You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Lindsay Dong and Version 3 by Lindsay Dong.
The main degenerative diseases of the retina include macular degeneration, proliferative vitreoretinopathy, retinitis pigmentosa, and glaucoma. Novel approaches for treating retinal diseases are based on cell replacement therapy using a variety of exogenous stem cells. An alternative and complementary approach is the potential use of retinal regeneration cell sources (RRCSs) containing retinal pigment epithelium, ciliary body, Müller glia, and retinal ciliary region. RRCSs in lower vertebrates in vivo and in mammals mostly in vitro are able to proliferate and exhibit gene expression and epigenetic characteristics typical for neural/retinal cell progenitors.
- retinal degenerative diseases
- retinal regeneration
- intrinsic cell sources
- molecular features and regulation
1. The Main Structure of the Retina
The vertebrate retina is organized by a single plan, which, however, has specific morphological and functional features [1][2]. The retina is a neural, highly structured, stratified tissue where different types of neurons have strict localization, maintaining a stereotypic pattern of the NR (Figure 1). Functionally, it is a sensory tissue consisting of ordered layers whose cells interact with one another and neurons of other layers to provide light perception, receiving, and transmitting visual information. The retina is formed by six major neural retina (NR) cell types and retinal pigmented epithelium (RPE) cells. NR cell populations are represented by photoreceptors (rods and cones), bipolars, and horizontal, amacrine, and ganglion cells. Müller glial cells (MGCs), microglia cells, astrocytes, and oligodendrocytes are integrated into the NR. The NR consists of three nuclear layers and two reticulate (plexiform) layers formed by fibers and synaptic contacts of neurons. The outer nuclear layer (ONL) is composed of bodies of photoreceptors, rods and cones. Their processes establish topological and functional connections with RPE. The inner nuclear layer (INL) contains bipolar, horizontal, and amacrine cells. INL interneurons are responsible for the visual signal transmission from photoreceptors to ganglion cells constituting the ganglion layer. The long processes of the ganglion cells compose the optic nerve that transmits information to the visual analyzer of the brain. The outer plexiform layer (OPL) is formed by fibers and synaptic contacts between photoreceptors and bipolar; the inner plexiform layer (IPL), by processes and synapses between bipolar and ganglion cells, as well as by horizontal processes forming connections between horizontal and amacrine cells. Bodies of the MGCs are located in the INL, extending their processes to the inner and outer limiting membranes of the NR, and are involved in their formation. In mammals and human the NR has two vascular supplies, the choroidal vasculature underlying RPE and the vessels of the inner retina (Figure 1). The blood supply to the inner retina is via the central retinal artery, whose branches radiate from the optic nerve head onto the inner retinal surface and then give rise to branches that penetrate into the retina through the INL, IPL, and OPL [3].
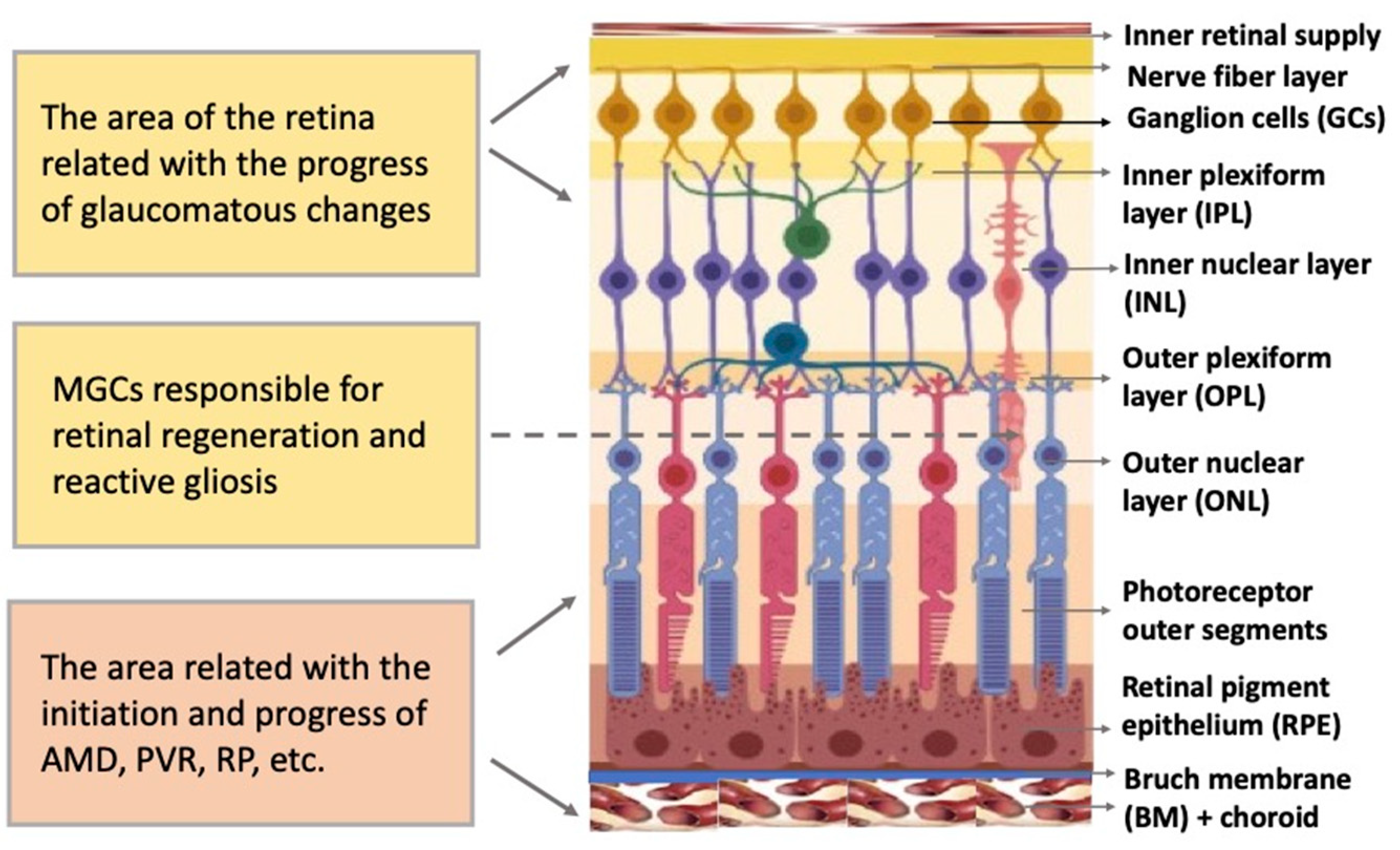
Figure 1. Structure of the retina and retinal compartments involved in degenerative diseases. MGCs—Müller glial cells, AMD—age-related macular degeneration, PVR—proliferative vitreoretinopathy, RP—retinitis pigmentosa.
