Alzheimer's disease (AD) is a multifactorial disease and the most common neurodegenerative disorder affecting the elderly population world-wide.The used medications treat the symptoms of AD, but without any delay on the progression of the disease. Curcumin presented favorable effects on AD. In the last decade curcumin analogues and derivatives have been synthesized in an attempt to optimize the beneficial properties of curcumin and improve its absorbance and distribution per os as a therapeutic agent.
Reviewing the bibliographical data of the last decade, information on the structures and / or chemical groups that are associated with specific action against AD, was gathered, derived from docking studies, (Q)SAR from vitro and in vivo tests. Phenolic hydroxyl groups might contribute to the anti-amyloidogenic activity. Phenyl methoxy groups seems to contribute to the suppression of Aβ42 and to the suppression of APP. Hydrophobic interactions revealed to be important. The presence of flexible moieties at the linker are crucial for the inhibition of Aβ aggregation. The inhibitory activity of derivatives is increased with the expansion of the aromatic rings. The keto-enol tautomer form offers as a new modification for the design of amyloid-binding agents.
Taking the above under consideration innovative design and synthesis will lead to more potent and specific curcumin analogues and derivatives against AD.
- Alzheimer Disease
- Curcumin analogues
- curcumin derivatives
- structural characteristics
- modeling
- QSAR
- therapy
- Introduction
1. Introduction
Alzheimer disease (AD) is characterized by serious loss of short-term memory and impaired cognition, followed by neurodegeneration. It is a multifactorial disease, still under research and a lot of causes have been suggested to be correlated to the onset of AD [1].
Alzheimer disease (AD) is characterized by serious loss of short-term memory and impaired cognition, followed by neurodegeneration. It is a multifactorial disease, still under research and a lot of causes have been suggested to be correlated to the onset of AD [1].
AD is mainly characterized by the accumulation of β-amyloid (Aβ) plaques (or senile plaques) and neurofibrillary tangles (NFTs) of Tau protein, in brain [2]. Chronic brain inflammation also is a distinctive feature of AD in which the microglia, astrocytes, and neurons extensively are thought to be strongly correlated in the inflammatory process [3,4]. Oxidative stress appears to be a major determinant of AD pathogenesis and progression [5]. In addition, lipid peroxidation and concentrations of several metalls as Fe, Cu, Al, and Hg in AD patients were found to be elevated [6].
AD is mainly characterized by the accumulation of β-amyloid (Aβ) plaques (or senile plaques) and neurofibrillary tangles (NFTs) of Tau protein, in brain [2]. Chronic brain inflammation also is a distinctive feature of AD in which the microglia, astrocytes, and neurons extensively are thought to be strongly correlated in the inflammatory process [3][4]. Oxidative stress appears to be a major determinant of AD pathogenesis and progression [5]. In addition, lipid peroxidation and concentrations of several metalls as Fe, Cu, Al, and Hg in AD patients were found to be elevated [6].
Low levels of Acetylocholine are observed in AD patients making Acetylcholinesterase (AChE) an important therapeutic player in AD disorder. Recently the role of neocortical acetylcholine (Ach) in spatial memory has been obtained. [7].
Low levels of Acetylocholine are observed in AD patients making Acetylcholinesterase (AChE) an important therapeutic player in AD disorder. Recently the role of neocortical acetylcholine (Ach) in spatial memory has been obtained [7].
Curcumin as well as curcuminoids are of great interest to researchers because it a pleiotropic-multitarget molecule, presenting a wide variety of bioactivities, including anti-inflammatory, antioxidant as well as anti-AD properties [8,9], inhibition activity against AChE, butrylcholinesterase (BChE) [10,11], β-secretase (BACE-1) [12] and glycogen synthase kinase 3 beta (GSK3β) [13].
Curcumin as well as curcuminoids are of great interest to researchers because it a pleiotropic-multitarget molecule, presenting a wide variety of bioactivities, including anti-inflammatory, antioxidant as well as anti-AD properties [8][9], inhibition activity against AChE, butrylcholinesterase (BChE) [10][11], β-secretase (BACE-1) [12][12] and glycogen synthase kinase 3 beta (GSK3β) [13].
