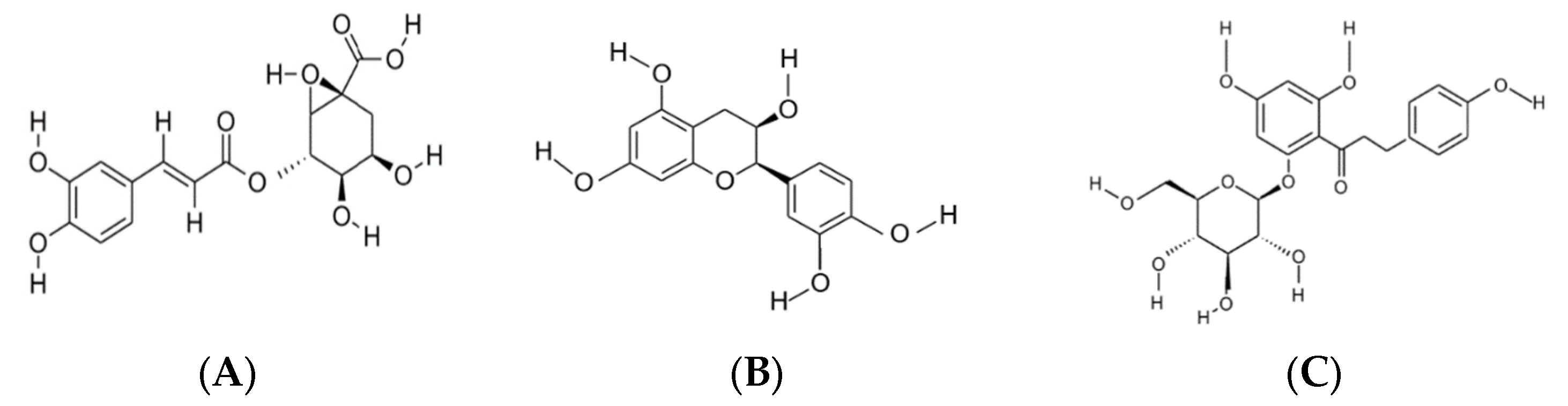
Polyphenols of plant origin are a broad family of secondary metabolites that range from basic phenolic acids to more complex compounds such as stilbenes, flavonoids, and tannins, all of which have several phenol units in their structure. Considerable health benefits, such as having prebiotic potential and cardio-protective and weight control effects, have been linked to diets based on polyphenol-enriched foods and plant-based products, indicating the potential role of these substances in the prevention or treatment of numerous pathologies. The most representative phenolic compounds in apple pomace are phloridzin, chlorogenic acid, and epicatechin, with major health implications in diabetes, cancer, and cardiovascular and neurocognitive diseases.
- phenolic compounds
- health effects
- apple-processing by-products
1. Introduction
2. Polyphenols in Apple-Processing By-Products
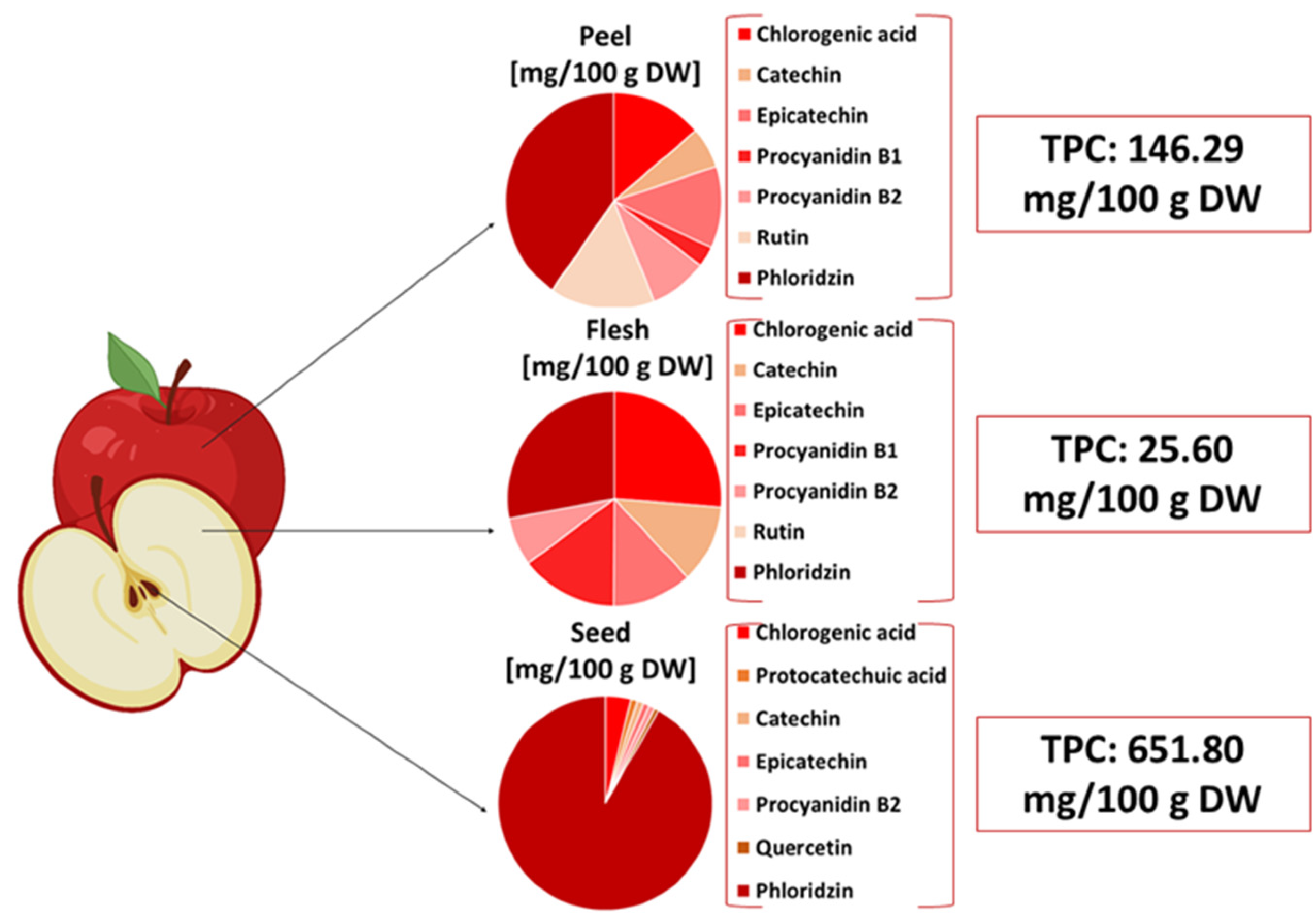
Apples are one of the most consumed fruits worldwide, both in industry and at the individual population level [31][5]. Approximately 11 million metric tons of apples is produced and used annually in the apple-processing industry and alcoholic beverage production in Europe [32][6]. Apple pomace is one of the most widely produced agrifood wastes, with an annual production rate of about 4 million tons worldwide [33][7]. The recovery rate for this by-product, however, is modest. Pomace is frequently discarded and dumped in landfills as waste, which causes environmental issues and presents a potential risk to public health [3,34][3][8]. The amount of pomace resulting after apple processing can be reused in biotechnological routs as a substrate for the production of different compounds, such as flavoring compounds, pigments, fuel, and citric acid, or as raw