2. Brief Characteristics of the Major Degenerative Disorders of the Retina
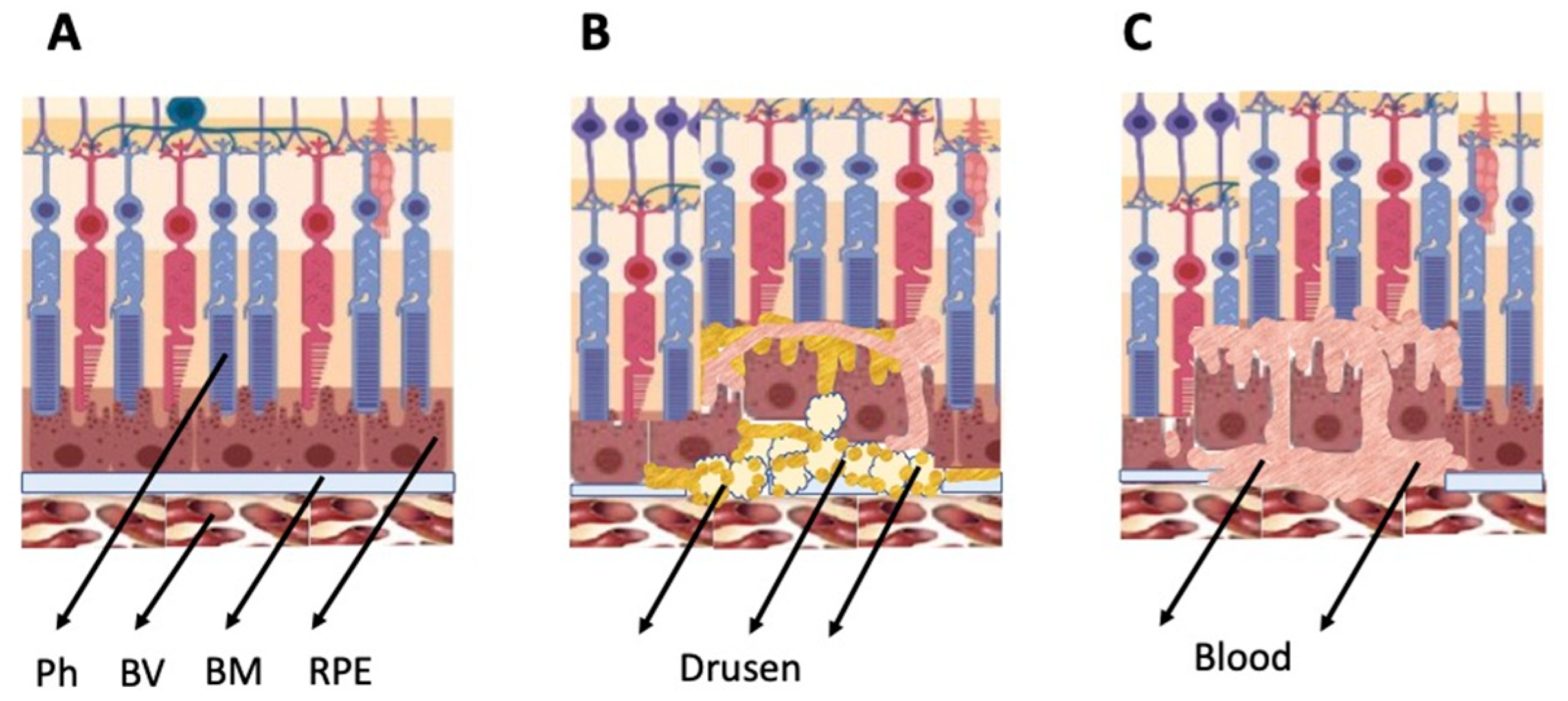
The general types of retinal degeneration include age-related macular degeneration (AMD), glaucoma, retinitis pigmentosa (RP), and proliferative vitreoretinopathy (PVR). All of these disorders, except glaucoma, are caused by the loss of cells and cell–cell interactions in the functional light perception system, RPE and NR (Figure 2). AMD, affecting, according to approximate estimates, a quarter or more of the global population aged 65+, is accompanied by the loss of photoreceptors in the maculae region, where the light rays are focused on the retina. There are two forms of AMD: the “dry” (prevailing) and “wet” AMD [4]. With the dry AMD in an atrophic form, extracellular matrix molecules accumulate in the space outside the RPE, which causes the formation of so-called druses, consisting of fats, vitronectin, amyloid proteins, and inflammatory proteins, accumulated inside the RPE layer (Figure 2). These changes occur in RPE within the maculae region, causing partial cell death, layer disorganization, disruption of RPE functions, and para-inflammatory reaction, which inevitably results in the loss of photoreceptors [5][6]. The wet AMD, also referred to as neovascular (exudative) form, is manifested as the proliferation of a network of blood vessels lining the RPE choroidal membrane in the maculae region. Vessels become dysfunctional and leaky, with fluid and blood accumulating in the maculae region [7] (Figure 2). This causes disjunction of RPE apical processes and photoreceptors, while the connections between them are mandatory for light perception. AMD treatment is diverse, involving neurotrophic factors, growth factors, cell viability factors, and also oxidative stress-preventing factors [8][9][10][11]. With the wet AMD, vascular endothelial growth factor (VEGF) inhibitors and the photodynamic therapy are mainly used [12][13]. Despite efforts aimed at developing adequate therapy, the challenges associated with the treatment of AMD to preserve vision are still substantial. In this regard, the idea of cell replacement with the help of cell sources, including intrinsic ones, to regenerate RPE and photoreceptors becomes highly relevant.