Curcumin presents poor absorbance, biodistribution bioavailbility per os and selectivity. Thus, its use is significantly limited. Its multi-interaction with several molecular targets, diminishes its selectivity. In the last decade researchers focused to curcumin analogues trying to optimize the beneficial properties of curcumin against AD and improve its pharmacokinetic profile. Curcumin analogues were designed and synthesized as multitarget anti-AD agents showing promising results in treatment and diagnosis [14-16] of AD.
Curcumin presents poor absorbance, biodistribution bioavailbility per os and selectivity. Thus, its use is significantly limited. Its multi-interaction with several molecular targets, diminishes its selectivity. In the last decade researchers focused to curcumin analogues trying to optimize the beneficial properties of curcumin against AD and improve its pharmacokinetic profile. Curcumin analogues were designed and synthesized as multitarget anti-AD agents showing promising results in treatment and diagnosis [16][14][15] of AD.
- Discussion-Results
2. Discussion-Results
Perusal of publications of the last decade, describing curcumin analogues and curcumin derivatives against AD are analyzed. Their structural characteristics, functional groups, modelling studies, structure activity relationships, biological evaluation
in vitro / in vivo
are discussed.
The following Schemes highlight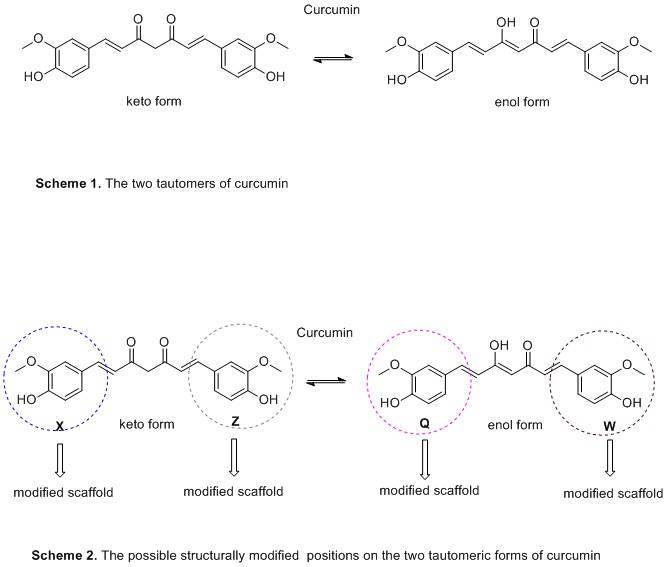
curcumin structure and indicates the possible positions for structural modifications.
So far the design of curcumin analogues is more focused on the inhibition of Αβ-amyloid and the suppression of oxidative stress. The attempts to design and synthesize molecules that inhibit both protein accumulation and AChE in the brain were fewer and the results very poor.
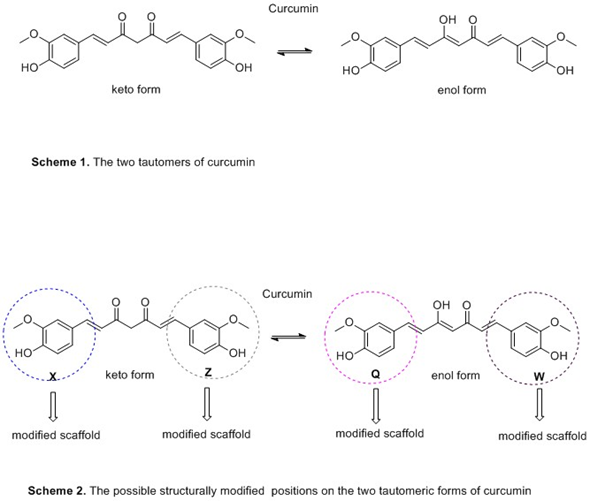
Judging the review findings, the presence of phenyl methoxy and hydroxyl groups seems to play an important role in inhibiting Αβ-amyloid accumulation. On the other hand, phenolic groups in combination with methoxyl moiety in ortho position and hydroxyl substituent are implicated to the oxidative stress suppression (Scheme 1). It seems that the styryl function and steric or electronic factors through the large aromatic structure are contributed to the anti-oxidant activity[17].(Scheme 2)
Curcumin and its derivatives due to the existence of two aromatic rings and the distance between them, could be able to favorably interact with both the quaternary and peripheral sites of AChE's catalytic site through hydrogen bonds formation. The keto-enol moiety is characterized as the best alternative modification to elevate the levels of acetyl-choline by the design of more potent AChE inhibitors (Scheme 1)[1][2][3][2][4][2][5][6][7][8][9][10][11][12][13][5][5][14][15][16][17].(Scheme 2)
Curcumin and its derivatives due to the existence of two aromatic rings and the distance between them, could be able to favorably interact with both the quaternary and peripheral sites of AChE's catalytic site through hydrogen bonds formation. The keto-enol moiety is characterized as the best alternative modification to elevate the levels of acetyl-choline by the design of more potent AChE inhibitors (Scheme 1) [17].(Scheme 2)
Curcumin hybrids, which combine tacrine, donepezil, rivastigmine melatonin with curcumin present a multifunctional profile, targeting many factors implicated in Alzheimer's disease. However, it should to be noticed that the permeability of the blood-brain barrier is not very successful for all the molecules of this type [17].