materials for the extraction of fibers and phenolic compounds [35,36,37,38][9][10][11][12]. From a nutritional point of view, apple pomace is a by-product rich in fibers, vitamins, minerals, phenolic compounds, and pigments [19][13]. All these macronutrients have a significant role in the human organism through their effects on metabolism. Therefore, apple pomace has attracted researchers’ consideration, as well as stakeholders’ attention, by virtue of its valuable composition and by presenting suitable properties for further sustainable use [39][14]. The nutritional profile of apple pomace is mainly represented by phenolic compounds, carbohydrates, and fibers, as presented in table Table 1. These constituents can help treat gastrointestinal disorders, decrease serum triglycerides and LDL-cholesterol, and regulate glycemia [40,41][15][16]. All these effects in the human organism can be explained through their high concentration of the beneficial compounds mentioned above, primarily exerting anti-inflammatory and antioxidant roles [42][17].|
Composition |
Amount (% DW) |
|---|---|
|
Total sugar |
45.1 ± 5.3 |
|
Total dietary fiber |
26.5 ± 0.8 |
|
Insoluble fiber |
18.4 ± 0.4 |
|
Soluble fiber |
8.2 ± 0.5 |
|
Total phenolic content (mg EGA/100 g AP) |
289.1 ± 24.2 |
|
Fat |
3.8 ± 0.2 |
|
Protein 2 |
3.8 ± 0.0 |
|
Polyphenolic profile |
(mg/100 g dry matter) |
|
Quercetin-3-O-galactoside |
22.55 ± 0.34 |
|
Quercetin-3-O-xyloside |
13.91 ± 0.03 |
|
Quercetin-3-O-rhamnoside |
19.21 ± 0.00 |
|
Chlorogenic acid |
20.55 ± 0.12 |
|
p-coumaroylquinic acid |
0.16 ± 0.03 |
|
Catechin |
1.44 ± 0.02 |
|
Procyanidin B2 |
2.61 ± 0.00 |
|
Phloretin-2-O-xylosyl-glucoside |
1.48 ± 0.14 |
|
Phlorizin |
15.52 ± 0.00 |
References
- Gu, B.; Zhang, X.; Bai, X.; Fu, B.; Chen, D. Four steps to food security for swelling cities. Nature 2019, 566, 31–33.
