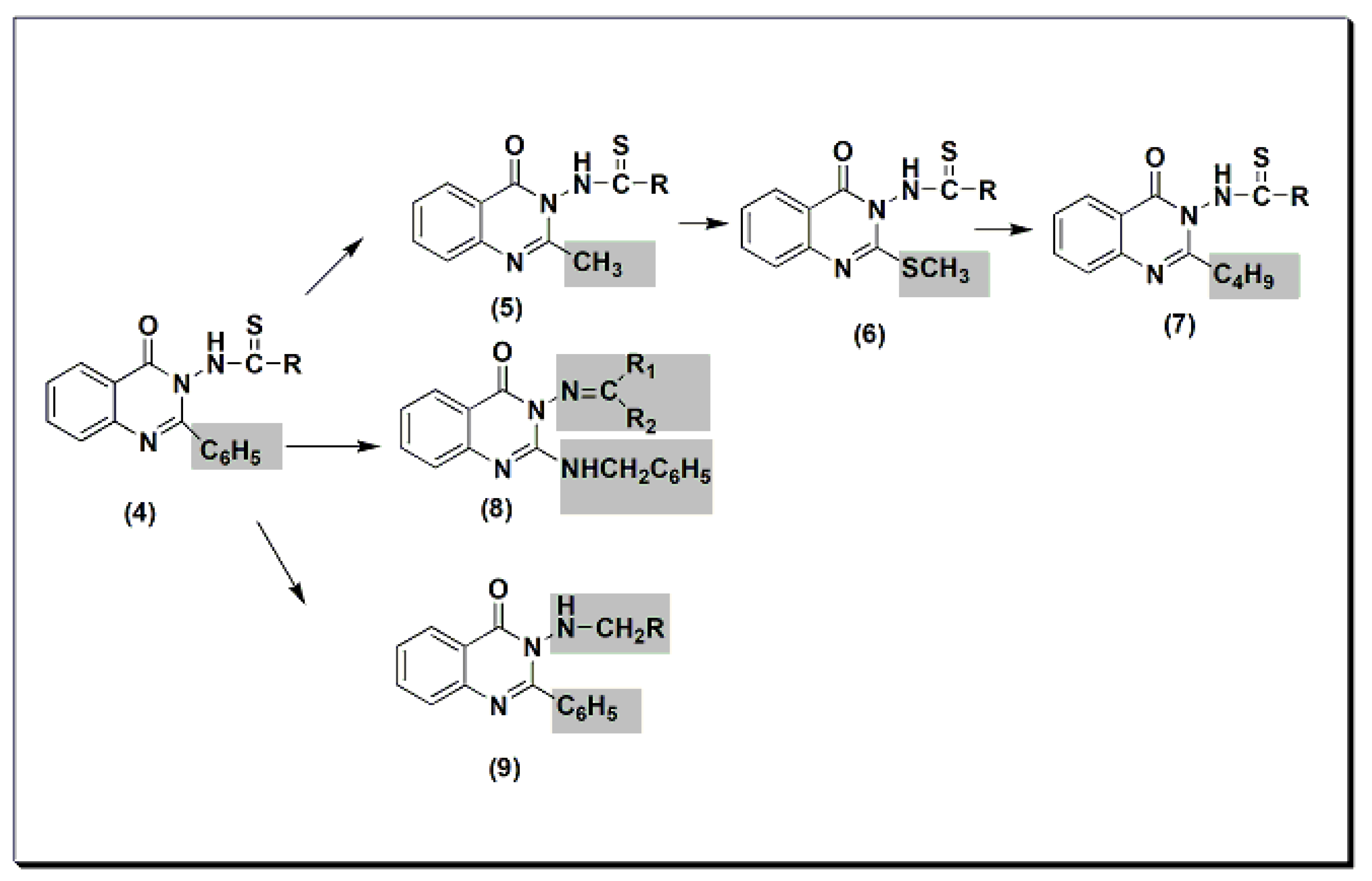
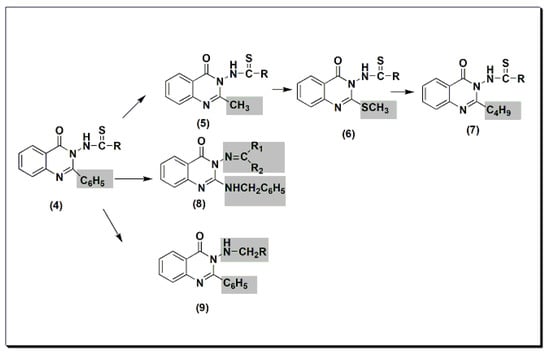 Figure 5.The analgesic quinazolines4,5,6,7,8, and9.
