Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Beatrix Zheng and Version 1 by Csaba Hancz.
Aquaculture plays an important role in food production for the world population and at the same time for the livelihood of the most needed globally. The concerns about sustainability and ecological health are growing in this extremely diversified sector just like in the whole agriculture industry. The use of probiotics in aquaculture already has a long history and has served from the beginning the goals of more sustainable production; however, the expansion of intensive systems along with global climate change produces new challenges.
- probiotics
- aquaculture
- microbiome
- metagenomics
1. Introduction
Aquaculture is one of the world’s largest and most rapidly developing food production sectors; it is practiced with a wide variety of intensities and techniques, culturing—besides plants—a plethora of vertebrate and invertebrate species in the sea and freshwater. It contributes significantly to the livelihood and food of the ever-increasing world population [1,2,3][1][2][3]. The Blue Food Assessment, a collaboration involving more than 100 researchers, systematically assessed how aquatic food contributes to global food security, providing protein and other valuable nutrients for more than 3.2 billion people [4]. “Blue food” is a rich source of the omega-3 long-chain polyunsaturated fatty acids docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), as well as vitamins A and B12 [5].
Since the aquaculture industry has been gradually shifting to crop-based feed ingredients that fundamentally link aquaculture production to terrestrial agriculture, multidisciplinary research is needed to study the ecological and environmental health implications [6]. However, the intensification of aquaculture practices has increased the stress for both the aquatic animals and the environment [7,8][7][8]. Various chemicals and antibiotics have been applied [9,10,11][9][10][11] that cause serious problems and indirectly affect human health and even directly by producing antibiotic-resistant bacterium strains. It was long ago that the European Union stated in Regulation (EC) No. 1831/2003 that “Antibiotics, other than coccidiostats or histomonostats, shall not be authorized as feed additives”, which prompted researchers to seek for alternatives to reduce the abuse of antibiotics, not only in Europe, and one of the most promising was the group of probiotics. Ensuring animals’ good health and well-being remains a cornerstone in all aquaculture systems. To achieve this goal, probiotics offer a sustainable and eco-friendly alternative by replacing antibiotics and synthetic chemicals [12]. Probiotics’ beneficial role in aquaculture is diverse but perhaps the best-known effect of them is the amelioration of feed utilization of the host, easily measurable by the feed conversion ratio (FCR). Lowering the FCR is crucial from an economic point of view but is also important to decrease the load on the environment. Fry et al. [6] discuss the limitations of simply using FCR to evaluate the effectiveness of feed utilization concluding that using multiple measures to compare the efficiency of various types of food including nutrient and calorie retention is advisable. Environmental footprint measures including resource use, greenhouse gas emissions, biodiversity loss, and water pollution have also to be considered. Avadí et al. [13] compared the environmental performance of different types of feed use by applying the Life Cycle Assessment (LCA), which provided a more complex picture than the simple consideration of FCR. The consideration of the environmental footprint of given production technologies is also a growing concern in aquaculture [14]. The Blue Growth Strategy of the European Union is turning out to be a global movement [15] and is in accordance with the United Nations Sustainable Development Goals set in 2015 [16].
Probiotics are relatively simple to define correctly: “a product containing microbiota in a quantity capable of producing a putative beneficial effect” [16]. As will be discussed later, this effect is mainly achieved by influencing the microbiome of the intestinal tract both in human medicine and animal husbandry. A wider definition was provided by Verschuere et al. [17] suggesting that: “it is a microbial supplement with living microorganisms, with beneficial effects on the host, by modifying its microbial community associated with the host or its cultivation environment, by ensuring improved utilization of the artificial feed or its nutritional value, enhancing the host response toward diseases and by improving its vigor in general”. Probiotics are the functional feed additives group derived from different sources and include prebiotics, probiotics, seaweeds, mushrooms, microalgae, enzymes, organic acids, mycotoxin binders, phytobiotic compounds, and yeasts [18].
El-Saadony et al. [18] also collected several works from the literature defining probiotics. Felis et al. [19] provide an excellent summary of probiotics taxonomy that includes fungi. Ran et al. [20] found that both live and heat-inactivated baker’s yeast had beneficial effects on Nile tilapia while live yeast showed advantages as a dietary supplement. Abdalkareem et al. [21] confirmed the probiotic role of the unicellular fungi Aspergillus niger in common carp since it improved growth, immunity, digestion, and fish hematology.