Figure 2. Schematic representation of AMD-related changes in the eye. (A)—normal eye; (B)—“dry” AMD; (C)—“wet” AMD. Ph—photoreceptors, BV—blood vessels, BM—Bruch membrane; RPE—retinal pigment epithelium.
PVR, often accompanying retinal rupture, is manifested as the withdrawal of RPE cells outside the layer, their epithelial–mesenchymal transition (EMT), transformation, and involvement in the epiretinal membrane (EM) formation [14][15][16]. Proliferative diabetic retinopathy [17] and subretinal fibrosis [18] are also known to be caused by mesenchymal transformation of RPE. In the treatment of this range of disorders, studies of mechanisms and methods for preventing the EMT of RPE cells and the EM formation with their involvement, which prevent normal functional connection between RPE and photoreceptors from being restored, are of particular importance [19].
Retinitis pigmentosa (RP) is characterized as a heterogeneous genetic disorder that leads to progressive devolution of the retina. This congenital disorder has a heterogeneous genetic origin. Approximately 100 genes are known whose mutations may result in RP [20]. It is often accompanied by the loss of peripheral and night vision. This is explained by the initial death of rods, as well as changes in the choroidal network, which, while progressing, leads to the degeneration of cones. Various approaches are currently being developed to slow down the progress of the disorder, including gene therapy, pharmacology, neuroprotection, electrical stimulation, retinal prostheses, and retinal transplantation [21].
Glaucoma is also one of the major retinal diseases leading to impairment of vision, often irreversible. Glaucoma represents a degenerative optic neuropathy characterized by the progressive degeneration of retinal ganglion cells and the retinal nerve fiber layer. Glaucomatous alterations, often associated with intraocular pressure increase, remain inconspicuous for a long time, but in the final stage, they lead to the death of bodies and axons of neurons, including those in the optic nerve head region [22]. The ocular hypertension and deleterious mechanical forces exerted at the back of the eye, at the level of the optic nerve head/optic disc, are the only modifiable risk factors associated with glaucoma that can be treated. The main approach to treatment is to reduce the intraocular pressure by administering prostaglandins and β-blockers [23][24].
3. Intrinsic Retinal Regeneration Cell Sources and Their Implication for Biomedicine of the Eye
3.1. Retinal Ciliary Zone Cells
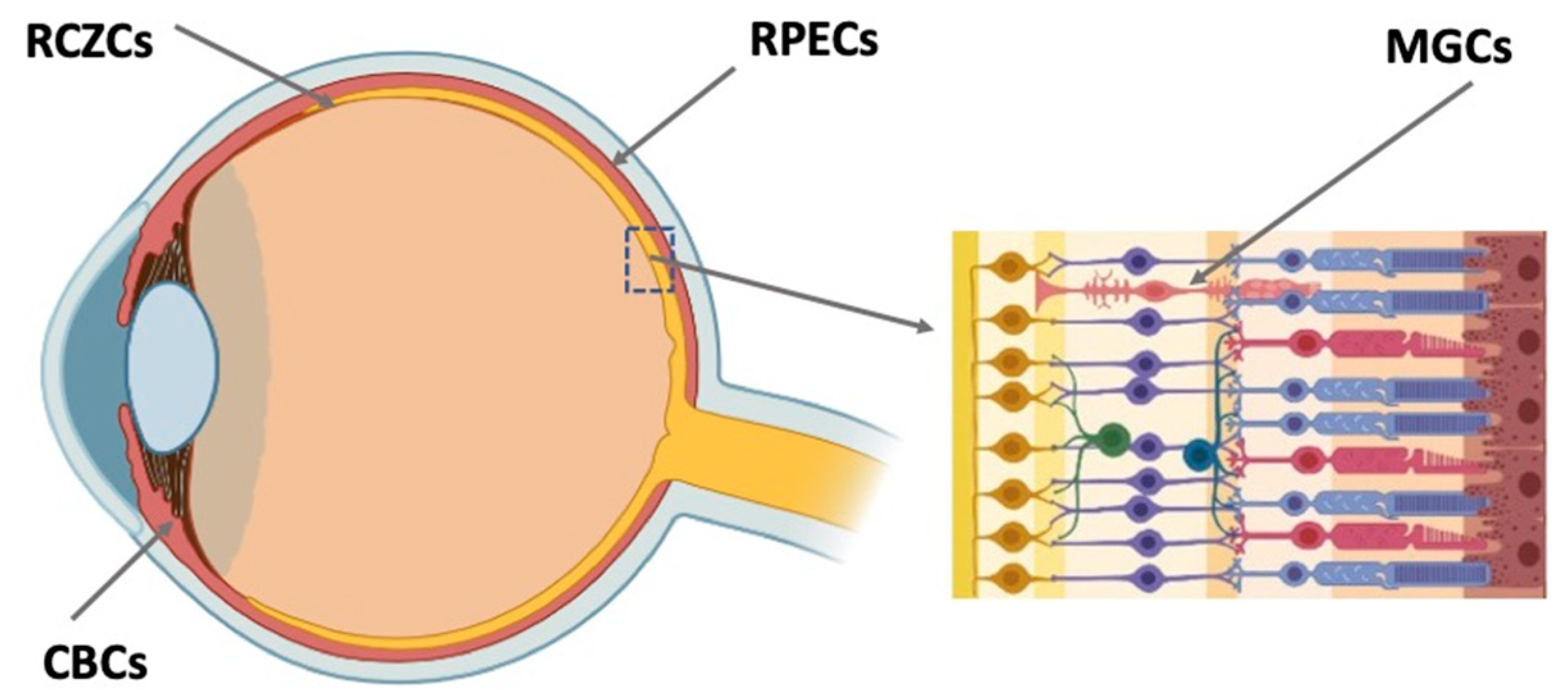
Retinal regeneration cell sources (RRCSs) of this category are located on the extreme periphery of the retina, in the so-called corner of the eye (Figure 3). In vertebrates, the ciliary zone cells exhibit pronounced, to varying degrees, features of stemness/early progenitors. There are differences in the size of this cell population, decreasing in the series from fish to mammals. In fish and amphibians, persistent neurogenesis occurs in the poorly differentiated ciliary marginal zone (CMZ) that provides recruitment of cells to the growing retina [25][26]. The cells of this zone are also involved in the regeneration of the retina in case of damage [25][27][28][29].
