Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 4 by Jessie Wu and Version 3 by Jessie Wu.
Neurocognitive impairment (NCI) associated with HIV infection of the brain impacts a large proportion of people with HIV (PWH) regardless of antiretroviral therapy (ART). While the number of people with HIV (PWH) and severe NCI has dropped considerably with the introduction of antiretroviral therapy (ART), the sole use of ART is not sufficient to prevent or arrest NCI in many PWH. As the HIV field continues to investigate cure strategies, adjunctive therapies are greatly needed. HIV imaging, cerebrospinal fluid, and pathological studies point to the presence of continual inflammation, and the presence of HIV RNA, DNA, and proteins in the brain despite ART.
- HIV
- brain
- neuroinflammation
1. Cognitive
Many clinicians and investigators who specialize in the neurological complications of HIV infection continue to use the Frascati criteria as a framework to categorize HIV associated NCI [1]. Three main categories of cognitive dysfunction within HIV associated neurocognitive disorders (HAND) are defined and include asymptomatic neurocognitive impairment (ANI), mild neurocognitive disorder (MND) and HIV associated dementia (HAD). ANI and mild neurocognitive disorder (MND) are essentially defined by specific abnormalities in neuropsychological testing. The primary distinction is that people with HIV (PWH) who have MND have mild functional deficits and those with ANI do not. Meanwhile, those with HAD have even more profound neurocognitive deficits than those with MND. Those with HAD have more significant impairment of activities of daily living (ADLs) than those with MND [2]. Clinical evidence indicates that ANI and MND can be reversed with initiation of antiretroviral therapy (ART), while HAD is often not completely reversible. Therefore, ANI and MND can be considered ‘mild’ HAND or mild neurocognitive impairment (NCI). Researchers focus on mild NCI in PWH because the primary emphasis of this research is to discuss the development of therapeutic strategies that reverse or stabilize mild NCI in PWH who are already on suppressive ART.
As a larger proportion of the PWH ages, risk factors for NCI and other medical comorbidities have a larger role in the PWH population with mild NCI [3][4][5]. In some cases, the presence of these comorbidities makes it difficult to discern whether the mild NCI is linked to HIV infection in the brain or another underlying condition. Comorbidities related to age may include hypertension, diabetes, cerebrovascular disease, or renal failure [3][4][5]. Another major comorbidity in the PWH population is the presence of traumatic brain injury (TBI) [6][7]. In a preliminary study of 41 veterans with HIV who were recruited sequentially at the Atlanta VA Medical Center, 44% had NCI and 20% of those had a history of TBI [8][9]. TBI appears to make cognitive impairment worse in PWH vs. HIV associated NCI without TBI as executive function and working memory are both significantly decreased [6]. Notably, it was found that approximately 1/5 of the participants in the CNS HIV Antiretroviral Therapy Effects Research (CHARTER) study group were reported to have TBI [7]. Other risk factors and comorbidities that are relevant to PWH with mild NCI also include ART-associated neurotoxicity, low CD4+ count, longer period of HIV infection, high systemic viral load, cardiovascular disease, alcohol and drug abuse, coinfection (e.g., syphilis), or other neurodegenerative diseases such as Alzheimer’s Disease and Parkinson’s Disease [10][11][12][13][14][15][16][17][18][19][20][21][22]. The list of possible comorbidities in PWH is extensive, which makes it currently difficult for the field to differentiate specific biomarkers linked to cognitive impairment in PWH.
2. Imaging
Autopsy studies provide invaluable information about the pathogenesis of HIV brain infection; however, they have limitations. These include alterations in pathophysiological findings related to various specific causes of death (e.g., systemic infections such as pneumonia), delays in times to autopsy that can result in spurious results, trouble with correlating mild NCI with pathology specifically related to it versus agonal effects in the dying brain, and relatively low number of cases coming to autopsy with mild NCI, making statistical considerations more challenging. Neuroimaging has the advantage of showing brain pathology without the above considerations and in live patients. This will be briefly reviewed herein, because there are extensive published reviews available [23][24]. Imaging has been used to study a wide variety of important features of the effects of HIV on the brain, including brain volume and neuroinflammation [23]. Drawbacks to current neuroimaging methods include the questionable ability to examine markers of neuroinflammation and neuronal injury and particularly the inability to detect subtle changes in finer structures like synapses or dendritic spines, which are critical features of mild NCI [24]. Magnetic resonance imaging (MRI) has been used to detect atrophy in areas such as the frontal and temporal cortex, which reflects neuronal loss and injury, in PWH [25]. These MRI studies lend support to the idea that ART is not sufficient in preventing white matter injury for some PWH [18][26].
Magnetic resonance spectroscopy (MRS) can be used in conjunction with MRI to examine the biochemical composition of tissue [27]. MRS measures such as N-acetylaspartate (NAA), choline (Cho), GABA, glutamate/glutamine complex (glx), Cr (creatine), and myo-inositol (Mi) have been used to characterize neuronal integrity and neuronal mitochondrial function, inflammation, neuronal receptors, metabolism, and glial activation, respectively [27][28][29][30]. MRS revealed that approximately 48% of HIV infected patients (neuro-asymptomatic and severe NCI) presented significant increases in inflammation and glial activation in the frontal white matter (FWM), basal ganglia (BG), and mid-frontal cortex (MFC) [31]. A decrease in neuronal integrity was observed in the severe NCI HIV-infected group [31]. As reiterated here and elsewhere in this review, conventional ART alone is not sufficient to prevent cognitive impairment and irregularities in brain metabolite levels and in addition, the long-term reduction in Glx and NAA in specific regions are associated with NCI [32]. This restudyearch suggests that despite HIV suppression, neuronal injury can still occur as indicated by the reduction in Glx and NAA in cortical and/or subcortical regions.
Nevertheless, following ART, patients can exhibit a decrease in inflammation and glial activation in the FWM, parietal gray matter, and BG [33]. The study gives further support for early ART initiation in dampening neuroinflammation and inhibiting further neuronal injury [33]. Other studies using MRS underline the role of glial inflammation in cognitive dysfunction [34][35][36][37].
Other groups have used positron emission tomography (PET) technology to study potential markers of neuroinflammation in PWH [38][39][40][41][42]. TPSO PET studies in PWH suggest associations with specific cognitive domains and microglial activity in brain regions such as frontal cortex, hippocampus, anterior and posterior cingulate, and corpus callosum [39][40][43]. However, while TPSO is found to be highly elevated in microglia and astrocytes during neuronal injury, the biological specificity and relevance of TPSO to neuroinflammatory activity has been debated, underscoring that a multitude of measures may better provide utility towards clarity of information gained in the setting of PWH [42][44][45].
3. Cerebrospinal Findings
Aside from autopsy and neuroimaging studies, cerebrospinal fluid (CSF) investigations can provide insight into the pathogenesis of HIV brain infection. However, CSF at best only roughly reflects virological and immunological events occurring in brain parenchyma [46]. Interferon-alpha (IFNa) has been shown to be significantly elevated in PWH that have severe NCI [47][48][49]. Additionally, a cross-sectional study by Anderson and colleagues demonstrated a correlation between cognitive impairment and increased IFNa levels in PWH and mild NCI [50]. The same study also found a strong correlation between a rise in CSF neurofilament light chain protein and IFNa levels [50]. Williams and colleagues published a comparative review on CSF markers for HIV infection of the CNS [51]. Briefly, the authors screened 1943 studies and narrowed the final review to 20 cross-sectional studies and 9 longitudinal studies based on defined criteria. On closer examination, the authors found higher levels of CSF markers neopterin, sCD163, sCD14, IFN-γ, IL-1α, IL-7, IL-8, and sTNFR-II while lower levels of IL-6 are associated most consistently with NCI in PWH. The authors acknowledge the limitations of their comparative review due to: (1) age and sex-matching is not consistent across each study, (2) CNS/plasma discordance (higher viral load in CSF vs. plasma) not determined across all studies, (3) sampling bias mostly restricted to U.S. studies, (4) low number of participants leading to less statistical power, (5) most studies do not correlate CSF markers with disease severity/stage, and (6) confounding factors such as coinfections/comorbidities are not regularly reported in the 29 studies reviewed [51].
There is considerable evidence through CSF (see above) and autopsy studies (see below) that HIV persists within the brain when replication is suppressed in plasma by ART [52][53][54]. The importance of HIV persistence in the brain is predicated on two issues: (1) the potential for persistent brain HIV to re-seed the circulation and peripheral tissues, and (2) the capacity of persistent HIV in the CNS to affect the development and progression of HIV associated NCI. HIV persistence can be further defined as a tissue reservoir that allows for continual viral replication despite ART [55][56]. Criteria for a persistent viral reservoir can include: (1) confirmation of integrated proviral DNA in long-lived cells, and (2) continual presence of virus in a quiescent state in the reservoir that can be stimulated to generate replication competent virions [57][58]. Brain mononuclear phagocytes (MPs) such as perivascular macrophages can survive for months [59], while microglia can persist for years [60][61]. The number of brain MPs containing integrated HIV ranges widely depending on the neuroanatomic site of sampling, duration of HIV infection, ART selection and timing, and the presence or absence of NCI, which satisfies the first criteria above [62]. The presence of viral RNA in patient brains [63], and the detection of proviral DNA in brain MPs [62] suggests that the CNS represents another HIV reservoir that can support viral persistence in the era of ART, thus highlighting the need for adjunctive therapies.