Figure 5.The analgesic quinazolines4,5,6,7,8, and9.5. Anti-Inflammatory Activity
The previously discussed compounds (4–23) were evaluated for their anti-inflammatory activity. All these derivatives showed anti-inflammatory activity except the compound 20. It showed low anti-inflammatory activity when it was unsubstituted, while substitution with Cl at C-6 and CH3at C-8 showed good activity.Two new derivatives of quinazolines, 25 and 26 (Figure 6), were designed, synthesized, and evaluated for their anti-inflammatory activity [44]. These derivatives were substituted isoquino-quinazolinone 25 and substituted quinazolino-quinazolinone 26. They were compared to the potent substituted 2-phenyl-quinazoline compound 24, but both were less active than compound 24.13), were designed, synthesized, and evaluated for their anti-inflammatory activity [56]. These derivatives were substituted isoquino-quinazolinone 25 and substituted quinazolino-quinazolinone 26. They were compared to the potent substituted 2-phenyl-quinazoline compound 24, but both were less active than compound 24.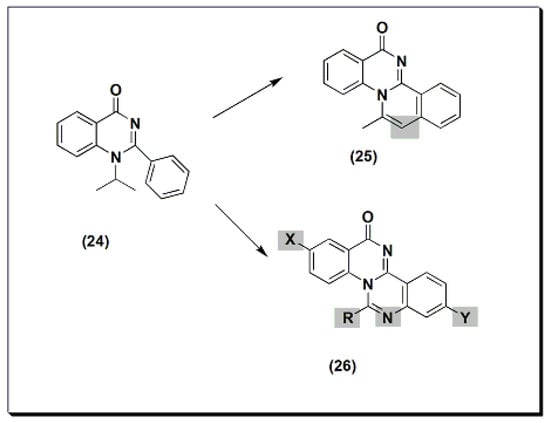 Figure 613.The anti-inflammatory quinazolines 24, 25, and 26.Other derivatives of compound 27 (Figure 7) were tested for their anti-inflammatory activity at 8 mg/kg using standard piroxicam at 4 mg/kg [45]. An SAR study showed that unsubstituted, 4-methyl, and 3-nitro derivatives of compound 27 yielded better activity than standard piroxicam. Using a 4 mg/kg dose yielded lower activity than piroxicam or diclofenac for the three derivatives. A structural modification was conducted by the introduction of the nicotinyl thiadiazole group at C-3 of 28 to improve the anti-inflammatory activity [46]. Nicotinyl-5-pyridyl thiadiazole of substituted 2-phenylquinazolinone yielded the highest activity, equivalent to standard ibuprofen. Substituted quino-quinazolinedione derivatives 29 and 30 showed an activity range of 12.7–58.2%. SAR studies showed that 10-iodosubstitution resulted in a significant increase in anti-inflammatory activity.14) were tested for their anti-inflammatory activity at 8 mg/kg using standard piroxicam at 4 mg/kg [57]. An SAR study showed that unsubstituted, 4-methyl, and 3-nitro derivatives of compound 27 yielded better activity than standard piroxicam. Using a 4 mg/kg dose yielded lower activity than piroxicam or diclofenac for the three derivatives. A structural modification was conducted by the introduction of the nicotinyl thiadiazole group at C-3 of 28 to improve the anti-inflammatory activity [58]. Nicotinyl-5-pyridyl thiadiazole of substituted 2-phenylquinazolinone yielded the highest activity, equivalent to standard ibuprofen. Substituted quino-quinazolinedione derivatives 29 and 30 showed an activity range of 12.7–58.2%. SAR studies showed that 10-iodosubstitution resulted in a significant increase in anti-inflammatory activity.