Figure 3. Potential endogenous cell sources for retinal regeneration (summarized data). RCZCs—cells of the retina ciliary zone; RPECs—retinal pigment epithelial cells; CBCs—cells of the ciliary body; MGCs—Müller glial cells.
3.2. Ciliary Body Cells
In adult mammals, the region similar to the CMZ is extremely reduced by the number of cells and does not normally exhibit any regenerative abilities [30]. A region, close in localization but not analogous to the CMZ of lower vertebrates, in adult mammals and humans is represented by the ciliary body (CB) (Figure 1). The CB in the mammalian eye has two cell layers and muscles. The outer pigmented layer is a continuation of the RPE; the inner, non-pigmented layer, a continuation of the NR. The cells constituting the CB have a specialization different from that of RPE and NR neurons. They produce components of vitreous fluid and are involved in visual accommodation [31][32]. However, in case of damage to the NR leading to the loss of ganglion and amacrine cells, cell proliferation is known to be activated in the non-pigmented CB layer [30][33][34][35][36]. In adult mice, some CB cells re-enter the cell cycle and change their phenotype, expressing TF Chx10 and also marker proteins of bipolar and photoreceptors as a response to the optic nerve transection causing the death of ganglion cells [34]. Expression of retinal progenitor genes was observed in the adult mouse CB after intraocular injections of regulators of Rho GTPase activity [37]. The injections enhanced the co-expression of TFs Pax6 and Chx10, but showed no effect on proliferation in the CB. The inactivation of Rho GTPases conversely increased the proliferation of CB cells, including those exposed to growth factors.
3.3. Retinal Pigment Epithelium Cells
In adult vertebrates and humans, RPE is a monolayer of pigmented, epithelial, and specialized cells. The RPE is oriented towards the NR with its apical side; on the basal side, it is limited by the Bruch’s membrane and the vascular membrane referred to as choroid (Figure 1 and Figure 3). The RPE is multifunctional: apart from transferring substances from the choroid to the NR, it protects against oxidative stress, produces growth factors, and metabolizes vitamin A derivatives. A major RPE function is phagocytosis of the outer segments of photoreceptors, their digestion by lysosomes, and retinoid metabolism, i.e., providing the processes required for light perception [38][39][40][41]. Among the well-known RRCSs, RPE has been studied to a greater extent, including the possible implications for practical use. Damage to the RPE layer and its cells, and also disturbance of their relationship with photoreceptors, are the causes of most degenerative diseases of the retina. In