4. Viral Persistence in CSerebrospinal Fluid and Viral Escape
The presence of inflammatory cytokines and other biomarkers in the CSF mentioned above points to the persistence of HIV in the CSF. CNS seeding of HIV, as detected by HIV RNA in the CSF, can occur as early as a week after primary infection [64]. HIV proviral DNA can be detected in the CSF of PWH despite early administration of ART [65]. Furthermore, HIV RNA can be detected in the CSF up to 10 years on ART when testing is performed with assays that quantitate virus to very low levels (single copy assays), reinforcing the idea that the CNS is a HIV reservoir [66]. HIV escape in the CSF can be defined as HIV viral load in the CNS/CSF compartment exceeding detectable HIV RNA in the plasma [67][68][69][70][71]. CSF viral escape can be accompanied by symptoms such as progression of memory and executive dysfunction, cerebellar ataxia, or headaches [72]. Several factors are thought to lead to CSF viral escape including drug resistance, noncompliance with ART regimen, or poor CNS-penetration effectiveness (described below) [72]. Aside from the detection of HIV RNA and DNA in the CSF, HIV viral proteins (e.g., Vpr, Tat, gp120) have also been detected in the CSF [73][74][75][76]. Vpr, Tat, gp120, and Nef are demonstrated to be neurotoxic in animal models or in vitro systems [77][78][79][80][81][82][83][84] (also see the Adjunctive Therapies in Animal Models and In Vitro Systems section below).
5. Treatment
Since the advent of combined ART in the mid 1990′s, the incidence of HAD has dramatically decreased, although mild NCI is common in PWH, who are now living long enough to develop increasing numbers of comorbidities (see above) that likely potentiate the adverse effects of HIV infection in the brain [85]. Notably, multiple studies have shown that ART is associated with improved cognition in PWH who do not have virologic control [86][87][88] (Table 1). However, a major concern is that PWH who have mild NCI despite ART will be more prone to progress to dementia because of comorbidities and the effects of aging [85]. Therefore, treatment regimens that work to further reduce (or ideally eliminate) brain viral load and moderate the CNS immune response are being developed (see Future Treatment Directions below). ART treatment of PWH with NCI has been influenced by the categorization of ART agents by their CNS penetration-effectiveness (CPE). ART drugs are ranked 1 (lowest) through 4 (highest) in terms of molecular properties (protein binding or molecular weight), efficacy based on CSF HIV suppression, and known CSF concentration of the specific ART agent [89][90]. The efficacy of ART may depend on the target cell type where some ART agents have been shown to have higher half maximal effective concentration (EC50) in microglia in vitro, the putative HIV reservoir in the brain, vs. T cells or macrophages [91].
Table 1. Summary of Selected Clinical Trials.
| Therapeutic | Anti-Inflammatory in CNS | AntiHIV in CNS | Other Effects | Randomized-Controlled? | Group n (Analyzed) and Summary Stats * | Outcome(s) |
|---|---|---|---|---|---|---|
| Antiretroviral | ||||||
| Maraviroc | No decrease in CSF neopterin and β2-microglobulin. Ref [92] |
NM | High EC50 in microglia in vitro. Ref [91] | ✓ | Maraviroc arm n = 9 Control arm n = 5 Time-treatment arm interaction: (p < 0.05) Effect size: (d = 0.77) and (d = 0.55) after 6 months or 12 months, respectively. |
Improved Cognition in PWH based on time-treatment arm interaction. Ref [92] |
| Cenicriviroc | NM | NM | N/A | Open label | Single arm (Cenicriviroc) n = 17 Cognitive domain of attention (p = 0.011) and working memory (p = 0.017). Decreased plasma levels of sCD163, sCD14 and neopterin (p < 0.01). |
Improved Cognition; decrease in myeloid activation markers. Ref [93] |
| Dolutegravir + Maraviroc | NM | NM | Increased CD4+ and CD8+ cell counts. | ✓ | Dual placebo n = 63 Dolutegravir and placebo n = 67 Dolutegravir and Maraviroc n = 60 cognitive testing (p > 0.10). |
No improvement in cognition in PWH + NCI. Ref [94] |
| Misc. drugs | ||||||
| Lithium | NM | NM | Lithium is well tolerated with ART. | ✓ | Placebo arm n = 31 Lithium arm n = 30 Summary Global Deficit Score-24 Weeks (p = 0.329) |
No improvement in cognition in PWH + NCI vs. placebo arm. Ref [95] |
| Selegiline transdermal system (STS) | NM | NM | Improvement in psychomotor speed in two prior pilot studies. See reference [96]. | ✓ | 3 mg/24 h STS (n = 42) 6 mg/24 h STS (n = 43) Placebo (n = 43) NPZ-8 score p = 0.35. NPZ total score p = 0.88. (Oxidative stress) CSF protein carbonyl concentration (p = 0.260) |
No improvement in cognition in STS vs placebo arm. No effect on oxidative stress. Ref [96][97] |
| Paroxetine, Fluconazole, or both | NM | NM | N/A | ✓ | Paroxetine + Fluconazole n = 11 Paroxetine + Placebo n = 11 Placebo + Fluconazole n = 9 Placebo + Placebo n = 10 Paroxetine arms improved summary NPZ-8 score (p = 0.023) |
Fluconazole did not have an observable additive effect with Paroxetine. Paroxetine in general improved cognition in PWH. Ref [98] |
| Atorvastatin | X | X | Reduction of blood lipids. | Open Label | Atorvastatin (no ART) single arm n = 7 viral load and CSF inflammatory markers (p > 0.05) Reduced cholesterol and LDL at 4–8 weeks (p < 0.01) or triglycerides at 4 weeks (p < 0.05). |
No effect on viral load or inflammatory markers in CSF. Ref [99] |
| Memantine | NM | NM | Significant increase in NAA/Cr in FWM (p= 0.040) and Parietal Cortex (p= 0.023) via MRS. | ✓ | Memantine arm n = 54 Placebo arm n = 56 NPZ-8 score p = 0.585 (week 16) |
No improvement in cognition in PWH. Ref [100] |
| Anti-inflammatory | ||||||
| Minocycline | NM | NM | Decrease in oxidative stress markers after 24 weeks. Ref [101] Decrease in various oxidative stress lipid markers (p ≤ 0.024). Ref [101] No effect on various markers of inflammation (p > 0.05) Ref [101]. |
✓ | Minocycline arm n = 26 Placebo arm n = 26 Cognitive analysis by UNP Sum criteria (p = 0.37) Ref [102]. Minocycline arm n = 8 Ref [101] Placebo arm n = 13 Ref [101] |
No improvement in cognition in PWH. Ref [102] |
Abbreviations: reference (Ref), central nervous system (CNS), people with HIV (PWH), neurocognitive impairment (NCI), antiretroviral therapy (ART), magnetic resonance spectroscopy (MRS), cerebrospinal fluid (CSF), N-acetyl aspartate to creatine ratio (NAA/Cr), frontal white matter (FWM), neuropsychological z score (NPZ), (NPZ-8) average z score of 8 neuropsychological tests, Uganda Neuropsychological Test Battery Summary Measure (UNP Sum). NM: not measured in referenced study or discussed in review. N/A: not applicable or no further comment. X = No. ✓ = Yes. * n’s reported for all studies only include patients who completed the clinical trial.
Asahchop and colleagues showed that the ART drug maraviroc, a CCR5 antagonist, is more effective at inhibiting HIV in mononuclear phagocytes (MPs) vs. T cells in vitro [91]. In a pilot study, the maraviroc treatment arm showed improved cognition in PWH [92] (Table 1). The CCR2 signaling pathway is also believed to be associated with cognitive dysfunction, as there is a correlation between CCR2+ monocytes and neurocognitive impairment in ART-untreated HIV-infected individuals [103]. An exploratory study on 17 virally suppressed HIV-infected individuals revealed that Cenicriviroc (CVC) (CCR2/CCR5 dual antagonist) also improved cognitive performance [93] (Table 1). Currently, it is unclear whether using ART with higher CPE scores can improve cognitive outcomes. Eighteen studies were reviewed regarding the relationship of CPE and cognitive performance. Eight studies concluded that a higher CPE score leads to better cognitive outcomes, three studies showed the opposite, while the remaining seven studies showed no relationship between CPE and improved cognition [104]. Few of these were controlled trials, but a small, randomized trial of 59 participants (some with virologic suppression at baseline, some without) did not show a cognitive benefit for CNS-targeted ART compared to non-CNS-targeted ART. More recently, a multi-national randomized controlled trial enrolled 191 people with HIV who had cognitive impairment and were taking suppressive ART [94] (Table 1). Participants were randomly assigned to intensify their existing ART regimen with a combination of (1) dolutegravir and maraviroc, (2) dolutegravir and placebo, or (3) dual placebo. Consistent with practice effect, cognitive performance improved in all three arms. However, the improvement in cognitive performance in the active treatment arms did not differ from that in the dual-placebo arm and thus did not support the conclusion that ART intensification benefits people with HIV who have cognitive impairment. Of note, the overall cognitive performance of the active treatment arms also did not decline, adding further evidence against the neurotoxicity of integrase inhibitors and maraviroc [94].
Unfortunately to date, many potential non-ART therapeutics tested in humans with HIV NCI have shown limited to no efficacy as well. In a 2016 clinical trial, lithium did not improve cognitive deficits or change MR spectroscopy results between treatment and placebo [95] (Table 1). Likewise, selegiline, another neuroprotectant with antioxidant properties, when given to PWH and NCI resulted in no significant cognitive improvement, and did not decrease oxidative stress biomarkers or result in beneficial changes in MRS parameters (e.g., a small rise in NAA/Cr in the BG and centrum semiovale or an increase Cho/Cr in the midfrontal cortex) [96][97] (Table 1). Sacktor and coworkers conducted a pilot randomized controlled trial to study monotherapy or combined therapy of a selective serotonin reuptake inhibitor, paroxetine, with an anti-fungal agent, fluconazole [98] (Table 1). This trial was based on results of paroxetine and fluconazole together having neuroprotective effects in vitro and protecting SIV-infected macaques from neuronal injury [105][106]. Paroxetine alone generally improved cognition in a battery of neuropsychological tests in PWH; however, its effects were not consistent over all cognitive domains tested and was not associated with consistent improvements in cellular stress markers and inflammation [98]. Fluconazole alone was associated with worsening in one of the neurocognitive tests and in CSF oxidative stress markers. The two treatments together were not associated with improvements in cognitive or laboratory tests compared to paroxetine alone. It is important to note that the authors go on to state in their Discussion that “Over the past 20 years, there have been over ten placebo-controlled trials of adjunctive agents to treat HIV-associated cognitive impairment, and none of them have shown improvement to enter clinical practice” [98].