References
- 1. Potter, P.E. Curcumin: a natural substance with potential efficacy in Alzheimer's disease. J Exp Pharmacol 2013, 5, 23-31, doi:10.2147/JEP.S26803.
- 2. Lee, I.; Yang, J.; Lee, J.H.; Choe, Y.S. Synthesis and evaluation of 1-(4-[18F]fluoroethyl)-7-(4′-methyl)curcumin with improved brain permeability for β-amyloid plaque imaging. Bioorganic & Medicinal Chemistry Letters 2011, 21, 5765-5769, doi:https://doi.org/10.1016/j.bmcl.2011.08.003.
- 2. Lee, I.; Yang, J.; Lee, J.H.; Choe, Y.S. Synthesis and evaluation of 1-(4-[18F]fluoroethyl)-7-(4′-methyl)curcumin with improved brain permeability for β-amyloid plaque imaging. Bioorganic & Medicinal Chemistry Letters 2011, 21, 5765-5769, doi:https://doi.org/10.1016/j.bmcl.2011.08.003.Decha Pinkaew; Chatchawan Changtam; Chainarong Tocharus; Sarinthorn Thummayot; Apichart Suksamrarn; Jiraporn Tocharus; Sirinthorn Thummayot; Apichart Suksamran; Di-O-demethylcurcumin protects SK-N-SH cells against mitochondrial and endoplasmic reticulum-mediated apoptotic cell death induced by Aβ25-35. Neurochemistry International 2015, 80, 110-119, 10.1016/j.neuint.2014.10.008.
- Decha Pinkaew; Chatchawan Changtam; Chainarong Tocharus; Sarinthorn Thummayot; Apichart Suksamrarn; Jiraporn Tocharus; Sirinthorn Thummayot; Apichart Suksamran; Di-O-demethylcurcumin protects SK-N-SH cells against mitochondrial and endoplasmic reticulum-mediated apoptotic cell death induced by Aβ25-35. NeurocGerbrand Ceder; Et Al. Et Al.; ChemInform Abstract: Sidorenkite (Na3MnPO4CO3): A New Intercalation Cathode Material for Na-Ion Batteries.. Chemistry International form 2015, 80, 110-119, 10.1016/j.neuint.2014.10.008.3, 44, , 10.1002/chin.201338012.
- Gerbrand Ceder; Et Al. Et Al.; ChemInform Abstract: Sidorenkite (Na3MnPO4CO3): A New Intercalation Cathode Material for Na-Ion Batteries.. Peng Zhai; Chun-Li Xia; Jia-Heng Tan; Ding Li; Tian-Miao Ou; Shi-Liang Huang; Lian Quan Gu; Zhi-Shu Huang; Syntheses And Evaluation Of Asymmetric Curcumin Analogues As Potential Multifunctional Agents For The Treatment Of Alzheimer's Disease.. Current AlzhemInform imer Research 2013, 44, , 10.1002/chin.201338012.5, 12, , null.