Probiotics are mostly living microbial cells, although heat-inactivated versions have also been proven to have benefits for the host. These beneficial microbes play a pivotal role in regulating health conditions directly and by helping the immune system, growth performance, and feed utilization of animals. Their use in aquafeeds was the first area of intense development that continues until now. However, the application of probiotics offers effective new and sustainable ways of maintaining good water quality and even increasing the biomass of natural food organisms in different pond cultures [22] as will be discussed later. In aquaculture, the use of probiotics is similar to that of terrestrial animals, but there are also significant differences due to the different environments. The more direct link between aquatic animals and their environment has even led to a broader interpretation of the concept of probiotics, including the environment [17]. This indicates the 109 exposures to aquatic pathogenic microbes (Vibrio sp., Plesiomonas shigelloides, Aeromonas sp.), which cause most mortality in cultivated crustacean and fish species and are also important from a human perspective as they can cause food poisoning. Limbu et al. [23] analyzed the systemic effects of antibiotics in cultured fish and their potential human health risk. In the spirit of this more holistic approach, Infante-Villamil et al. [24] stress the importance of maintaining a high diversity level in the microbiome of aquaculture animals (vertebrate and invertebrate).
Probiotics used in aquaculture constitute a significant part of a huge market of global probiotics in animal feed. This market was valued at USD 4.4 billion in 2020 and is estimated to reach USD 7.3 billion by 2026. The demand is projected to remain high due to the increasing awareness of their benefits [25]. This sound background certainly fosters scientific research and product development, too.
Developments in microbiome science have certainly opened new frontiers of research for probiotics, prebiotics, and postbiotics not only in human healthcare but also in aquaculture. Novel products and applications may significantly change the profile of good practice in many fields and even portend a new era in sustainable development. It is a difficult task to provide an overall summary of the recently published literature on this field—an ever-growing mass of information—where even excellent reviews can be found in great numbers such as [12,18,24,26,27,28,29,30,31,32,33[12][18][24][26][27][28][29][30][31][32][33][34][35][36][37][38],34,35,36,37,38], not mentioning some earlier ones.
2. The Microbiome of Aquatic Animals and Its Functioning
2.1. Main Characteristics of the Aquatic Microbiome
Natural water bodies and aquatic organisms living in them are occupied by a galaxy of microorganisms. The interaction between the microbes of the environment and the vertebrate and invertebrate organisms is constant. The interactions with positive or negative effects among microbial species in the gut of the animals also play an important role that profoundly affects health and vitality. The exogenous microbes also enter the host organism through the skin and gills that harbor symbiotic, commensal, and pathogenic bacterial communities thereafter. Protective mucous covers the surfaces that, with its resident microbiome, can protect against deleterious microbes [39]. The persistent microbiome provides the host with both immunogenic and metabolic integrity and functionality [37].
The main site of host–microbe interactions, however, is the gastrointestinal tract (GIT) and its colonization and functioning have major importance [37,40][37][40]. The concept and role of the core microbiome resulting from the coevolution of host and microbiota are discussed by Wuertz et al. [37]. The GIT of fish is populated by a bacterial load of about 108 bacterial cells per gram, which represent approximately 500 mainly aerobic or facultative anaerobe microbes [41]. Ringø et al. [42], for example, summarize results obtained using lactic acid bacteria and bacilli (LAB) in the aquaculture of crustaceans and fish listing in 14 papers published only between 2017 and 2019 that discuss the beneficial effects of Lactobacillus and Enterococcus species. The oxygen content in the fish gut is higher than in the human gut, which can explain the low abundance of anaerobic bacteria. Bacteroidetes, Firmicutes, and Proteobacteria comprise the dominant proportion of the gut microbiota in most fish [1,43][1][43]. Herbivores’ guts have the most diversified microbiomes because the digestion of cellulose needs bacteria such as Clostridium, Leptotrichia, or Citrobacter [44].