Statins exhibit neuroprotective properties by reducing neuroinflammation in a rat model [107] and reducing monocyte chemotaxis in human peripheral blood monocyte culture [108]. Lipid metabolism dysregulation is associated with the pathogenesis of HAND [109]. The neuroprotective effect elicited by statins has potential for clinical treatment or prevention for HIV-1-associated neuropathogenicity. However, recent clinic studies indicated that statin use may not protect PWH from the development of NCI. Atorvastatin failed to significantly reduce neopterin (MP proinflammatory marker) and HIV RNA in CSF after high dose administration (80 mg) per day for 8 weeks (Table 1) [99]. Another clinical report with 1407 participants recruited in an AIDS cohort study, also did not indicate any benefit from statin treatment on cognitive performance [110].
Minocycline, an antibiotic with anti-inflammatory properties, was also hypothesized to have neuroprotective properties in HIV. However, a randomized controlled trial with minocycline failed to show a significant cognitive benefit in PWH with impairment despite effective ART [101][102]. Similarly, a randomized placebo-controlled trial testing the NMDAR antagonist memantine did not significantly ameliorate NCI in PWH [100]. However, memantine was associated with a significant increase in NAA/Cr (marker for neuronal integrity) ratios in the frontal white matter and parietal cortex, of mild NCI participants, by week 16 as measured by MR spectroscopy. Additionally, patients with lower baseline HIV RNA in the CSF exhibited even higher NAA/Cr in the frontal white matter compared to patients with higher baseline HIV RNA suggesting the importance of adjunctive use of ART [100].
In a randomized clinical trial, Marconi and coworkers showed that ruxolitinib (Rux), a JAK 1/2 inhibitor that is FDA approved for myelofibrosis and polycythema vera, is tolerated in PWH on ART [111] and decreased immune activation markers such as IL-18 and sCD14, which mechanistically relates to activated monocytes and have been implicated in trafficking to the CNS compartment [112][113][114]. Rux reduced CD4+/CD38+/HLA-DR+ cells that are associated with cell death and comorbid disease [111][115]. Rux can also reduce the number CD3+/CD4+/Bcl2+ cells, which are thought to maintain the latent viral reservoir [116]. Notably, Bcl-2 is a modulator of cell survival, and its upregulation may promote a further long-lived phenotype among cells harboring the viral reservoir. Downregulating this key cell survival marker, along with immune activation markers associated with disease progression, reservoir size, and systemic persistence including across the CNS, could provide a valuable therapeutic option to reverse immune dysfunction that prevents natural control of the latent reservoir, along with conferring a reduced lifespan of already-infected cells [117][118][119]. An initial concern was that combining an immunomodulator such as Rux may suppress the immune response resulting in viral recrudescence despite ART, although this was not found [111]. Recently, studies with a second generation JAK 1/2 inhibitor, baricitinib, which researcher's team has also demonstrated confers anti-HIV effects including reversal of cognitive deficits in murine model [120] (see Animal Models below), has been repurposed for COVID-19, garnering full FDA approval (first and only immunomodulator with this designation to date), a “strong recommendation from the World Health Organization; WHO), and first line treatment status worldwide. Studies with baricitinib have shown that baricitinib reverses immune dysfunction conferred by COVID-19, allowing the natural immune response to function without chronic inflammation from the virus, resulting in an increase in COVID-specific antibodies and concomitant restoration of immune activity [121][122][123][124]. These data underscore the delicate interplay between virally induced inflammation, functional immunity, and restoration of key immune functions in the presence of baricitinib in the viral infection setting. Future studies should explore whether immunomodulators such as baricitinib, which like Rux is a JAK 1/2 inhibitor but better tolerated, in combination with ART can improve performance in neuropsychological testing in patients with mild NCI (See Future Treatment Directions below).
6. Pathology
HIV encephalitis (HIVE) is typically defined as including HIV-infected cells within the brain, presence of multinucleated giant cells containing HIV antigens, which are overwhelmingly represented by MPs (i.e., macrophages and microglia), and also usually includes reactive or inflammatory glia (including MP and astrocytes) and neuronal abnormalities [125]. The neuronal abnormalities depend on the extent or severity of HIVE, but range from dendritic alterations to neuronal death [126][127][128]. Currently, virally suppressed patients with mild NCI do not exhibit apparent neuronal death, may or may not exhibit HIVE, and neuronal death and HIVE are not necessarily correlative factors for milder forms of NCI [125][129][130]. A study found that AIDS patients with progressive dementia exhibited no significant difference in neuronal loss when compared to HIV patients not diagnosed with dementia [131]. This suggests that more elusive mechanisms drive mild NCI in PWH. One possibility is that there is a smoldering infection accompanied by a persistent, low level of neuroinflammation, which drives synaptodendritic injury and scaling [132][133][134][135]. A neuronal synapse is composed of at least one axon (i.e., the longest process/branch of a neuron) projected onto a dendritic spine (i.e., bulbous protrusion on a shorter branch) of a neighboring neuron (Figure 1). The end of the axon is the presynaptic terminal while the dendritic spine is the postsynaptic terminal and both terminals are separated by a small space, the synaptic cleft. As an electrical signal travels down the axon (depolarization), this triggers the release of vesicles containing neurotransmitters (e.g., glutamate) at the presynaptic terminal, which can bind a cognate receptor (e.g., NMDA or AMPA) at the postsynaptic terminal. The strength of electrical conductance in a network of synapses, within the brain, is intrinsically linked to cognition, learning, memory, etc. which underlies everything discussed in this review [136].
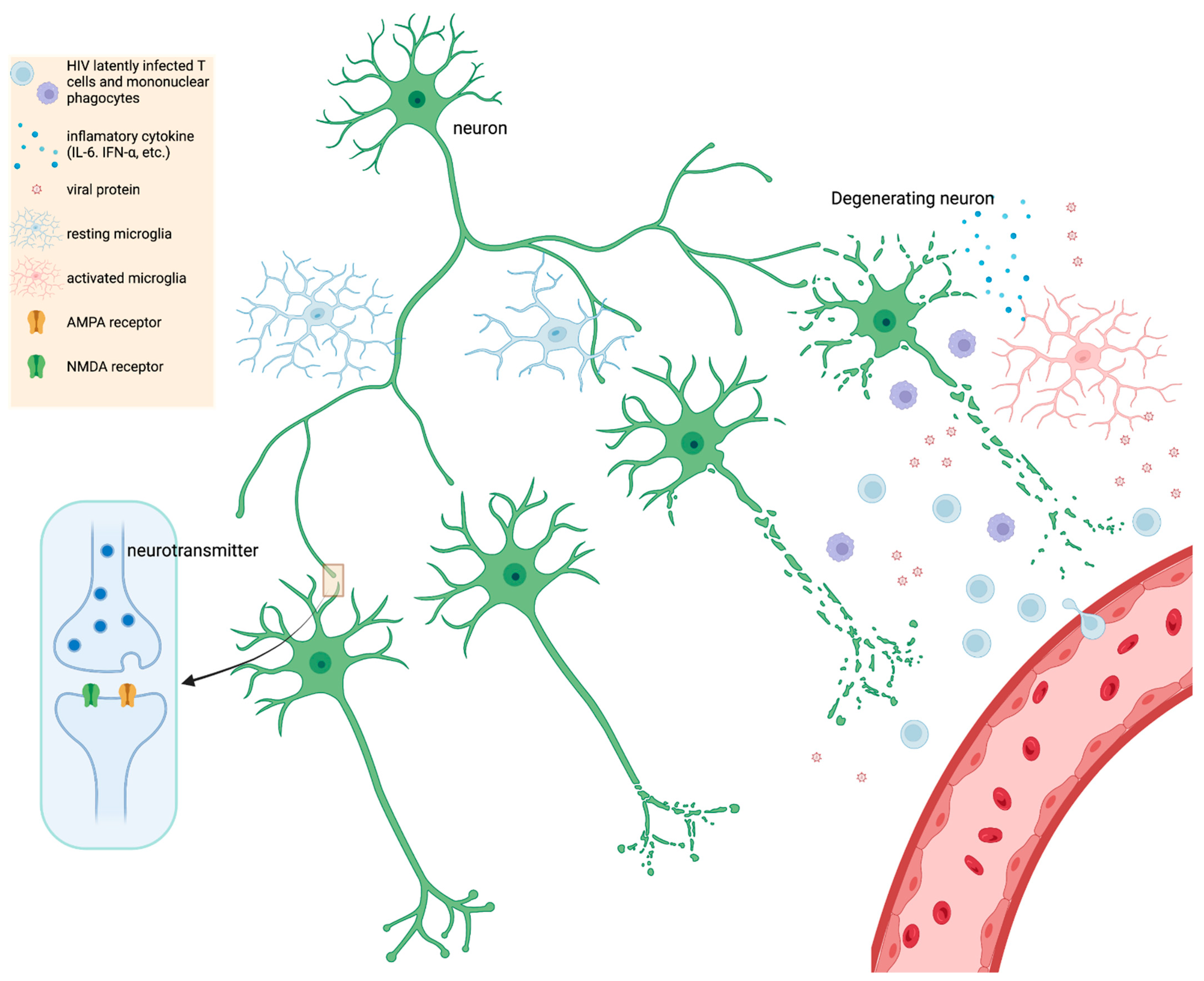
Figure 1. A simple schematic illustrating HIV infection within the CNS. HIV latently infects T cells and mononuclear phagocytes that migrate across the BBB; various stimuli trigger reactivation of viral latency in CNS. Upon activation, elevated levels of viral proteins (gp120, Nef, Tat) trigger resting microglia, which further leads to proinflammatory cytokine secretion, such as IL-6, IFN-α, etc. Chronic inflammation and HIV protein expression contribute to neurocognitive impairment (NCI) and injury of synapses and dendrites (inset diagram). Synaptodendritic injury may include less branching of dendrites, shortening of neurites (axons and dendrites), or downregulation of glutamate receptors such as NMDA or AMPA. In severe but rarer cases of HIV-induced inflammation and NCI, death of neurons can occur. Figure created in BioRender.