this regard, and taking into account the common origin of RPE and NR in the development of the eye and the possibility of their mutual conversion [19], RPE has been extensively studied using animal models and in humans. Studies have been conducted in various directions: cell functions, differentiation of RPE and its changes during regeneration, and congenital and acquired eye pathologies; RPE behavior in vitro and under transplantation conditions has also been investigated [39][42][43][44][45][46][47][48].3.4. Müller Glial Cells
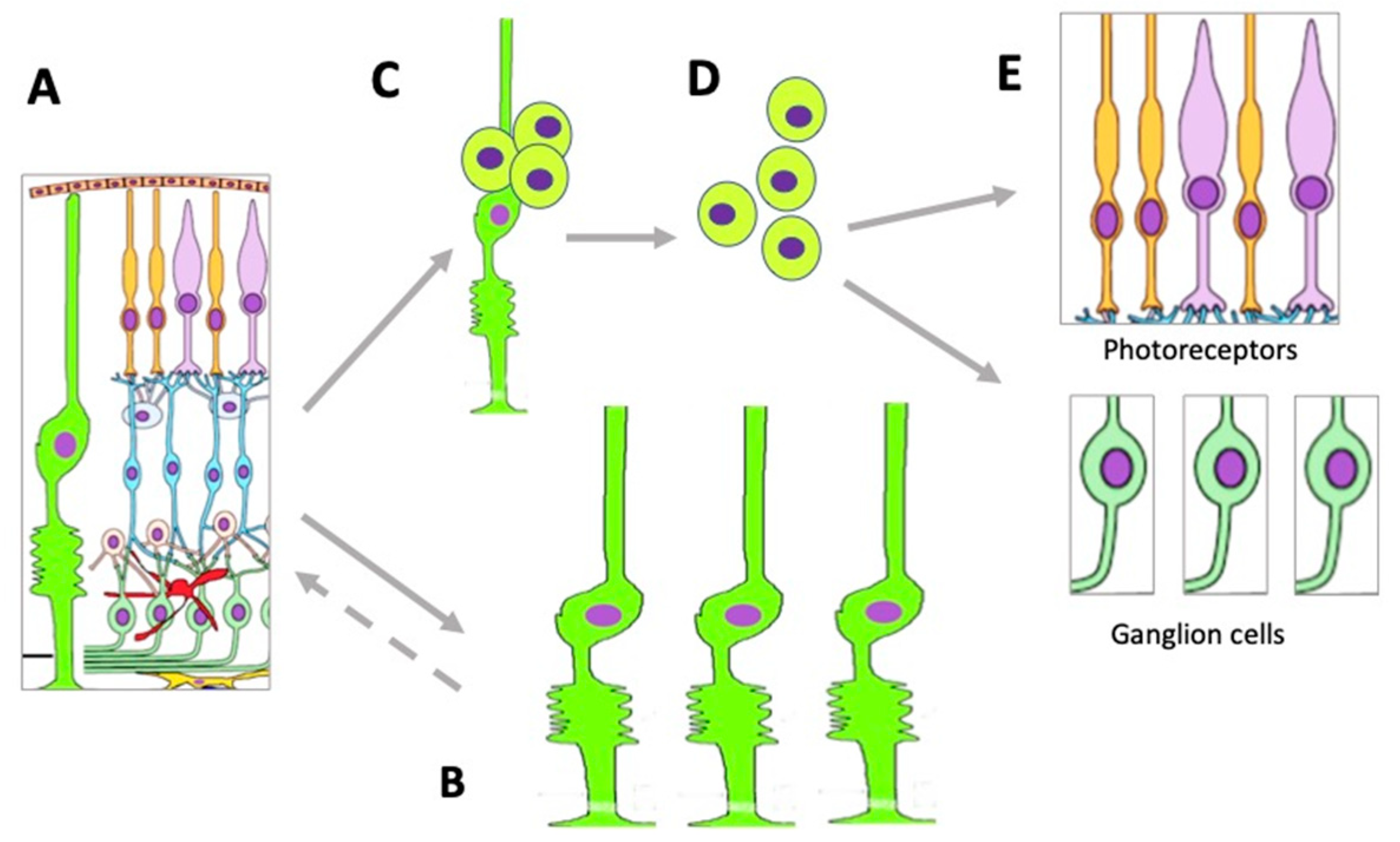
Müller glial cells (MGCs) are well-known and widely studied as latent RRCSs (see [49][50][51][52][53]). According to data obtained recently, MGCs that have undergone age-related changes can still be stimulated to regenerate cells lost after acute NR damage in aged zebrafish [54]. MGCs are very promising for regenerating cell losses in the NR [52][55][56][57]. MGC bodies are located radially in the INL, extending long processes to the outer and inner limiting membranes of the NR (Figure 2). The Müller glia is a cell population specialized in performing a wide range of functions, including neurotrophic and structural ones, and also maintaining synaptic connections with NR neurons. It is involved in both NR cleaning and light perception [58]. Furthermore, extensive evidence indicates that MGCs are a population that can exhibit the properties of neural progenitor cells. If NR is damaged, they re-express TFs (six3, pax6, rx1, olig2, and vsx2) characteristic of NR progenitors and immature macroglial cells [59]. In the case of surgical excision of zebrafish NR, MGCs proliferate and produce retinal progenitors capable of differentiating into photoreceptors (cones) and interneurons [60]. After thermal or light-induced damage to the fish NR causing loss of photoreceptors, MGCs re-enter the cell cycle and subsequently up-regulate the expression of stem and progenitor cell-specific proteins [61][62][63]. During the life-long growth of the eye in fish, MGCs maintain the rod photoreceptor lineage, and in case of regeneration, they are able to produce precursors for photoreceptors and ganglion cells [49]. Emerging evidence shows that inflammation plays an essential role in the multi-step process of retinal regeneration [64]. The zebrafish model, with its extensive experimental manipulation capabilities, has been accepted for the study of MGC reprogramming in order to stimulate MGC conversion in mammals [65] (Figure 4).
Figure 4. Changes occurring in the MGC population under conditions of retinal damage. (A)—MGCs in the structure of normal retina; (B)—MGC hypertrophy and proliferation in conditions of reactive gliosis; (C)—MGC reprogramming and proliferation during retinal regeneration in vivo and after directed stimulation in vitro; (D)—MGC-derived retinal cell precursors emerging during retinal regeneration in vivo and after directed stimulation in vitro; (E)—retinal neurons formed from MGC-derived retinal cell precursors.