Figure 613.The anti-inflammatory quinazolines 24, 25, and 26.Other derivatives of compound 27 (Figure 7) were tested for their anti-inflammatory activity at 8 mg/kg using standard piroxicam at 4 mg/kg [45]. An SAR study showed that unsubstituted, 4-methyl, and 3-nitro derivatives of compound 27 yielded better activity than standard piroxicam. Using a 4 mg/kg dose yielded lower activity than piroxicam or diclofenac for the three derivatives. A structural modification was conducted by the introduction of the nicotinyl thiadiazole group at C-3 of 28 to improve the anti-inflammatory activity [46]. Nicotinyl-5-pyridyl thiadiazole of substituted 2-phenylquinazolinone yielded the highest activity, equivalent to standard ibuprofen. Substituted quino-quinazolinedione derivatives 29 and 30 showed an activity range of 12.7–58.2%. SAR studies showed that 10-iodosubstitution resulted in a significant increase in anti-inflammatory activity.14) were tested for their anti-inflammatory activity at 8 mg/kg using standard piroxicam at 4 mg/kg [57]. An SAR study showed that unsubstituted, 4-methyl, and 3-nitro derivatives of compound 27 yielded better activity than standard piroxicam. Using a 4 mg/kg dose yielded lower activity than piroxicam or diclofenac for the three derivatives. A structural modification was conducted by the introduction of the nicotinyl thiadiazole group at C-3 of 28 to improve the anti-inflammatory activity [58]. Nicotinyl-5-pyridyl