Synaptodendritic injury refers to dysfunction of synapses which may include reduction in receptors (e.g., NMDA or AMPA), decreased branching and length of dendrites, or disintegration of dendritic architecture such as loss of dendritic spine density or dysfunction of the neuronal cytoskeleton (Figure 1) [24][135][137]. Synaptic scaling is a specific type of reaction to synaptodendritic injury where synapses try to reestablish balance between inhibitory and excitatory synaptic transmission [135]. When neuronal calcium channels go awry and neurons are more susceptible to excitotoxicity, synapses usually scale down excitatory synapses (e.g., NMDA and upregulate inhibitory neurons e.g., GABA [135]. Synaptodendritic injury is often associated with HIV protein neurotoxicity (e.g., gp120) or from inflammatory cytokines and chemokines [24][138].
Notably, HIV proteins such as gp120, Tat, Vpr, and Nef can be detected in autopsy brains and have been associated with neurotoxicity [139][140][141][142][143]. In a recent report, Donoso and colleagues observed that residual production of HIV mRNA and proteins can still occur in latently infected cells (brain MPs and astrocytes) of brain autopsy samples despite long-term ART [144].
A more detailed review on the neurotoxicity of HIV proteins is beyond the scope of this review. An earlier autopsy study showed that synaptodendritic injury associated with NeuroHIV can correlate with the degree of cognitive impairment [145]. Similarly, Masliah et al. observed that dendritic injury in postmortem samples correlated with mild NCI [128]. The postmortem data suggests that disruption of synapses is probably linked to mild NCI in PWH.
Brain MPs are critical cell types driving NCI in PWH (Figure 1). In support of this hypothesis Gelman and colleagues examined autopsy samples from 140 infected human donors with mild NCI and the RNA and DNA pool present in brain tissue [63]. In this restudyearch, HIV RNA and DNA could be correlated with worse cognitive impairment if there was HIVE and microglial nodule encephalitis (MNE) in the brain (i.e., severe NCI). MNE is characterized as an encephalitis where activated microglial cells accumulate in nodules of varying sizes [125]. The authors concluded that reducing viral load through ART is more beneficial in improving cognitive function [63]. In contrast, in patients with mild NCI, without HIVE and MNE, the authors found minimal to no correlation between HIV RNA and DNA and neuropsychological test performance [63]. This suggests that viral replication alone is not the main driver of mild NCI in PWH.
In another postmortem study, tissue samples from PWH who had severe NCI (i.e., dementia), were usually found to have HIVE, which was defined as including HIV p24 immunopositivity and MP activation (Figure 1) [146]. The MP activity was intense as indicated by CD16, CD163, and MHCII staining in the brain parenchyma and perivascular regions [146]. Furthermore, HIVE included macrophage/microglial nodules showing morphology consistent with pronounced activation. In mild NCI postmortem brain autopsy samples typically there was an absence of HIV immunoreactivity, and MPs were less activated, although they were still more activated than control brain autopsy specimens [146]. This suggests that despite HIV suppression with ART, MP activity is an important component in mild NCI in PWH.
Ginsberg et al. further extended the findings by Tavazzi and colleagues by performing a microarray analysis on autopsy samples from HIVE (mostly severe NCI), PWH without HIVE (mostly mild NCI), and HIV-negative donors [147]. Briefly, the study isolated MPs from the brain parenchyma of the three groups and analyzed >500 transcripts related to inflammation, cellular stress, and apoptosis. Proinflammatory cytokines such as IL-6 and the primary TNFa receptor (Tnfrsf1a) were upregulated in HIVE and HIV+ without HIVE samples. Importantly, while the study outlines that the HIV+ mild NCI group upregulates macrophage/microglial transcripts to a lesser degree compared to the HIVE (i.e., severe NCI) group, it also further highlights the role that brain MPs play in HIV infection in the CNS as it relates to mild NCI [147].
References
- Antinori, A.; Arendt, G.; Becker, J.T.; Brew, B.J.; Byrd, D.A.; Cherner, M.; Clifford, D.B.; Cinque, P.; Epstein, L.G.; Goodkin, K.; et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology 2007, 69, 1789–1799.
- Rumbaugh, J.A.; Tyor, W. HIV-associated neurocognitive disorders: Five new things. Neurol. Clin. Pract. 2015, 5, 224–231.
- Guaraldi, G.; Orlando, G.; Zona, S.; Menozzi, M.; Carli, F.; Garlassi, E.; Berti, A.; Rossi, E.; Roverato, A.; Palella, F. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin. Infect. Dis. 2011, 53, 1120–1126.
- Lagathu, C.; Cossarizza, A.; Béréziat, V.; Nasi, M.; Capeau, J.; Pinti, M. Basic science and pathogenesis of ageing with HIV: Potential mechanisms and biomarkers. AIDS 2017, 31 (Suppl. 2), S105–S119.
- Pathai, S.; Bajillan, H.; Landay, A.L.; High, K.P. Is HIV a model of accelerated or accentuated aging? J. Gerontol. A Biol. Sci. Med. Sci 2014, 69, 833–842.
- Lin, K.; Taylor, M.J.; Heaton, R.; Franklin, D.; Jernigan, T.; Fennema-Notestine, C.; McCutchan, A.; Atkinson, J.H.; Ellis, R.J.; McArthur, J.; et al. Effects of traumatic brain injury on cognitive functioning and cerebral metabolites in HIV-infected individuals. J. Clin. Exp. Neuropsychol. 2011, 33, 326–334.
- Heaton, R.K.; Clifford, D.B.; Franklin, D.R., Jr.; Woods, S.P.; Ake, C.; Vaida, F.; Ellis, R.J.; Letendre, S.L.; Marcotte, T.D.; Atkinson, J.H.; et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 2010, 75, 2087–2096.
- Bayer, C.; Tyor, W.; Tan, A.; Zola, S.; Marconi, V.; Hu, W.; Penna, S. Estimating the Prevalence and Frequency of HIV-associated Neurocognitive Disorders (HAND) in a Veteran Population. In Proceedings of the 17th Conference of the American Academy of Clinical Neuropsychology, Chicago, IL, USA, 5–8 June 2019.
- Bayer, C.; Tyor, W.; Tan, A.; Zola, S.; Hu, W.; Bott, N.; Marconi, V.; Penna, S. Utility of the Neurotrack Visual Paired Comparison (VPC) Task as a Screening Tool for HIV-associated Neurocognitive Disorders (HAND). In Proceedings of the 17th Conference of the American Academy of Clinical Neuropsychology, Chicago, IL, USA, 5–8 June 2019.
- Nightingale, S.; Winston, A. Measuring and managing cognitive impairment in HIV. AIDS 2017, 31 (Suppl. 2), S165–S172.
- Robertson, K.R.; Su, Z.; Margolis, D.M.; Krambrink, A.; Havlir, D.V.; Evans, S.; Skiest, D.J. Neurocognitive effects of treatment interruption in stable HIV-positive patients in an observational cohort. Neurology 2010, 74, 1260–1266.
- Ciavatta, V.T.; Bichler, E.K.; Speigel, I.A.; Elder, C.C.; Teng, S.L.; Tyor, W.R.; García, P.S. In vitro and Ex vivo Neurotoxic Effects of Efavirenz are Greater than Those of Other Common Antiretrovirals. Neurochem. Res. 2017, 42, 3220–3232.
- Valcour, V.; Watters, M.R.; Williams, A.E.; Sacktor, N.; McMurtray, A.; Shikuma, C. Aging exacerbates extrapyramidal motor signs in the era of highly active antiretroviral therapy. J. Neurovirol. 2008, 14, 362–367.
- Kumar, A.M.; Fernandez, J.B.; Singer, E.J.; Commins, D.; Waldrop-Valverde, D.; Ownby, R.L.; Kumar, M. Human immunodeficiency virus type 1 in the central nervous system leads to decreased dopamine in different regions of postmortem human brains. J. Neurovirol. 2009, 15, 257–274.
- Fabbiani, M.; Ciccarelli, N.; Tana, M.; Farina, S.; Baldonero, E.; Di Cristo, V.; Colafigli, M.; Tamburrini, E.; Cauda, R.; Silveri, M.C.; et al. Cardiovascular risk factors and carotid intima-media thickness are associated with lower cognitive performance in HIV-infected patients. HIV Med. 2013, 14, 136–144.
- Wendelken, L.A.; Jahanshad, N.; Rosen, H.J.; Busovaca, E.; Allen, I.; Coppola, G.; Adams, C.; Rankin, K.P.; Milanini, B.; Clifford, K.; et al. ApoE ε4 Is Associated With Cognition, Brain Integrity, and Atrophy in HIV Over Age 60. J. Acquir. Immune Defic. Syndr. 2016, 73, 426–432.
- Valcour, V.; Shikuma, C.; Shiramizu, B.; Watters, M.; Poff, P.; Selnes, O.A.; Grove, J.; Liu, Y.; Abdul-Majid, K.B.; Gartner, S.; et al. Age, apolipoprotein E4, and the risk of HIV dementia: The Hawaii Aging with HIV Cohort. J. Neuroimmunol. 2004, 157, 197–202.
- Cysique, L.A.; Soares, J.R.; Geng, G.; Scarpetta, M.; Moffat, K.; Green, M.; Brew, B.J.; Henry, R.G.; Rae, C. White matter measures are near normal in controlled HIV infection except in those with cognitive impairment and longer HIV duration. J. Neurovirol. 2017, 23, 539–547.