4. Conclusions
Intrinsic retinal regeneration cell sources (RRCSs), which include RPE, CB, MG, and the NR ciliary region, have intrinsic genetic features that determine their potencies for retinal neuron production. These potencies are implemented, to varying degrees, from complete or partial retinal regeneration by RRCSs in fish, amphibians, and bird embryos to the manifestation of certain progenitor properties, proliferation, and change of the RRCS phenotype in mammals. In the latter, as well as in humans, such regenerative responses are most frequently found under conditions of directed induction in vitro. Identification of RRCSs and mechanisms of regulation of their behavior have long been conducted in various studies on animal models and humans. The studies have shown that the mechanisms regulating the manifestation/inhibition of regenerative responses of RRCSs in animals and humans are common. In general, these include external signaling, changing transcription patterns, and the epigenetic landscape. The pathways of conversion through proliferation and acquisition of a progenitor state that cells can leave by acquiring a new differentiation of one or more retinal phenotypes are also common for latent RRCSs (RPE, CB, and MGCs). In the progenitor state phase, RRCSs express “developmental” TFs and multipotency genes that are controlled by intracellular mechanisms of genome regulation. These mechanisms, in turn, depend on a wide range of external effects such as signaling molecules (growth factors, inflammation, viability, cell death, hormones, etc.). Modulation of immune responses during degenerative processes in the NR can also affect the course of NR regeneration.
The major degenerative diseases of the retina are associated with changes, death, and loss of function by the RPE and photoreceptors. Diseases of this kind include AMD, PVR, and RP. Glaucoma is caused by the loss of ganglion cells, while reactive gliosis, accompanying many NR pathologies, is caused by cell hypertrophy and an increase in the MGC population. To translate data to biomedicine of the eye, studies of RRCSs are being conducted in two main areas. The first is a search for technologies to provide replacement of dead/degenerating NR cells with healthy ones obtained from endogenous RRCSs. Designing the methods to promote the production of autologous retinal cells de novo under in vivo or in vitro conditions is an important alternative to the use of stem cells for these purposes. The use of exogenous stem cells in ophthalmology is widely studied currently. Nevertheless, despite marked technological advances, the risks of mutations, tumor growth, undesirable benign transformations of these cells, etc. have been recorded. Obtaining the required cell types from stem cells with integration of new cells into the cell ensemble and, at last, the issue of immune rejection, still pose serious challenges. The natural retinal regeneration process achieved through the use of RRCSs may be more successful for therapy, including the facilitated integration of endogenous cells and proper synaptic targeting. However, the issue of competition between the use of endogenous RRCSs and stem cells is not addressed here. As can be seen from the examples, discussed in the paper these methods can be coupled and complement each other.
References
- Masland, R.H. The Neuronal Organization of the Retina. Neuron 2012, 76, 266–280.
- Hoon, M.; Okawa, H.; Della Santina, L.; Wong, R.O. Functional architecture of the retina: Development and disease. Prog. Retin. Eye Res. 2014, 42, 44–84.
- Alm, A. Ocular circulation. In Adler’s Physiology of the Eye: Clinical Application; Hart, W.M., Ed.; Mosby: St. Louis, MO, USA, 1992; pp. 198–227.
- Fernandes, A.-R.; Zielińska, A.; Sanchez-Lopez, E.; dos Santos, T.; Garcia, M.L.; Silva, A.M.; Karczewski, J.; Souto, E.B. Exudative versus Nonexudative Age-Related Macular Degeneration: Physiopathology and Treatment Options. Int. J. Mol. Sci. 2022, 23, 2592.
- Al-Hussaini, H.; Schneiders, M.; Lundh, P.; Jeffery, G. Drusen are associated with local and distant disruptions to human retinal pigment epithelium cells. Exp. Eye Res. 2009, 88, 610–612.
- Ding, X.; Patel, M.; Chan, C.C. Molecular pathology of age-related macular degeneration. Prog. Retin. Eye Res. 2009, 28, 1–18.
- Chirco, K.; Sohn, E.; Stone, E.; Tucker, B.; Mullins, R. Structural and molecular changes in the aging choroid: Implications for age-related macular degeneration. Eye 2017, 31, 10.
- Pardue, M.T.; Allen, R.S. Neuroprotective strategies for retinal disease. Prog. Retin. Eye Res. 2018, 65, 50–76.
- Novikova, Y.P.; Gancharova, O.S.; Eichler, O.V.; Philippov, P.P.; Grigoryan, E.N. Preventive and Therapeutic Effects of SkQ1-Containing Visomitin Eye Drops against Light-Induced Retinal Degeneration. Biochemistry 2014, 79, 1101–1110.
- Jaffe, G.J.; Ciulla, T.A.; Ciardella, A.P.; Devin, F.; Dugel, P.U.; Eandi, C.M.; Masonson, H.; Monés, J.; Pearlman, J.A.; Quaranta-El Maftouhi, M.; et al. Dual antagonism of PDGF and VEGF in neovascular age-related macular degeneration: A phase IIb, multicenter, randomized controlled trial. Ophthalmology 2017, 124, 224–234.
- Ammar, M.J.; Hsu, J.; Chiang, A.; Ho, A.C.; Regillo, C.D. Age-related macular degeneration therapy: A review. Curr. Opin. Ophthalmol. 2020, 31, 215–221.
- Han, J.W.; Choi, J.; Kim, Y.S.; Kim, J.; Brinkmann, R.; Lyu, J.; Park, T.K. Comparison of the neuroinflammatory responses to selective retina therapy and continuous-wave laser photocoagulation in mouse eyes. Graefe’s Arch. Clin. Exp. Ophthalmol. 2018, 256, 341–353.
- Khanna, S.; Komati, R.; Eichenbaum, D.A.; Hariprasad, I.; Ciulla, T.A.; Hariprasad, S.M. Current and upcoming anti-VEGF therapies and dosing strategies for the treatment of neovascular AMD: A comparative review. BMJ Open Ophthalmol. 2019, 4, e000398.