thiadiazole of substituted 2-phenylquinazolinone yielded the highest activity, equivalent to standard ibuprofen. Substituted quino-quinazolinedione derivatives 29 and 30 showed an activity range of 12.7–58.2%. SAR studies showed that 10-iodosubstitution resulted in a significant increase in anti-inflammatory activity.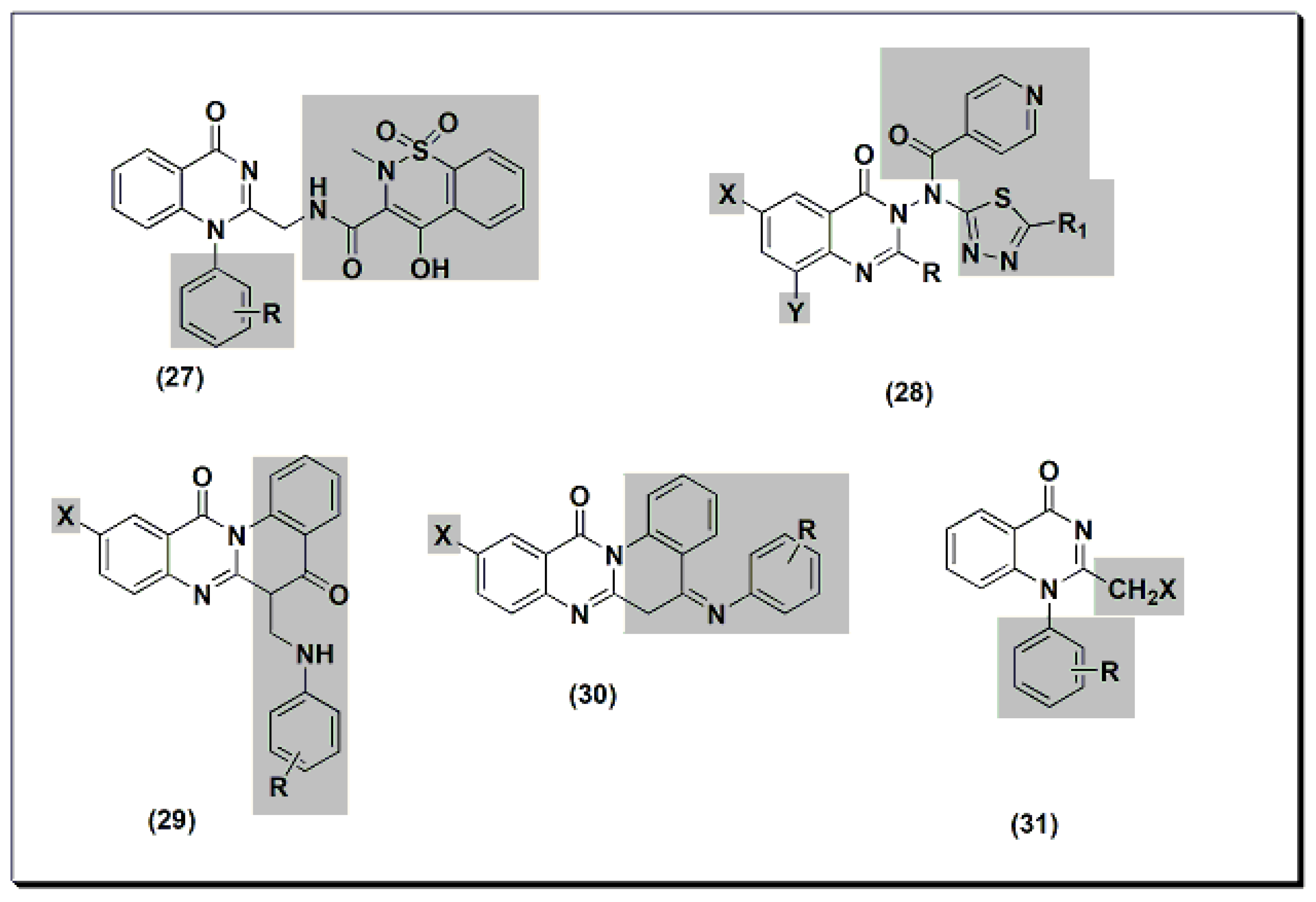
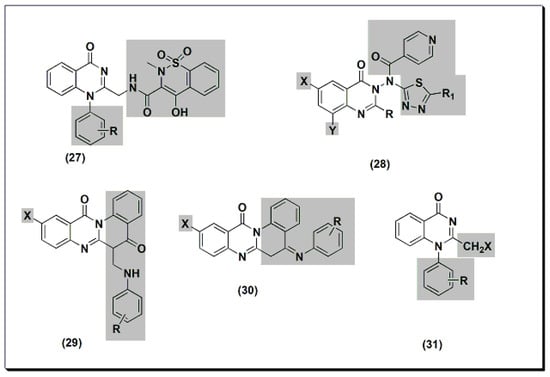 Figure 714. The anti-inflammatory quinazolines 27, 28, 29, 30, and 31.2,3,6-trisubstituted quinazolinone derivatives 32–36 (Figure 8) were designed, synthesized, and tested as anti-inflammatory agents [47]. These derivatives showed a variable activity range of 10.28–53.33%. Compounds with o-methoxyphenyl substituents at C-3 and p-dimethylaminophenyl at C-2 showed activity higher than standard phenylbutazone. In addition, these derivatives were tested for their ulcerogenic activity. The highly active anti-inflammatory derivatives were less ulcerogenic compared to standard phenylbutazone.15) were designed, synthesized, and tested as anti-inflammatory agents [61]. These derivatives showed a variable activity range of 10.28–53.33%. Compounds with o-methoxyphenyl substituents at C-3 and p-dimethylaminophenyl at C-2 showed activity higher than standard phenylbutazone. In addition, these derivatives were tested for their ulcerogenic activity. The highly active anti-inflammatory derivatives were less ulcerogenic compared to standard phenylbutazone.