- Qi, Y.; Li, R.L.; Wang, Y.Y.; Wang, W.; Liu, X.Z.; Liu, J.; Li, X.; Zhang, X.D.; Yu, W.; Liu, J.J.; et al. Characteristics of Brain White Matter Microstructure in HIV Male Patients With Primary Syphilis Co-Infection. Front. Neurol. 2021, 12, 776818.
- Marra, C.M.; Deutsch, R.; Collier, A.C.; Morgello, S.; Letendre, S.; Clifford, D.; Gelman, B.; McArthur, J.; McCutchan, J.A.; Simpson, D.M.; et al. Neurocognitive impairment in HIV-infected individuals with previous syphilis. Int. J. STD AIDS 2013, 24, 351–355.
- Monick, A.J.; Joyce, M.R.; Chugh, N.; Creighton, J.A.; Morgan, O.P.; Strain, E.C.; Marvel, C.L. Characterization of basal ganglia volume changes in the context of HIV and polysubstance use. Sci. Rep. 2022, 12, 4357.
- Smail, R.C.; Brew, B.J. HIV-associated neurocognitive disorder. Handb. Clin. Neurol. 2018, 152, 75–97.
- Chang, L.; Shukla, D.K. Imaging studies of the HIV-infected brain. Handb. Clin. Neurol. 2018, 152, 229–264.
- Irollo, E.; Luchetta, J.; Ho, C.; Nash, B.; Meucci, O. Mechanisms of neuronal dysfunction in HIV-associated neurocognitive disorders. Cell Mol. Life Sci. 2021, 78, 4283–4303.
- Hassanzadeh-Behbahani, S.; Shattuck, K.F.; Bronshteyn, M.; Dawson, M.; Diaz, M.; Kumar, P.; Moore, D.J.; Ellis, R.J.; Jiang, X. Low CD4 nadir linked to widespread cortical thinning in adults living with HIV. Neuroimage Clin. 2020, 25, 102155.
- van Zoest, R.A.; Underwood, J.; De Francesco, D.; Sabin, C.A.; Cole, J.H.; Wit, F.W.; Caan, M.W.A.; Kootstra, N.A.; Fuchs, D.; Zetterberg, H.; et al. Structural Brain Abnormalities in Successfully Treated HIV Infection: Associations With Disease and Cerebrospinal Fluid Biomarkers. J. Infect. Dis. 2017, 217, 69–81.
- Chaganti, J.; Brew, B.J. MR spectroscopy in HIV associated neurocognitive disorder in the era of cART: A review. AIDS Res. Ther. 2021, 18, 65.
- Fisher, S.K.; Novak, J.E.; Agranoff, B.W. Inositol and higher inositol phosphates in neural tissues: Homeostasis, metabolism and functional significance. J. Neurochem. 2002, 82, 736–754.
- Chang, L.; Ernst, T.; Poland, R.E.; Jenden, D.J. In vivo proton magnetic resonance spectroscopy of the normal aging human brain. Life Sci. 1996, 58, 2049–2056.
- Salvan, A.M.; Vion-Dury, J.; Confort-Gouny, S.; Nicoli, F.; Lamoureux, S.; Cozzone, P.J. Brain proton magnetic resonance spectroscopy in HIV-related encephalopathy: Identification of evolving metabolic patterns in relation to dementia and therapy. AIDS Res. Hum. Retrovir. 1997, 13, 1055–1066.
- Harezlak, J.; Buchthal, S.; Taylor, M.; Schifitto, G.; Zhong, J.; Daar, E.; Alger, J.; Singer, E.; Campbell, T.; Yiannoutsos, C.; et al. Persistence of HIV-associated cognitive impairment, inflammation, and neuronal injury in era of highly active antiretroviral treatment. AIDS 2011, 25, 625–633.
- Gongvatana, A.; Harezlak, J.; Buchthal, S.; Daar, E.; Schifitto, G.; Campbell, T.; Taylor, M.; Singer, E.; Algers, J.; Zhong, J.; et al. Progressive cerebral injury in the setting of chronic HIV infection and antiretroviral therapy. J. Neurovirol. 2013, 19, 209–218.
- Young, A.C.; Yiannoutsos, C.T.; Hegde, M.; Lee, E.; Peterson, J.; Walter, R.; Price, R.W.; Meyerhoff, D.J.; Spudich, S. Cerebral metabolite changes prior to and after antiretroviral therapy in primary HIV infection. Neurology 2014, 83, 1592–1600.
- Chang, L.; Ernst, T.; Witt, M.D.; Ames, N.; Gaiefsky, M.; Miller, E. Relationships among brain metabolites, cognitive function, and viral loads in antiretroviral-naïve HIV patients. Neuroimage 2002, 17, 1638–1648.
- Meyerhoff, D.J.; Bloomer, C.; Cardenas, V.; Norman, D.; Weiner, M.W.; Fein, G. Elevated subcortical choline metabolites in cognitively and clinically asymptomatic HIV+ patients. Neurology 1999, 52, 995–1003.
- Chang, L.; Ernst, T.; St Hillaire, C.; Conant, K. Antiretroviral treatment alters relationship between MCP-1 and neurometabolites in HIV patients. Antivir. Ther. 2004, 9, 431–440.
- López-Villegas, D.; Lenkinski, R.E.; Frank, I. Biochemical changes in the frontal lobe of HIV-infected individuals detected by magnetic resonance spectroscopy. Proc. Natl. Acad. Sci. USA 1997, 94, 9854–9859.
- Coughlin, J.M.; Wang, Y.; Ma, S.; Yue, C.; Kim, P.K.; Adams, A.V.; Roosa, H.V.; Gage, K.L.; Stathis, M.; Rais, R.; et al. Regional brain distribution of translocator protein using DPA-713 PET in individuals infected with HIV. J. Neurovirol. 2014, 20, 219–232.
- Vera, J.H.; Guo, Q.; Cole, J.H.; Boasso, A.; Greathead, L.; Kelleher, P.; Rabiner, E.A.; Kalk, N.; Bishop, C.; Gunn, R.N.; et al. Neuroinflammation in treated HIV-positive individuals: A TSPO PET study. Neurology 2016, 86, 1425–1432.
- Rubin, L.H.; Sacktor, N.; Creighton, J.; Du, Y.; Endres, C.J.; Pomper, M.G.; Coughlin, J.M. Microglial activation is inversely associated with cognition in individuals living with HIV on effective antiretroviral therapy. AIDS 2018, 32, 1661–1667.
- Kim, E.J.; Yu, S.W. Translocator protein 18 kDa (TSPO): Old dogma, new mice, new structure, and new questions for neuroprotection. Neural. Regen. Res. 2015, 10, 878–880.
- Lee, Y.; Park, Y.; Nam, H.; Lee, J.W.; Yu, S.W. Translocator protein (TSPO): The new story of the old protein in neuroinflammation. BMB Rep. 2020, 53, 20–27.
- Garvey, L.J.; Pavese, N.; Politis, M.; Ramlackhansingh, A.; Brooks, D.J.; Taylor-Robinson, S.D.; Winston, A. Increased microglia activation in neurologically asymptomatic HIV-infected patients receiving effective ART. AIDS 2014, 28, 67–72.
- Boerwinkle, A.; Ances, B.M. Molecular Imaging of Neuroinflammation in HIV. J. Neuroimmune Pharmacol. 2019, 14, 9–15.
- Boerwinkle, A.H.; Strain, J.F.; Burdo, T.; Doyle, J.; Christensen, J.; Su, Y.; Wisch, J.K.; Cooley, S.A.; Vaida, F.; Smith, M.D.; et al. Comparison of -PBR28 Binding Between Persons Living With HIV and HIV-Uninfected Individuals. J. Acquir. Immune Defic. Syndr. 2020, 85, 244–251.
- Moench, T.R.; Griffin, D.E. Immunocytochemical identification and quantitation of the mononuclear cells in the cerebrospinal fluid, meninges, and brain during acute viral meningoencephalitis. J. Exp. Med. 1984, 159, 77–88.
- Rho, M.B.; Wesselingh, S.; Glass, J.D.; McArthur, J.C.; Choi, S.; Griffin, J.; Tyor, W.R. A potential role for interferon-alpha in the pathogenesis of HIV-associated dementia. Brain Behav. Immun. 1995, 9, 366–377.
- Liu, H.; Zhou, R.H.; Liu, Y.; Guo, L.; Wang, X.; Hu, W.H.; Ho, W.Z. HIV infection suppresses TLR3 activation-mediated antiviral immunity in microglia and macrophages. Immunology 2020, 160, 269–279.
- Xu, L.; Xu, Y.; Zheng, Y.; Peng, X.; Yang, Z.; Cao, Q.; Xiang, D.; Zhao, H. Differences in cytokine and chemokine profiles in cerebrospinal fluid caused by the etiology of cryptococcal meningitis and tuberculous meningitis in HIV patients. Clin. Exp. Immunol. 2021, 206, 82–90.
- Anderson, A.M.; Lennox, J.L.; Mulligan, M.M.; Loring, D.W.; Zetterberg, H.; Blennow, K.; Kessing, C.; Koneru, R.; Easley, K.; Tyor, W.R. Cerebrospinal fluid interferon alpha levels correlate with neurocognitive impairment in ambulatory HIV-Infected individuals. J. Neurovirol. 2017, 23, 106–112.
- Williams, M.E.; Stein, D.J.; Joska, J.A.; Naudé, P.J.W. Cerebrospinal fluid immune markers and HIV-associated neurocognitive impairments: A systematic review. J. Neuroimmunol. 2021, 358, 577649.
- Alammar, L.; Gama, L.; Clements, J.E. Simian immunodeficiency virus infection in the brain and lung leads to differential type I IFN signaling during acute infection. J. Immunol. 2011, 186, 4008–4018.
- Hellmuth, J.; Valcour, V.; Spudich, S. CNS reservoirs for HIV: Implications for eradication. J. Virus Erad. 2015, 1, 67–71.