- Szabo, K.; Teleky, E.B.; Ranga, F.; Simon, E.; Pop, L.O.; Babalau-Fuss, V.; Kapsalis, N.; Cristian, D. Bioaccessibility of microencapsulated carotenoids, recovered from tomato processing industrial by-products, using in vitro digestion model. LWT 2021, 152, 112285.
- Martău, G.A.; Teleky, B.E.; Ranga, F.; Pop, I.D.; Vodnar, D.C. Apple Pomace as a Sustainable Substrate in Sourdough Fermentation. Front. Microbiol. 2021, 12, 3850.
- Iriondo-Dehond, M.; Miguel, E.; Del Castillo, M.D. Food byproducts as sustainable ingredients for innovative and healthy dairy foods. Nutrients 2018, 10, 1358.
- Francini, A.; Sebastiani, L. Phenolic compounds in apple (Malus x domestica borkh.): Compounds characterization and stability during postharvest and after processing. Antioxidants 2013, 2, 181–193.
- Bchir, B.; Karoui, R.; Danthine, S.; Blecker, C.; Besbes, S.; Attia, H. Date, Apple, and Pear By-Products as Functional Ingredients in Pasta: Cooking Quality Attributes and Physicochemical, Rheological, and Sensorial Properties. Foods 2022, 11, 1393.
- Gołębiewska, E.; Kalinowska, M.; Yildiz, G. Sustainable Use of Apple Pomace (AP) in Different Industrial Sectors. Materials 2022, 15, 1788.
- Lyu, F.; Luiz, S.F.; Azeredo, D.R.P.; Cruz, A.G.; Ajlouni, S.; Ranadheera, C.S. Apple pomace as a functional and healthy ingredient in food products: A review. Processes 2020, 8, 319.
- Ricci, A.; Cirlini, M.; Guido, A.; Liberatore, C.M.; Ganino, T.; Lazzi, C.; Chiancone, B. From byproduct to resource: Fermented apple pomace as beer flavoring. Foods 2019, 8, 309.
- Le Deun, E.; Van Der Werf, R.; Le Bail, G.; Le Quéré, J.M.; Guyot, S. HPLC-DAD-MS Profiling of Polyphenols Responsible for the Yellow-Orange Color in Apple Juices of Different French Cider Apple Varieties. J. Agric. Food Chem. 2015, 63, 7675–7684.