- Morescalchi, F.; Duse, S.; Gambicorti, E.; Romano, M.R.; Costagliola, C.; Semeraro, F. Proliferative Vitreoretinopathy after Eye Injuries: An Overexpression of Growth Factors and Cytokines Leading to a Retinal Keloid. Mediat. Inflamm. 2013, 2013, 269787.
- Idrees, S.; Sridhar, J.; Kuriyan, A.E. Proliferative Vitreoretinopathy: A Review. Int. Ophthalmol. Clin. 2019, 59, 221–240.
- Zou, H.; Shan, C.; Ma, L.; Liu, J.; Yang, N.; Zhao, J. Polarity and epithelial-mesenchymal transition of retinal pigment epithelial cells in proliferative vitreoretinopathy. Peer J. 2020, 8, e10136.
- Abu El-Asrar, A.M.; Midena, E.; Al-Shabrawey, M.; Mohammad, G. New Developments in the Pathophysiology and Management of Diabetic Retinopathy. J. Diabetes Res. 2013, 2013, 424258.
- Lopez, P.F.; Yan, Q.; Kohen, L.; Rao, N.A.; Spee, C.; Black, J.; Oganesian, A. Retinal pigment epithelial wound healing in vivo. Arch Ophthalmol. 1995, 113, 1437–1446.
- Grigoryan, E.N. Pigment Epithelia of the Eye: Cell-Type Conversion in Regeneration and Disease. Life 2022, 12, 382.
- Hartong, D.T.; Berson, E.L.; Dryja, T.P. Retinitis pigmentosa. Lancet 2006, 368, 1795–1809.
- Pach, J.; Gekeler, F. Therapeutic approaches for retinitis pigmentosa. Klin. Monbl. Augenheilkd. 2013, 230, 512–518.
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The pathophysiology and treatment of glaucoma: A review. JAMA 2014, 311, 1901–1911.
- Cholkar, K.; Trinh, H.M.; Pal, D.; Mitra, A.K. Discovery of novel inhibitors for the treatment of glaucoma. Expert Opin. Drug Discov. 2015, 10, 293–313.
- Prum, B.E., Jr.; Herndon, L.W., Jr.; Moroi, S.E.; Mansberger, S.L.; Stein, J.D.; Lim, M.C.; Rosenberg, L.F.; Gedde, S.J.; Williams, R.D. Primary Angle Closure Preferred Practice Pattern® Guidelines. Ophthalmology 2016, 123, P1–P40.
- Harris, W.A.; Perron, M. Molecular recapitulation: The growth of the vertebrate retina. Int. J. Dev. Biol. 1998, 42, 299–304.
- Hitchcock, P.F.; Raymond, P.A. The teleost retina as a model for developmental and regeneration biology. Zebrafish 2004, 1, 257–271.
- Johns, P.R. Growth of the adult goldfish eye. III. Source of the new retinal cells. J. Comp. Neurol. 1977, 176, 343–357.
- Mitashov, V.I.; Panova, I.G.; Koussoulakos, S. Transdifferentiation potencies of ciliary and pigment epithelium cells of lower vertebrates and mammals. Russ. J. Dev. Biol. 2004, 35, 395–403.
- Fischer, A.J.; Bosse, J.L.; El-Hodiri, H.M. The ciliary marginal zone (CMZ) in development and regeneration of the vertebrate eye. Exp. Eye Res. 2013, 116, 199–204.
- Karl, M.O.; Hayes, S.; Nelson, B.R.; Tan, K.; Buckingham, B.; Reh, T.A. Stimulation of neural regeneration in the mouse retina. Proc. Natl. Acad. Sci. USA 2008, 105, 19508–19513.
- Napier, H.R.; Kidson, S.H. Molecular events in early development of the ciliary body: A question of folding. Exp. Eye Res. 2007, 84, 615–625.
- McDougal, D.H.; Gamlin, P.D. Autonomic control of the eye. Comp. Physiol. 2015, 5, 439–473.
- Wohl, S.G.; Schmeer, C.W.; Isenmann, S. Neurogenic potential of stem/progenitor-like cells in the adult mammalian eye. Prog. Retin. Eye Res. 2012, 31, 213–242.
- Nickerson, P.E.; Emsley, J.G.; Myers, T.; Clarke, D.B. Proliferation and expression of progenitor and mature retinal phenotypes in the adult mammalian ciliary body after retinal ganglion cell injury. Investig. Ophthalmol. Vis. Sci. 2007, 48, 5266–5275.
- Ooto, S.; Akagi, T.; Kageyama, R.; Akita, J.; Mandai, M.; Honda, Y.; Takahashi, M. Potential for neural regeneration after neurotoxic injury in the adult mammalian retina. Proc. Natl. Acad. Sci. USA 2004, 101, 13654–13659.
- Wohl, S.G.; Schmeer, C.W.; Kretz, A.; Witte, O.W.; Isenmann, S. Optic nerve lesion increases cell proliferation and nestin expression in the adult mouse eye in vivo. Exp. Neurol. 2009, 219, 175–186.
- Del Debbio, C.B.; Santos, M.F.; Yan, C.Y.; Ahmad, I.; Hamassaki, D.E. Rho GTPases control ciliary epithelium cells proliferation and progenitor profile induction in vivo. Investig. Ophthalmol. Vis. Sci. 2014, 55, 2631–2641.
- Strauss, O. The Retinal Pigment Epithelium in Visual Function. Physiol. Rev. 2005, 85, 845–881.
- Sparrow, J.R.; Hicks, D.; Hamel, C.P. The Retinal Pigment Epithelium in Health and Disease. Curr. Mol. Med. 2010, 10, 802–823.