- Mallard, J.; Williams, K. Correction to: An SIV macaque model of SIV and HAND: The need for adjunctive therapies in HIV that target activated monocytes and macrophages. J. Neurovirol. 2018, 24, 664.
- Eisele, E.; Siliciano, R.F. Redefining the viral reservoirs that prevent HIV-1 eradication. Immunity 2012, 37, 377–388.
- Balcom, E.F.; Roda, W.C.; Cohen, E.A.; Li, M.Y.; Power, C. HIV-1 persistence in the central nervous system: Viral and host determinants during antiretroviral therapy. Curr. Opin. Virol. 2019, 38, 54–62.
- Blankson, J.N.; Persaud, D.; Siliciano, R.F. The challenge of viral reservoirs in HIV-1 infection. Annu. Rev. Med. 2002, 53, 557–593.
- Marban, C.; Forouzanfar, F.; Ait-Ammar, A.; Fahmi, F.; El Mekdad, H.; Daouad, F.; Rohr, O.; Schwartz, C. Targeting the Brain Reservoirs: Toward an HIV Cure. Front. Immunol. 2016, 7, 397.
- Koppensteiner, H.; Brack-Werner, R.; Schindler, M. Macrophages and their relevance in Human Immunodeficiency Virus Type I infection. Retrovirology 2012, 9, 82.
- Soulet, D.; Rivest, S. Bone-marrow-derived microglia: Myth or reality? Curr. Opin. Pharmacol. 2008, 8, 508–518.
- Réu, P.; Khosravi, A.; Bernard, S.; Mold, J.E.; Salehpour, M.; Alkass, K.; Perl, S.; Tisdale, J.; Possnert, G.; Druid, H.; et al. The Lifespan and Turnover of Microglia in the Human Brain. Cell Rep. 2017, 20, 779–784.
- Roda, W.C.; Li, M.Y.; Akinwumi, M.S.; Asahchop, E.L.; Gelman, B.B.; Witwer, K.W.; Power, C. Modeling brain lentiviral infections during antiretroviral therapy in AIDS. J. Neurovirol. 2017, 23, 577–586.
- Gelman, B.B.; Lisinicchia, J.G.; Morgello, S.; Masliah, E.; Commins, D.; Achim, C.L.; Fox, H.S.; Kolson, D.L.; Grant, I.; Singer, E.; et al. Neurovirological correlation with HIV-associated neurocognitive disorders and encephalitis in a HAART-era cohort. J. Acquir. Immune Defic. Syndr. 2013, 62, 487–495.
- Valcour, V.; Chalermchai, T.; Sailasuta, N.; Marovich, M.; Lerdlum, S.; Suttichom, D.; Suwanwela, N.C.; Jagodzinski, L.; Michael, N.; Spudich, S.; et al. Central nervous system viral invasion and inflammation during acute HIV infection. J. Infect. Dis. 2012, 206, 275–282.
- Oliveira, M.F.; Chaillon, A.; Nakazawa, M.; Vargas, M.; Letendre, S.L.; Strain, M.C.; Ellis, R.J.; Morris, S.; Little, S.J.; Smith, D.M.; et al. Early Antiretroviral Therapy Is Associated with Lower HIV DNA Molecular Diversity and Lower Inflammation in Cerebrospinal Fluid but Does Not Prevent the Establishment of Compartmentalized HIV DNA Populations. PLoS Pathog. 2017, 13, e1006112.
- Dahl, V.; Peterson, J.; Fuchs, D.; Gisslen, M.; Palmer, S.; Price, R.W. Low levels of HIV-1 RNA detected in the cerebrospinal fluid after up to 10 years of suppressive therapy are associated with local immune activation. AIDS 2014, 28, 2251–2258.
- Ferretti, F.; Gisslen, M.; Cinque, P.; Price, R.W. Cerebrospinal Fluid HIV Escape from Antiretroviral Therapy. Curr. HIV/AIDS Rep. 2015, 12, 280–288.
- Nightingale, S.; Geretti, A.M.; Beloukas, A.; Fisher, M.; Winston, A.; Else, L.; Nelson, M.; Taylor, S.; Ustianowski, A.; Ainsworth, J.; et al. Discordant CSF/plasma HIV-1 RNA in patients with unexplained low-level viraemia. J. Neurovirol. 2016, 22, 852–860.
- Canestri, A.; Lescure, F.X.; Jaureguiberry, S.; Moulignier, A.; Amiel, C.; Marcelin, A.G.; Peytavin, G.; Tubiana, R.; Pialoux, G.; Katlama, C. Discordance between cerebral spinal fluid and plasma HIV replication in patients with neurological symptoms who are receiving suppressive antiretroviral therapy. Clin. Infect. Dis. 2010, 50, 773–778.
- Peluso, M.J.; Ferretti, F.; Peterson, J.; Lee, E.; Fuchs, D.; Boschini, A.; Gisslén, M.; Angoff, N.; Price, R.W.; Cinque, P.; et al. Cerebrospinal fluid HIV escape associated with progressive neurologic dysfunction in patients on antiretroviral therapy with well controlled plasma viral load. AIDS 2012, 26, 1765–1774.
- Kugathasan, R.; Collier, D.A.; Haddow, L.J.; El Bouzidi, K.; Edwards, S.G.; Cartledge, J.D.; Miller, R.F.; Gupta, R.K. Diffuse White Matter Signal Abnormalities on Magnetic Resonance Imaging Are Associated With Human Immunodeficiency Virus Type 1 Viral Escape in the Central Nervous System Among Patients With Neurological Symptoms. Clin. Infect. Dis. 2017, 64, 1059–1065.
- Mastrangelo, A.; Turrini, F.; de Zan, V.; Caccia, R.; Gerevini, S.; Cinque, P. Symptomatic cerebrospinal fluid escape. AIDS 2019, 33 (Suppl. 2), S159–S169.
- Levy, D.N.; Refaeli, Y.; MacGregor, R.R.; Weiner, D.B. Serum Vpr regulates productive infection and latency of human immunodeficiency virus type 1. Proc. Natl. Acad. Sci. USA 1994, 91, 10873–10877.
- Mamik, M.K.; Hui, E.; Branton, W.G.; McKenzie, B.A.; Chisholm, J.; Cohen, E.A.; Power, C. HIV-1 Viral Protein R Activates NLRP3 Inflammasome in Microglia: Implications for HIV-1 Associated Neuroinflammation. J. Neuroimmune Pharmacol. 2017, 12, 233–248.
- Evering, T.H.; Kamau, E.; St Bernard, L.; Farmer, C.B.; Kong, X.P.; Markowitz, M. Single genome analysis reveals genetic characteristics of Neuroadaptation across HIV-1 envelope. Retrovirology 2014, 11, 65.
- Henderson, L.J.; Johnson, T.P.; Smith, B.R.; Reoma, L.B.; Santamaria, U.A.; Bachani, M.; Demarino, C.; Barclay, R.A.; Snow, J.; Sacktor, N.; et al. Presence of Tat and transactivation response element in spinal fluid despite antiretroviral therapy. AIDS 2019, 33 (Suppl. 2), S145–S157.
- Jones, G.J.; Barsby, N.L.; Cohen, E.A.; Holden, J.; Harris, K.; Dickie, P.; Jhamandas, J.; Power, C. HIV-1 Vpr causes neuronal apoptosis and in vivo neurodegeneration. J. Neurosci. 2007, 27, 3703–3711.
- Kitayama, H.; Miura, Y.; Ando, Y.; Hoshino, S.; Ishizaka, Y.; Koyanagi, Y. Human immunodeficiency virus type 1 Vpr inhibits axonal outgrowth through induction of mitochondrial dysfunction. J. Virol. 2008, 82, 2528–2542.
- Zou, W.; Kim, B.O.; Zhou, B.Y.; Liu, Y.; Messing, A.; He, J.J. Protection against human immunodeficiency virus type 1 Tat neurotoxicity by Ginkgo biloba extract EGb 761 involving glial fibrillary acidic protein. Am. J. Pathol. 2007, 171, 1923–1935.
- Kim, B.O.; Liu, Y.; Ruan, Y.; Xu, Z.C.; Schantz, L.; He, J.J. Neuropathologies in transgenic mice expressing human immunodeficiency virus type 1 Tat protein under the regulation of the astrocyte-specific glial fibrillary acidic protein promoter and doxycycline. Am. J. Pathol. 2003, 162, 1693–1707.
- Bachis, A.; Wenzel, E.; Boelk, A.; Becker, J.; Mocchetti, I. The neurotrophin receptor p75 mediates gp120-induced loss of synaptic spines in aging mice. Neurobiol. Aging 2016, 46, 160–168.
- Toggas, S.M.; Masliah, E.; Rockenstein, E.M.; Rall, G.F.; Abraham, C.R.; Mucke, L. Central nervous system damage produced by expression of the HIV-1 coat protein gp120 in transgenic mice. Nature 1994, 367, 188–193.
- Thaney, V.E.; O’Neill, A.M.; Hoefer, M.M.; Maung, R.; Sanchez, A.B.; Kaul, M. IFNβ Protects Neurons from Damage in a Murine Model of HIV-1 Associated Brain Injury. Sci. Rep. 2017, 7, 46514.
- Ditiatkovski, M.; Mukhamedova, N.; Dragoljevic, D.; Hoang, A.; Low, H.; Pushkarsky, T.; Fu, Y.; Carmichael, I.; Hill, A.F.; Murphy, A.J.; et al. Modification of lipid rafts by extracellular vesicles carrying HIV-1 protein Nef induces redistribution of amyloid precursor protein and Tau, causing neuronal dysfunction. J. Biol. Chem. 2020, 295, 13377–13392.
- Rosenthal, J.; Tyor, W. Aging, comorbidities, and the importance of finding biomarkers for HIV-associated neurocognitive disorders. J. Neurovirol. 2019, 25, 673–685.