- Peng Zhai; Chun-Li Xia; Jia-Heng Tan; Ding Li; Tian-Miao Ou; Shi-Liang Huang; Lian Quan Gu; Zhi-Shu Huang; Syntheses And Evaluation Of Asymmetric Curcumin Analogues As Potential Multifunctional Agents For The Treatment Of Alzheimer's Disease.. CuShang-Ying Chen; Yuan Chen; Yan-Ping Li; Shu-Han Chen; Jia-Heng Tan; Tian-Miao Ou; Lian Gu; Zhi-Shu Huang; Design, synthesis, and biological evaluation of curcumin analogues as multifunctional agents for the treatment of Alzheimer’s disease. Bioorrganic & Ment Alzheimer Research dicinal Chemistry 2015, 1, 12, , null.9, 5596-5604, 10.1016/j.bmc.2011.07.033.
- Shang-Ying Chen; Yuan Chen; Yan-Ping Li; Shu-Han Chen; Jia-Heng Tan; Tian-Miao Ou; Lian Gu; Zhi-Shu Huang; Design, synthesis, and biological evaluation of curcumin analogues as multifunctional agents for the treatment of Alzheimer’s disease. BSophie Sokolow; Krisztina Marosi; Kwangsik Nho; Jerome I. Rotter; Liana G. Apostolova; Alzheimer’S Disease Neuroimaging Initiative; P2-003: ACETYLCHOLINESTERASE INHIBITOR THERAPY IN MILD COGNITIVE IMPAIRMENT: YES OR NO?. Alzheioomerganic & Medicin's & Demential Chemistry 2011, 06, 19, 5596-5604, 10.1016/j.bmc.2011.07.033.4, P665-P666, 10.1016/j.jalz.2018.06.687.
- Sophie Sokolow; Krisztina Marosi; Kwangsik Nho; Jerome I. Rotter; Liana G. Apostolova; Alzheimer’S Disease Neuroimaging Initiative; P2-003: ACETYLCHOLINESTERASE INHIBITOR THERAPY IN MILD COGNITIVE IMPAIRMENT: YES OR NO?. AlzheLei Fang; Shaohua Gou; Xuying Liu; Feng Cao; Lin Cheng; Design, synthesis and anti-Alzheimer properties of dimethylaminomethyl-substituted curcumin derivatives. Bimeoor's & Dementia ganic & Medicinal Chemistry Letters 2006, 114, 24, P665-P666, 10.1016/j.jalz.2018.06.687., 40-43, 10.1016/j.bmcl.2013.12.011.
- Lei Fang; Shaohua Gou; Xuying Liu; Feng Cao; Lin Cheng; Design, synthesis and anti-Alzheimer properties of dimethylaminomethyl-substituted curcumin derivatives. BioMadhuri Venigalla; Sandra Sonego; Erika Gyengesi; Matt Sharman; Gerald Münch; Novel promising therapeutics against chronic neuroinflammation and neurodegeneration in Alzheimer's disease. Neurorganic & Medicinal Chemistry Letters International 2014, 24, 40-43, 10.1016/j.bmcl.2013.12.011.6, 95, 63-74, 10.1016/j.neuint.2015.10.011.
- Madhuri Venigalla; Sandra Sonego; Erika Gyengesi; Matt Sharman; Gerald Münch; Novel promising therapeutics against chronic neuroinflammation and neurodegeneration in Alzheimer's disease. Zeynep Kalaycıoğlu; Işıl Gazioğlu; Fatma Bedia Erim; Comparison of antioxidant, anticholinesterase, and antidiabetic activities of three curcuminoids isolated from Curcuma longa L.. Neaturochemistry International al Product Research 2016, 95, 63-74, 10.1016/j.neuint.2015.10.011.7, 31, 1-4, 10.1080/14786419.2017.1299727.