- Fuhrmann, S.; Zou, C.J.; Levine, E.M. Retinal pigment epithelium development, plasticity, and tissue homeostasis. Exp. Eye Res. 2014, 123, 141–150.
- Lakkaraju, A.; Umapathy, A.; Tan, L.X.; Daniele, L.; Philp, N.J.; Boesze-Battaglia, K.; Williams, D.S. The cell biology of the retinal pigment epithelium. Prog. Retin. Eye Res. 2020, 78, 100846.
- Koster, C.; Wever, K.E.; Wagstaff, P.E.; van den Hirk, K.T.; Hooijmans, C.R.; Bergen, A.A. A Systematic Review on Transplantation Studies of the Retinal Pigment Epithelium in Animal Models. Int. J. Mol. Sci. 2020, 21, 2719.
- Caceres, P.S.; Rodriguez-Boulan, E. Retinal Pigment Epithelium Polarity in Health and Blinding Diseases. Curr. Opin. Cell Biol. 2020, 62, 37–45.
- Markitantova, Y.; Simirskii, V. Inherited Eye Diseases with Retinal Manifestations through the Eyes of Homeobox Genes. Int. J. Mol. Sci. 2020, 21, 1602.
- Grigoryan, E.N.; Markitantova, Y.V. Molecular Strategies for Transdifferentiation of Retinal Pigment Epithelial Cells in Amphibians and Mammals In Vivo. Russ. J. Dev. Biol. 2021, 52, 20–43.
- George, S.; Lu, F.; Rao, M.; Leach, L.; Gross, J.M. The retinal pigment epithelium: Development, injury responses, and regenerative potential in mammalian and non-mammalian systems. Prog. Retin. Eye Res. 2021, 85, 100969.
- Rizzolo, L.J.; Nasonkin, I.O.; Adelman, R.A. Retinal Cell Transplantation, Biomaterials, and In Vitro Models for Developing Next-generation Therapies of Age-related Macular Degeneration. Stem Cells Transl. Med. 2022, 11, 269–281.
- Grigoryan, E.N. Self-Organization of the Retina during Eye Development, Retinal Regeneration In Vivo, and in Retinal 3D Organoids In Vitro. Biomedicines 2022, 10, 1458.
- Lenkowski, J.R.; Raymond, P.A. Muller glia: Stem cells for generation and regeneration of retinal neurons in teleost fish. Prog. Retin. Eye Res. 2014, 40, 94–123.
- Goldman, D. Muller glial cell reprogramming and retina regeneration. Nat. Rev. Neurosci. 2014, 15, 431–442.
- Hamon, A.; Roger, J.E.; Yang, X.J.; Perron, M. Muller glial cell-dependent regeneration of the neural retina: An overview across vertebrate model systems. Dev. Dyn. 2016, 245, 727–738.
- Chohan, A.; Singh, U.; Kumar, A.; Kaur, J. Müller stem cell dependent retinal regeneration. Clin. Chim. Acta 2017, 464, 160–164.
- Gao, H.; Huang, X.; Chen, X.; Xu, H. Müller Glia-Mediated Retinal Regeneration. Mol. Neurobiol. 2021, 58, 2342–2361.
- Martins, R.R.; Zamzam, M.; Tracey-White, D.; Moosajee, M.; Thummel, R.; Henriques, C.M.; MacDonald, R.B. Müller Glia maintain their regenerative potential despite degeneration in the aged zebrafish retina. Aging Cell 2022, 21, e13597.
- Madelaine, R.; Mourrain, P. Endogenous retinal neural stem cell reprogramming for neuronal regeneration. Neural. Reg. Res. 2017, 12, 1765–1767.
- Otteson, D.C. Talkin’ about my (re)generation: The who of intrinsic retinal stem cells. Neuroscience 2017, 34627, 447–449.
- Devoldere, J.; Peynshaert, K.; De Smedt, S.C.; Remaut, K. Müller cells as a target for retinal therapy. Drug Discov. Today 2019, 24, 1483–1498.
- de Hoz, R.; Rojas, B.; Ramirez, A.I.; Salazar, J.J.; Gallego, B.I.; Triviño, A.; Ramírez, J.M. Retinal macroglial responses in health and disease. BioMed Res. Int. 2016, 2016, 2954721.
- Reichenbach, A.; Bringmann, A. New functions of Müller cells. Glia 2013, 61, 651–678.
- Cameron, D.A. Cellular proliferation and neurogenesis in the injured retina of adult zebrafish. Vis. Neurosci. 2000, 17, 789–797.
- Raymond, P.; Barthel, L.K.; Bernardos, R.L.; Perkowski, J.J. Molecular characterization of retinal stem cells and their niches in adult zebrafish. BMC Dev. Biol. 2006, 6, 36.
- Thummel, R.; Kassen, S.C.; Enright, J.M.; Nelson, C.M.; Montgomery, J.E.; Hyde, D.R. Characterization of Müller glia and neuronal progenitors during adult zebrafish retinal regeneration. Exp. Eye Res. 2008, 87, 433–444.
- Nagashima, M.; Barthel, L.K.; Raymond, P.A. A self-renewing division of zebrafish Müller glial cells generates neuronal progenitors that require N-cadherin to regenerate retinal neurons. Development 2013, 140, 4510–4521.
- Nagashima, M.; Hitchcock, P. Inflammation Regulates the Multi-Step Process of Retinal Regeneration in Zebrafish. Cells 2021, 10, 783.
- Lahne, M.; Nagashima, M.; Hyde, D.R.; Hitchcock, P.F. Reprogramming Müller Glia to Regenerate Retinal Neurons. Annu. Rev. Vis. Sci. 2020, 6, 171–193.
More