- Anderson, A.M.; Jang, J.H.; Easley, K.A.; Fuchs, D.; Gisslen, M.; Zetterberg, H.; Blennow, K.; Ellis, R.J.; Franklin, D.; Heaton, R.K.; et al. Cognitive and Neuronal Link With Inflammation: A Longitudinal Study in People With and Without HIV Infection. J. Acquir. Immune Defic. Syndr. 2020, 85, 617–625.
- Robertson, K.R.; Robertson, W.T.; Ford, S.; Watson, D.; Fiscus, S.; Harp, A.G.; Hall, C.D. Highly active antiretroviral therapy improves neurocognitive functioning. J. Acquir. Immune Defic. Syndr. 2004, 36, 562–566.
- Schmitt, F.A.; Bigley, J.W.; McKinnis, R.; Logue, P.E.; Evans, R.W.; Drucker, J.L. Neuropsychological outcome of zidovudine (AZT) treatment of patients with AIDS and AIDS-related complex. N. Engl. J. Med. 1988, 319, 1573–1578.
- Letendre, S.; Marquie-Beck, J.; Capparelli, E.; Best, B.; Clifford, D.; Collier, A.C.; Gelman, B.B.; McArthur, J.C.; McCutchan, J.A.; Morgello, S.; et al. Validation of the CNS Penetration-Effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch. Neurol. 2008, 65, 65–70.
- Letendre, S.L.; Ellis, R.J.; Ances, B.M.; McCutchan, J.A. Neurologic complications of HIV disease and their treatment. Top HIV Med. 2010, 18, 45–55.
- Asahchop, E.L.; Meziane, O.; Mamik, M.K.; Chan, W.F.; Branton, W.G.; Resch, L.; Gill, M.J.; Haddad, E.; Guimond, J.V.; Wainberg, M.A.; et al. Reduced antiretroviral drug efficacy and concentration in HIV-infected microglia contributes to viral persistence in brain. Retrovirology 2017, 14, 47.
- Gates, T.M.; Cysique, L.A.; Siefried, K.J.; Chaganti, J.; Moffat, K.J.; Brew, B.J. Maraviroc-intensified combined antiretroviral therapy improves cognition in virally suppressed HIV-associated neurocognitive disorder. AIDS 2016, 30, 591–600.
- D’Antoni, M.L.; Paul, R.H.; Mitchell, B.I.; Kohorn, L.; Fischer, L.; Lefebvre, E.; Seyedkazemi, S.; Nakamoto, B.K.; Walker, M.; Kallianpur, K.J.; et al. Improved Cognitive Performance and Reduced Monocyte Activation in Virally Suppressed Chronic HIV After Dual CCR2 and CCR5 Antagonism. J. Acquir. Immune Defic. Syndr. 2018, 79, 108–116.
- Letendre, S.L.; Roa, J.; Chen, H.; McKhann, A.; Marra, C.M.; Daar, E.S.; Hunt, P.W.; Campbell, T.; Swaminathan, S.; Ha, B.; et al. ACTG A5324: A Randomized Trial of ART Intensification for Cognitive Impairment in PWH. In Proceedings of the Conference on Retroviruses and Opportunistic Infections, Virtual, 12–16 February 2022.
- Decloedt, E.H.; Freeman, C.; Howells, F.; Casson-Crook, M.; Lesosky, M.; Koutsilieri, E.; Lovestone, S.; Maartens, G.; Joska, J.A. Moderate to severe HIV-associated neurocognitive impairment: A randomized placebo-controlled trial of lithium. Medicine 2016, 95, e5401.
- Schifitto, G.; Yiannoutsos, C.T.; Ernst, T.; Navia, B.A.; Nath, A.; Sacktor, N.; Anderson, C.; Marra, C.M.; Clifford, D.B. Selegiline and oxidative stress in HIV-associated cognitive impairment. Neurology 2009, 73, 1975–1981.
- Schifitto, G.; Zhang, J.; Evans, S.R.; Sacktor, N.; Simpson, D.; Millar, L.L.; Hung, V.L.; Miller, E.N.; Smith, E.; Ellis, R.J.; et al. A multicenter trial of selegiline transdermal system for HIV-associated cognitive impairment. Neurology 2007, 69, 1314–1321.
- Sacktor, N.; Skolasky, R.L.; Moxley, R.; Wang, S.; Mielke, M.M.; Munro, C.; Steiner, J.; Nath, A.; Haughey, N.; McArthur, J. Paroxetine and fluconazole therapy for HIV-associated neurocognitive impairment: Results from a double-blind, placebo-controlled trial. J. Neurovirol. 2018, 24, 16–27.
- Probasco, J.C.; Spudich, S.S.; Critchfield, J.; Lee, E.; Lollo, N.; Deeks, S.G.; Price, R.W. Failure of atorvastatin to modulate CSF HIV-1 infection: Results of a pilot study. Neurology 2008, 71, 521–524.
- Schifitto, G.; Navia, B.A.; Yiannoutsos, C.T.; Marra, C.M.; Chang, L.; Ernst, T.; Jarvik, J.G.; Miller, E.N.; Singer, E.J.; Ellis, R.J.; et al. Memantine and HIV-associated cognitive impairment: A neuropsychological and proton magnetic resonance spectroscopy study. AIDS 2007, 21, 1877–1886.
- Sacktor, N.; Miyahara, S.; Evans, S.; Schifitto, G.; Cohen, B.; Haughey, N.; Drewes, J.L.; Graham, D.; Zink, M.C.; Anderson, C.; et al. Impact of minocycline on cerebrospinal fluid markers of oxidative stress, neuronal injury, and inflammation in HIV-seropositive individuals with cognitive impairment. J. Neurovirol. 2014, 20, 620–626.
- Nakasujja, N.; Miyahara, S.; Evans, S.; Lee, A.; Musisi, S.; Katabira, E.; Robertson, K.; Ronald, A.; Clifford, D.B.; Sacktor, N. Randomized trial of minocycline in the treatment of HIV-associated cognitive impairment. Neurology 2013, 80, 196–202.
- Ndhlovu, L.C.; D’Antoni, M.L.; Ananworanich, J.; Byron, M.M.; Chalermchai, T.; Sithinamsuwan, P.; Tipsuk, S.; Ho, E.; Slike, B.M.; Schuetz, A.; et al. Loss of CCR2 expressing non-classical monocytes are associated with cognitive impairment in antiretroviral therapy-naïve HIV-infected Thais. J. Neuroimmunol. 2015, 288, 25–33.
- Lin, S.P.; Calcagno, A.; Letendre, S.L.; Ma, Q. Clinical Treatment Options and Randomized Clinical Trials for Neurocognitive Complications of HIV Infection: Combination Antiretroviral Therapy, Central Nervous System Penetration Effectiveness, and Adjuvants. Curr. Top Behav. Neurosci. 2021, 50, 517–545.
- Steiner, J.P.; Bachani, M.; Wolfson-Stofko, B.; Lee, M.H.; Wang, T.; Li, G.; Li, W.; Strayer, D.; Haughey, N.J.; Nath, A. Interaction of paroxetine with mitochondrial proteins mediates neuroprotection. Neurotherapeutics 2015, 12, 200–216.
- Meulendyke, K.A.; Queen, S.E.; Engle, E.L.; Shirk, E.N.; Liu, J.; Steiner, J.P.; Nath, A.; Tarwater, P.M.; Graham, D.R.; Mankowski, J.L.; et al. Combination fluconazole/paroxetine treatment is neuroprotective despite ongoing neuroinflammation and viral replication in an SIV model of HIV neurological disease. J. Neurovirol. 2014, 20, 591–602.
- Ashraf, T.; Jiang, W.; Hoque, M.T.; Henderson, J.; Wu, C.; Bendayan, R. Role of anti-inflammatory compounds in human immunodeficiency virus-1 glycoprotein120-mediated brain inflammation. J. Neuroinflammation 2014, 11, 91.
- Yadav, A.; Betts, M.R.; Collman, R.G. Statin modulation of monocyte phenotype and function: Implications for HIV-1-associated neurocognitive disorders. J. Neurovirol. 2016, 22, 584–596.
- Bandaru, V.V.; Mielke, M.M.; Sacktor, N.; McArthur, J.C.; Grant, I.; Letendre, S.; Chang, L.; Wojna, V.; Pardo, C.; Calabresi, P.; et al. A lipid storage-like disorder contributes to cognitive decline in HIV-infected subjects. Neurology 2013, 81, 1492–1499.
- Saylor, D.; Molsberry, S.A.; Seaberg, E.C.; Cheng, Y.; Levine, A.; Martin, E.; Munro, C.; Palella, F.; Becker, J.; Sacktor, N. Statin Use and Cognitive Performance in the Multicenter Aids Cohort Study. In Proceedings of the Conference on Retroviruses and Opportunistic Infections, Virtual, 6–11 March 2021.
- Marconi, V.C.; Moser, C.; Gavegnano, C.; Deeks, S.G.; Lederman, M.M.; Overton, E.T.; Tsibris, A.; Hunt, P.W.; Kantor, A.; Sekaly, R.P.; et al. Randomized Trial of Ruxolitinib in Antiretroviral-Treated Adults With Human Immunodeficiency Virus. Clin. Infect. Dis. 2022, 74, 95–104.
- Bastard, J.P.; Soulié, C.; Fellahi, S.; Haïm-Boukobza, S.; Simon, A.; Katlama, C.; Calvez, V.; Marcelin, A.G.; Capeau, J. Circulating interleukin-6 levels correlate with residual HIV viraemia and markers of immune dysfunction in treatment-controlled HIV-infected patients. Antivir. Ther. 2012, 17, 915–919.
- Lichtfuss, G.F.; Cheng, W.J.; Farsakoglu, Y.; Paukovics, G.; Rajasuriar, R.; Velayudham, P.; Kramski, M.; Hearps, A.C.; Cameron, P.U.; Lewin, S.R.; et al. Virologically suppressed HIV patients show activation of NK cells and persistent innate immune activation. J. Immunol. 2012, 189, 1491–1499.