Figure 714. The anti-inflammatory quinazolines 27, 28, 29, 30, and 31.2,3,6-trisubstituted quinazolinone derivatives 32–36 (Figure 8) were designed, synthesized, and tested as anti-inflammatory agents [47]. These derivatives showed a variable activity range of 10.28–53.33%. Compounds with o-methoxyphenyl substituents at C-3 and p-dimethylaminophenyl at C-2 showed activity higher than standard phenylbutazone. In addition, these derivatives were tested for their ulcerogenic activity. The highly active anti-inflammatory derivatives were less ulcerogenic compared to standard phenylbutazone.15) were designed, synthesized, and tested as anti-inflammatory agents [61]. These derivatives showed a variable activity range of 10.28–53.33%. Compounds with o-methoxyphenyl substituents at C-3 and p-dimethylaminophenyl at C-2 showed activity higher than standard phenylbutazone. In addition, these derivatives were tested for their ulcerogenic activity. The highly active anti-inflammatory derivatives were less ulcerogenic compared to standard phenylbutazone.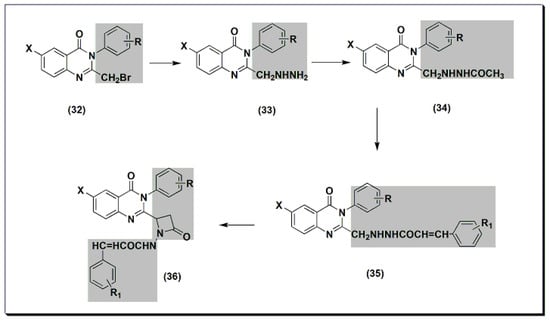 Figure 815. The anti-inflammatory quinazolines 32, 33, 34, 35, and 36.
Figure 815. The anti-inflammatory quinazolines 32, 33, 34, 35, and 36.6. Analgesic and Anti-Inflammatory Quinazolines Marketed Drugs