- Molinuevo-Salces, B.; Riaño, B.; Hijosa-Valsero, M.; González-García, I.; Paniagua-García, A.I.; Hernández, D.; Garita-Cambronero, J.; Díez-Antolínez, R.; García-González, M.C. Valorization of apple pomaces for biofuel production: A biorefinery approach. Biomass Bioenergy 2020, 142, 105785.
- Shojaosadati, S.A.; Babaeipour, V. Citric acid production from apple pomace in multi-layer packed bed solid-state bioreactor. Process Biochem. 2002, 37, 909–914.
- Rabetafika, H.N.; Bchir, B.; Blecker, C.; Richel, A. Fractionation of apple by-products as source of new ingredients: Current situation and perspectives. Trends Food Sci. Technol. 2014, 40, 99–114.
- Sato, M.F.; Vieira, R.G.; Zardo, D.M.; Falcão, L.D.; Nogueira, A.; Wosiacki, G. Apple pomace from eleven cultivars: An approach to identify sources of bioactive compounds. Acta Sci. Agron. 2010, 32, 29–35.
- Kasprzak-Drozd, K.; Oniszczuk, T.; Stasiak, M.; Oniszczuk, A. Beneficial effects of phenolic compounds on gut microbiota and metabolic syndrome. Int. J. Mol. Sci. 2021, 22, 3715.
- Sergent, T.; Piront, N.; Meurice, J.; Toussaint, O.; Schneider, Y.J. Anti-inflammatory effects of dietary phenolic compounds in an in vitro model of inflamed human intestinal epithelium. Chem. Biol. Interact. 2010, 188, 659–667.
- Skinner, R.C.; Gigliotti, J.C.; Ku, K.M.; Tou, J.C. A comprehensive analysis of the composition, health benefits, and safety of apple pomace. Nutr. Rev. 2018, 76, 893–909.
- Wang, X.; Kristo, E.; LaPointe, G. The effect of apple pomace on the texture, rheology and microstructure of set type yogurt. Food Hydrocoll. 2019, 91, 83–91.
- Gumul, D.; Ziobro, R.; Korus, J.; Kruczek, M. Apple pomace as a source of bioactive polyphenol compounds in gluten-free breads. Antioxidants 2021, 10, 807.
- Walia, M.; Rawat, K.; Bhushan, S.; Padwad, Y.S.; Singh, B. Fatty acid composition, physicochemical properties, antioxidant and cytotoxic activity of apple seed oil obtained from apple pomace. J. Sci. Food Agric. 2014, 94, 929–934.
- Bolarinwa, I.F.; Orfila, C.; Morgan, M.R.A. Determination of amygdalin in apple seeds, fresh apples and processed apple juices. Food Chem. 2015, 170, 437–442.
- Opyd, P.M.; Jurgoński, A.; Juśkiewicz, J.; Milala, J.; Zduńczyk, Z.; Król, B. Nutritional and health-related effects of a diet containing apple seed meal in rats: The case of amygdalin. Nutrients 2017, 9, 1091.
- Montañés, F.; Catchpole, O.J.; Tallon, S.; Mitchell, K.A.; Scott, D.; Webby, R.F. Extraction of apple seed oil by supercritical carbon dioxide at pressures up to 1300 bar. J. Supercrit. Fluids 2018, 141, 128–136.
- Feng, S.; Yi, J.; Li, X.; Wu, X.; Zhao, Y.; Ma, Y.; Bi, J. Systematic Review of Phenolic Compounds in Apple Fruits: Compositions, Distribution, Absorption, Metabolism, and Processing Stability. J. Agric. Food Chem. 2021, 69, 7–27.
- Pollini, L.; Cossignani, L.; Juan, C.; Mañes, J. Extraction of phenolic compounds from fresh apple pomace by different non-conventional techniques. Molecules 2021, 26, 4272.
- Lavelli, V.; Corti, S. Phloridzin and other phytochemicals in apple pomace: Stability evaluation upon dehydration and storage of dried product. Food Chem. 2011, 129, 1578–1583.