- Kamat, A.; Misra, V.; Cassol, E.; Ancuta, P.; Yan, Z.; Li, C.; Morgello, S.; Gabuzda, D. A plasma biomarker signature of immune activation in HIV patients on antiretroviral therapy. PLoS ONE 2012, 7, e30881.
- Lederman, M.M.; Funderburg, N.T.; Sekaly, R.P.; Klatt, N.R.; Hunt, P.W. Residual immune dysregulation syndrome in treated HIV infection. Adv. Immunol. 2013, 119, 51–83.
- Gavegnano, C.; Brehm, J.H.; Dupuy, F.P.; Talla, A.; Ribeiro, S.P.; Kulpa, D.A.; Cameron, C.; Santos, S.; Hurwitz, S.J.; Marconi, V.C.; et al. Novel mechanisms to inhibit HIV reservoir seeding using Jak inhibitors. PLoS Pathog. 2017, 13, e1006740.
- Vier, J.; Groth, M.; Sochalska, M.; Kirschnek, S. The anti-apoptotic Bcl-2 family protein A1/Bfl-1 regulates neutrophil survival and homeostasis and is controlled via PI3K and JAK/STAT signaling. Cell Death Dis. 2016, 7, e2103.
- Renault, T.T.; Chipuk, J.E. Getting away with murder: How does the BCL-2 family of proteins kill with immunity? Ann. N. Y. Acad. Sci. 2013, 1285, 59–79.
- Chetoui, N.; Boisvert, M.; Gendron, S.; Aoudjit, F. Interleukin-7 promotes the survival of human CD4+ effector/memory T cells by up-regulating Bcl-2 proteins and activating the JAK/STAT signalling pathway. Immunology 2010, 130, 418–426.
- Gavegnano, C.; Haile, W.B.; Hurwitz, S.; Tao, S.; Jiang, Y.; Schinazi, R.F.; Tyor, W.R. Baricitinib reverses HIV-associated neurocognitive disorders in a SCID mouse model and reservoir seeding in vitro. J. Neuroinflammation 2019, 16, 182.
- Marconi, V.C.; Ramanan, A.V.; de Bono, S.; Kartman, C.E.; Krishnan, V.; Liao, R.; Piruzeli, M.L.B.; Goldman, J.D.; Alatorre-Alexander, J.; de Cassia Pellegrini, R.; et al. Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): A randomised, double-blind, parallel-group, placebo-controlled phase 3 trial. Lancet Respir. Med. 2021, 9, 1407–1418.
- Titanji, B.K.; Farley, M.M.; Mehta, A.; Connor-Schuler, R.; Moanna, A.; Cribbs, S.K.; O’Shea, J.; DeSilva, K.; Chan, B.; Edwards, A.; et al. Use of Baricitinib in Patients With Moderate to Severe Coronavirus Disease 2019. Clin. Infect. Dis. 2021, 72, 1247–1250.
- Bronte, V.; Ugel, S.; Tinazzi, E.; Vella, A.; De Sanctis, F.; Canè, S.; Batani, V.; Trovato, R.; Fiore, A.; Petrova, V.; et al. Baricitinib restrains the immune dysregulation in patients with severe COVID-19. J. Clin. Investig. 2020, 130, 6409–6416.
- Kubo, S.; Nakayamada, S.; Sakata, K.; Kitanaga, Y.; Ma, X.; Lee, S.; Ishii, A.; Yamagata, K.; Nakano, K.; Tanaka, Y. Janus Kinase Inhibitor Baricitinib Modulates Human Innate and Adaptive Immune System. Front. Immunol. 2018, 9, 1510.
- Gelman, B.B. Neuropathology of HAND With Suppressive Antiretroviral Therapy: Encephalitis and Neurodegeneration Reconsidered. Curr. HIV/AIDS Rep. 2015, 12, 272–279.
- Kolson, D.L.; Sabnekar, P.; Baybis, M.; Crino, P.B. Gene expression in TUNEL-positive neurons in human immunodeficiency virus-infected brain. J. Neurovirol. 2004, 10 (Suppl. 1), 102–107.
- Masliah, E.; Ge, N.; Morey, M.; DeTeresa, R.; Terry, R.D.; Wiley, C.A. Cortical dendritic pathology in human immunodeficiency virus encephalitis. Lab Investig. 1992, 66, 285–291.
- Masliah, E.; Heaton, R.K.; Marcotte, T.D.; Ellis, R.J.; Wiley, C.A.; Mallory, M.; Achim, C.L.; McCutchan, J.A.; Nelson, J.A.; Atkinson, J.H.; et al. Dendritic injury is a pathological substrate for human immunodeficiency virus-related cognitive disorders. HNRC Group. The HIV Neurobehavioral Research Center. Ann. Neurol. 1997, 42, 963–972.
- Adle-Biassette, H.; Chretien, F.; Wingertsmann, L.; Hery, C.; Ereau, T.; Scaravilli, F.; Tardieu, M.; Gray, F. Neuronal apoptosis does not correlate with dementia in HIV infection but is related to microglial activation and axonal damage. Neuropathol. Appl. Neurobiol. 1999, 25, 123–133.
- Ellis, R.; Langford, D.; Masliah, E. HIV and antiretroviral therapy in the brain: Neuronal injury and repair. Nat. Rev. Neurosci. 2007, 8, 33–44.
- Weis, S.; Haug, H.; Budka, H. Neuronal damage in the cerebral cortex of AIDS brains: A morphometric study. Acta Neuropathol. 1993, 85, 185–189.
- Carroll, A.; Brew, B. HIV-associated neurocognitive disorders: Recent advances in pathogenesis, biomarkers, and treatment. F1000Res 2017, 6, 312.
- Manji, H.; Jäger, H.R.; Winston, A. HIV, dementia and antiretroviral drugs: 30 years of an epidemic. J. Neurol. Neurosurg. Psychiatry 2013, 84, 1126–1137.
- Spudich, S.; Gisslen, M.; Hagberg, L.; Lee, E.; Liegler, T.; Brew, B.; Fuchs, D.; Tambussi, G.; Cinque, P.; Hecht, F.M.; et al. Central nervous system immune activation characterizes primary human immunodeficiency virus 1 infection even in participants with minimal cerebrospinal fluid viral burden. J. Infect. Dis. 2011, 204, 753–760.
- Green, M.V.; Raybuck, J.D.; Zhang, X.; Wu, M.M.; Thayer, S.A. Scaling Synapses in the Presence of HIV. Neurochem. Res. 2019, 44, 234–246.
- Brown, J.C.; Higgins, E.S.; George, M.S. Synaptic Plasticity 101: The Story of the AMPA Receptor for the Brain Stimulation Practitioner. Neuromodulation 2021.
- Koneru, R.; Bimonte-Nelson, H.; Ciavatta, V.; Haile, W.; Elmore, K.; Ward, J.; Maroun, L.; Tyor, W.R. Reversing interferon-alpha neurotoxicity in a HIV-associated neurocognitive disorder mouse model. AIDS 2018, 32, 1403–1411.
- Cornell, J.; Salinas, S.; Huang, H.Y.; Zhou, M. Microglia regulation of synaptic plasticity and learning and memory. Neural. Regen. Res. 2022, 17, 705–716.
- Hudson, L.; Liu, J.; Nath, A.; Jones, M.; Raghavan, R.; Narayan, O.; Male, D.; Everall, I. Detection of the human immunodeficiency virus regulatory protein tat in CNS tissues. J. Neurovirol. 2000, 6, 145–155.
- Jones, M.V.; Bell, J.E.; Nath, A. Immunolocalization of HIV envelope gp120 in HIV encephalitis with dementia. AIDS 2000, 14, 2709–2713.
- Wheeler, E.D.; Achim, C.L.; Ayyavoo, V. Immunodetection of human immunodeficiency virus type 1 (HIV-1) Vpr in brain tissue of HIV-1 encephalitic patients. J. Neurovirol. 2006, 12, 200–210.
- Ranki, A.; Nyberg, M.; Ovod, V.; Haltia, M.; Elovaara, I.; Raininko, R.; Haapasalo, H.; Krohn, K. Abundant expression of HIV Nef and Rev proteins in brain astrocytes in vivo is associated with dementia. AIDS 1995, 9, 1001–1008.
- Pushkarsky, T.; Ward, A.; Ivanov, A.; Lin, X.; Sviridov, D.; Nekhai, S.; Bukrinsky, M.I. Abundance of Nef and p-Tau217 in Brains of Individuals Diagnosed with HIV-Associated Neurocognitive Disorders Correlate with Disease Severance. Mol. Neurobiol. 2022, 59, 1088–1097.
- Donoso, M.; D’Amico, D.; Valdebenito, S.; Hernandez, C.A.; Prideaux, B.; Eugenin, E.A. Identification, Quantification, and Characterization of HIV-1 Reservoirs in the Human Brain. Cells 2022, 11, 2379.
- Everall, I.P.; Heaton, R.K.; Marcotte, T.D.; Ellis, R.J.; McCutchan, J.A.; Atkinson, J.H.; Grant, I.; Mallory, M.; Masliah, E. Cortical synaptic density is reduced in mild to moderate human immunodeficiency virus neurocognitive disorder. HNRC Group. HIV Neurobehavioral Research Center. Brain Pathol. 1999, 9, 209–217.
- Tavazzi, E.; Morrison, D.; Sullivan, P.; Morgello, S.; Fischer, T. Brain inflammation is a common feature of HIV-infected patients without HIV encephalitis or productive brain infection. Curr. HIV Res. 2014, 12, 97–110.
- Ginsberg, S.D.; Alldred, M.J.; Gunnam, S.M.; Schiroli, C.; Lee, S.H.; Morgello, S.; Fischer, T. Expression profiling suggests microglial impairment in human immunodeficiency virus neuropathogenesis. Ann. Neurol. 2018, 83, 406–417.
More