- Zeynep Kalaycıoğlu; Işıl Gazioğlu; Fatma Bedia Erim; Comparison of antioxidant, anticholinesterase, and antidiabetic activities of three curcuminoids isolated from Curcuma longa L.. NatuJun Yan; Jinhui Hu; Anqiu Liu; Lin He; Xingshu Li; Hui Wei; Design, synthesis, and evaluation of multitarget-directed ligands against Alzheimer’s disease based on the fusion of donepezil and curcumin. Bioorgal Product Research nic & Medicinal Chemistry 2017, 31, 1-4, 10.1080/14786419.2017.1299727., 25, 2946-2955, 10.1016/j.bmc.2017.02.048.
- Jun Yan; Jinhui Hu; Anqiu Liu; Lin He; Xingshu Li; Hui Wei; Design, synthesis, and evaluation of multitarget-directed ligands against Alzheimer’s disease based on the fusion of donepezil and curcumin. Hiroyuki Konno; Hitoshi Endo; Satomi Ise; Keiki Miyazaki; Hideo Aoki; Akira Sanjoh; Kazuya Kobayashi; Yasunao Hattori; Kenichi Akaji; Synthesis and evaluation of curcumin derivatives toward an inhibitor of beta-site amyloid precursor protein cleaving enzyme 1. Bioorganic & Medicinal Chemistry Letters 2017, 4, 25, 2946-2955, 10.1016/j.bmc.2017.02.048.4, 685-690, 10.1016/j.bmcl.2013.11.039.
- Hiroyuki Konno; Hitoshi Endo; Satomi Ise; Keiki Miyazaki; Hideo Aoki; Akira Sanjoh; Kazuya Kobayashi; Yasunao Hattori; Kenichi Akaji; Synthesis and evaluation of curcumin derivatives toward an inhibitor of beta-site amyloid precursor protein cleaving enzyme 1. BXueli Zhang; Yanli Tian; Can Zhang; Xiaoyu Tian; Alana W. Ross; Robert D. Moir; HongBin Sun; Rudolph E. Tanzi; Anna Moore; Chongzhao Ran; Near-infrared fluorescence molecular imaging of amyloid beta species and monitoring therapy in animal models of Alzheimer's disease.. Proceedioornganic & Medicinal Chemistry Letters of the National Academy of Sciences 2014, 5, 1124, 685-690, 10.1016/j.bmcl.2013.11.039., 9734-9, 10.1073/pnas.1505420112.
- Xueli Zhang; Yanli Tian; Can Zhang; Xiaoyu Tian; Alana W. Ross; Robert D. Moir; HongBin Sun; Rudolph E. Tanzi; Anna Moore; Chongzhao Ran; Near-infrared fluorescence molecular imaging of amyloid beta species and monitoring therapy in animal models of Alzheimer's disease.. PXueli Zhang; Yanli Tian; Zeng Li; Xiaoyu Tian; HongBin Sun; Hong Liu; Anna Moore; Chongzhao Ran; Design and Synthesis of Curcumin Analogues for in Vivo Fluorescence Imaging and Inhibiting Copper-Induced Cross-Linking of Amyloid Beta Species in Alzheimer’s Disease. Jouroceedingsal of the National Academy of Sciences American Chemical Society 2015, 3, 112, 9734-9, 10.1073/pnas.1505420112.35, 16397-16409, 10.1021/ja405239v.
- Xueli Zhang; Yanli Tian; Zeng Li; Xiaoyu Tian; HongBin Sun; Hong Liu; Anna Moore; Chongzhao Ran; Design and Synthesis of Curcumin Analogues for in Vivo Fluorescence Imaging and Inhibiting Copper-Induced Cross-Linking of Amyloid Beta Species in Alzheimer’s Disease. Journal of the American Chemical Society 2013, 135, 16397-16409, 10.1021/ja405239v.Chainoglou,E.; Hadjipavlou-Litina, D.; Curcumin in Health and Diseases: Alzheimer’s Disease and Curcumin Analogues, Derivatives, and Hybrids. Int. J. Mol. Sci. 2020, 21(6), 1975; https://doi.org/10.3390/ijms21061975
- Chainoglou,E.; Hadjipavlou-Litina, D.; Curcumin in Health and Diseases: Alzheimer’s Disease and Curcumin Analogues, Derivatives, and Hybrids. Int. J. Mol. Sci. 2020, 21(6), 1975; https://doi.org/10.3390/ijms21061975