- Hrubá, M.; Baxant, J.; Čížková, H.; Smutná, V.; Kovařík, F.; Ševčík, R.; Hanušová, K.; Rajchl, A. Phloridzin as a marker for evaluation of fruit product’s authenticity. Czech J. Food Sci. 2021, 39, 49–57.
- Rana, S.; Gupta, S.; Rana, A.; Bhushan, S. Functional properties, phenolic constituents and antioxidant potential of industrial apple pomace for utilization as active food ingredient. Food Sci. Hum. Wellness 2015, 4, 180–187.
- Táborský, J.; Sus, J.; Lachman, J.; Šebková, B.; Adamcová, A.; Šatínský, D. Dynamics of phloridzin and related compounds in four cultivars of apple trees during the vegetation period. Molecules 2021, 26, 3816.
- Najafian, M.; Jahromi, M.Z.; Nowroznejhad, M.J.; Khajeaian, P.; Kargar, M.M.; Sadeghi, M.; Arasteh, A. Phloridzin reduces blood glucose levels and improves lipids metabolism in streptozotocin-induced diabetic rats. Mol. Biol. Rep. 2012, 39, 5299–5306.
- Kamdi, S.P.; Badwaik, H.R.; Raval, A.; Ajazuddin; Nakhate, K.T. Ameliorative potential of phloridzin in type 2 diabetes-induced memory deficits in rats. Eur. J. Pharmacol. 2021, 913, 174645.
- Masumoto, S.; Akimoto, Y.; Oike, H.; Kobori, M. Dietary Phloridzin Reduces Blood Glucose Levels and Reverses Sglt1 Expression in the Small Intestine in Streptozotocin-lnduced Diabetic Mice. J. Agric. Food Chem. 2009, 57, 4651–4656.
- Lu, Y.Y.; Liang, J.; Chen, S.X.; Wang, B.X.; Yuan, H.; Li, C.T.; Wu, Y.Y.; Wu, Y.F.; Shi, X.G.; Gao, J.; et al. Phloridzin alleviate colitis in mice by protecting the intestinal brush border and improving the expression of sodium glycogen transporter 1. J. Funct. Foods 2018, 45, 348–354.
- Fang, Y.Z.; Yang, S.; Wu, G. Free radicals, antioxidants, and nutrition. Nutrition 2002, 18, 872–879.
- Upadhyay, R.; Mohan Rao, L.J. An Outlook on Chlorogenic Acids-Occurrence, Chemistry, Technology, and Biological Activities. Crit. Rev. Food Sci. Nutr. 2013, 53, 968–984.
- Cropley, V.; Croft, R.; Silber, B.; Neale, C.; Scholey, A.; Stough, C.; Schmitt, J. Does coffee enriched with chlorogenic acids improve mood and cognition after acute administration in healthy elderly? A pilot study. Psychopharmacology 2012, 219, 737–749.
- Mubarak, A.; Catherine, P.B.; Liu, A.H.; Considine, M.J.; Rich, L.; Mas, E.; Croft, K.D.; Hodgson, J.M. Acute effects of chlorogenic acid on nitric oxide status, endothelial function and blood pressure in healthy volunteers: A randomised trial. J. Agric. Food Chem. 2012, 60, 9130–9136.
- Qu, Z.; Liu, A.; Li, P.; Liu, C.; Xiao, W.; Huang, J.; Liu, Z.; Zhang, S. Advances in physiological functions and mechanisms of (-)-epicatechin. Crit. Rev. Food Sci. Nutr. 2021, 61, 211–233.
- Cilleros, D.Á.; Martín, M.Á.; Ramos, S. (−)-Epicatechin and the colonic 2,3-dihydroxybenzoic acid metabolite regulate glucose uptake, glucose production, and improve insulin signalling in renal NRK-52E cells. Mol. Nutr. Food Res. 2018, 62, 1700470.
- Cordero-Herrera, I.; Chen, X.; Ramos, S.; Devaraj, S. (−)-Epicatechin attenuates high-glucose-induced inflammation by epigenetic modulation in human monocytes. Eur. J. Nutr. 2017, 56, 1369–1373.