The marketed drug proquazone 50 (Figure 9) [48] is a quinazoline-based non-steroidal anti-inflammatory agent (NSAI). It is a 4-aryl-1-alkyl-quinazolinone derivative [48]. It is the first effective anti-inflammatory drug of a non-acidic nature. It is used in the treatment of rheumatoid arthritis and osteoarthritis. The dose of 300 to 900 mg/day is as effective as aspirin and diclofenac in the patients with osteoarthritis.22) [68] is a quinazoline-based non-steroidal anti-inflammatory agent (NSAI). It is a 4-aryl-1-alkyl-quinazolinone derivative [68]. It is the first effective anti-inflammatory drug of a non-acidic nature. It is used in the treatment of rheumatoid arthritis and osteoarthritis. The dose of 300 to 900 mg/day is as effective as aspirin and diclofenac in the patients with osteoarthritis.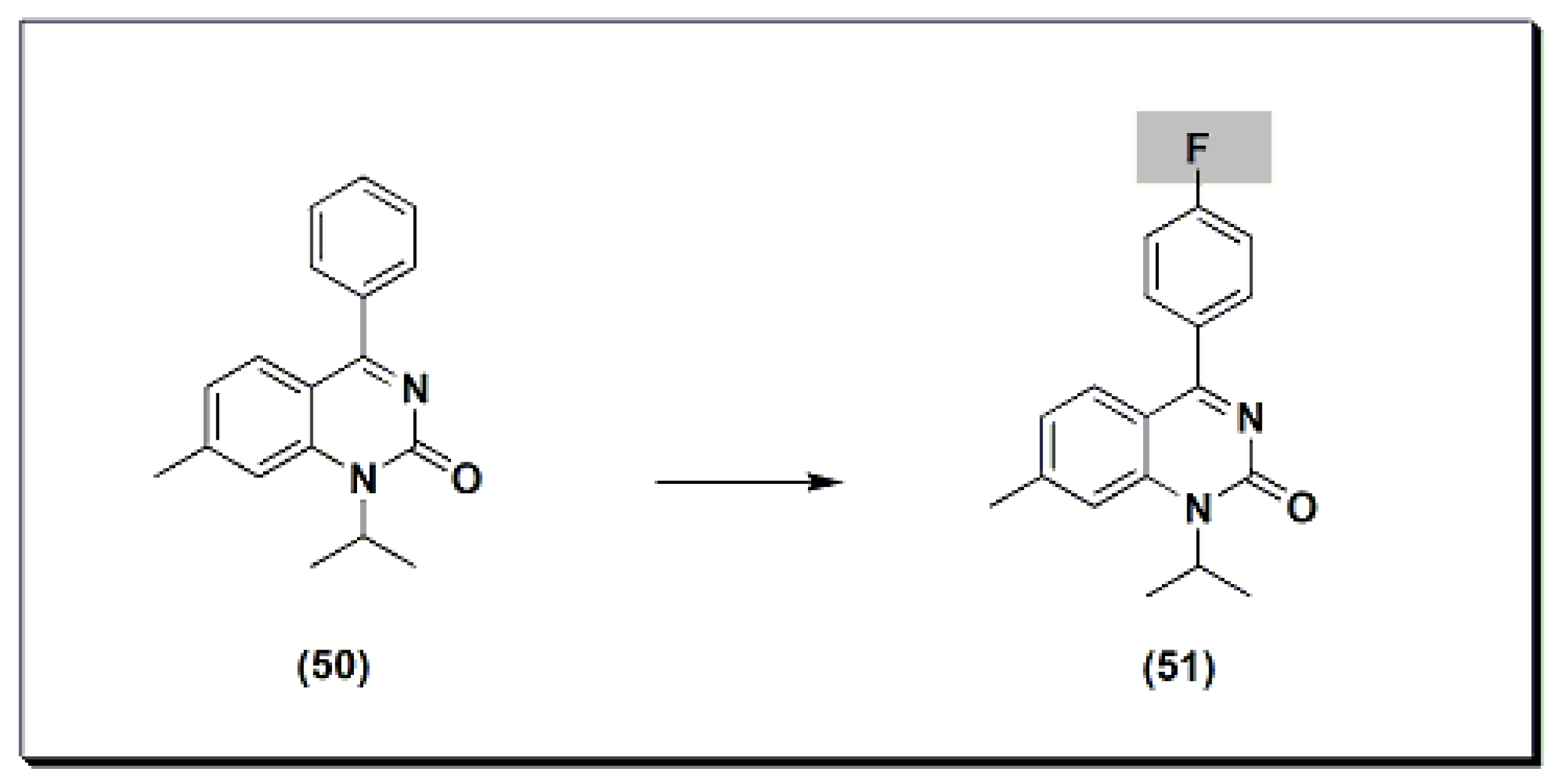
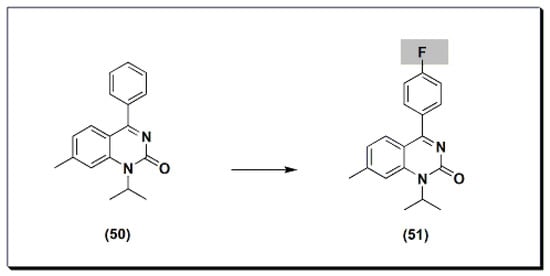 Figure 922.The molecular structure of proquazone and fluproquazone.The marketed quinazoline anti-inflammatory agent NSC127213 (Figure 1023) [48][68] is a derivative of tetrazolo quinazoline [48][68]. NSC127213 works by inhibiting the histamine-1 receptor (H1R) and the histamine-4 receptor (H4R) for the treatment and prevention of inflammatory, autoimmune, and allergic diseases. The molecular structure of these marketed drugs was based on previous studies on analgesic anti-inflammatory quinazolines.
Figure 922.The molecular structure of proquazone and fluproquazone.The marketed quinazoline anti-inflammatory agent NSC127213 (Figure 1023) [48][68] is a derivative of tetrazolo quinazoline [48][68]. NSC127213 works by inhibiting the histamine-1 receptor (H1R) and the histamine-4 receptor (H4R) for the treatment and prevention of inflammatory, autoimmune, and allergic diseases. The molecular structure of these marketed drugs was based on previous studies on analgesic anti-inflammatory quinazolines.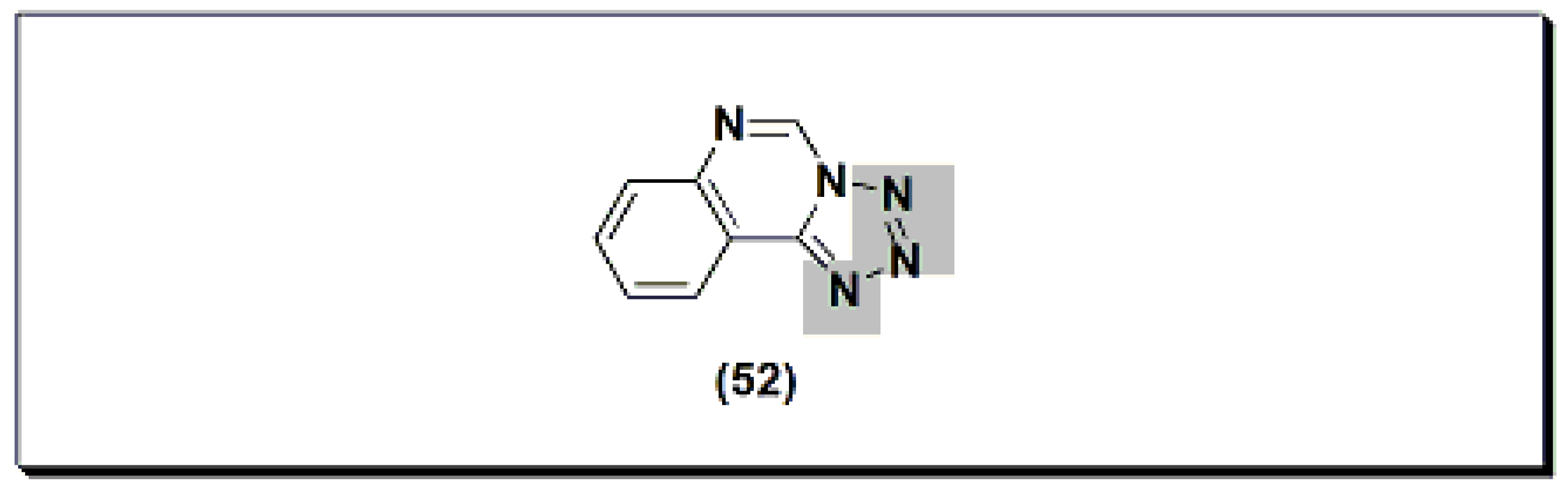
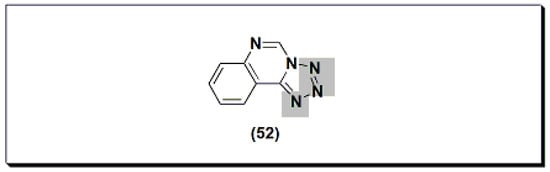 Figure 1023.The molecular structure of NSC127213.
Figure 1023.The molecular structure of NSC127213.78. Conclusions
As mentioned above, the quinazoline system has a wide range of biological activities, such as being analgesic, anti-inflammatory, anti-diabetic, anti-hyperlipidemic, anti-hypertensive, anti-bacterial, anti-fungal, anti-viral, anti-malarial, anti-convulsant, anti-cancer, anti-depressant, and anti-tubercular agents, as well as other activities. Therefore, it has great therapeutic potential for the treatment of different types of diseases and infections. The study of the structural–activity relationship (SAR) is the key for the improvement of efficient quinazoline therapeutic agents. Optimized quinazoline derivatives will be produced via an SAR-based study. Structural modifications can be performed by different techniques, such as the molecular hybridization or bioisosteric replacement techniques.