2.2. Probiotics’ Role in Disease Control
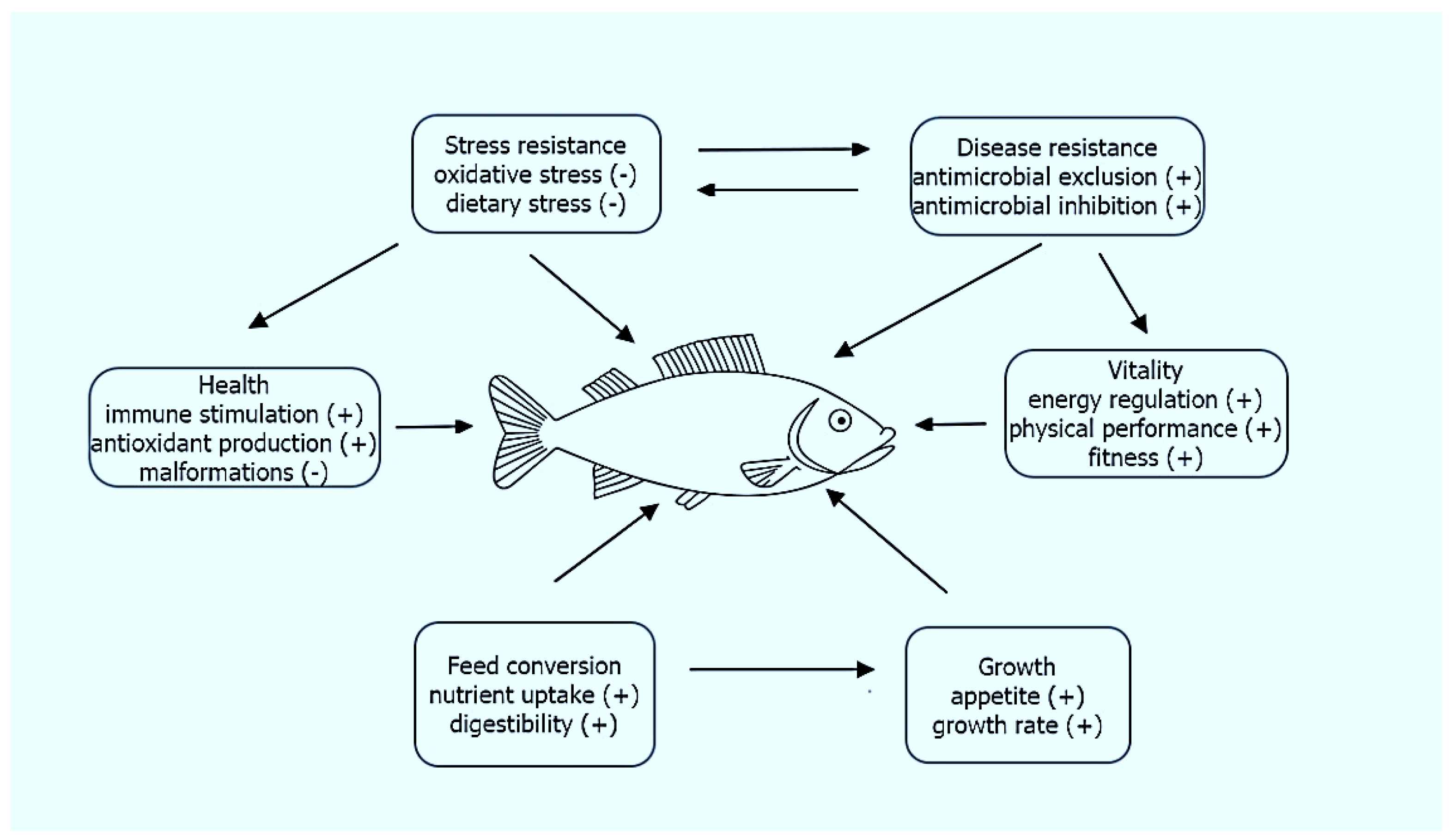
Probiotics are used extensively in aquaculture for disease control, notably against bacterial diseases [45]. Probiotics’ pathogen antagonism works in various ways. Probiotics can produce materials that prevent the reproduction of pathogens or kill them directly [46]. The most important postbiotics group is bacteriocins, which are proteinaceous or peptidic toxins produced by bacteria to inhibit the growth of similar or closely related bacterial strain(s). Although bacteriocins have been predominantly used as food preservatives, they are now receiving better attention as potential clinical antimicrobials and as potential immune-modulating agents [47,48,49][47][48][49]. Besides bacteriocins, the production of exoenzymes can also be an issue in the evaluation of potential probiotics [50]. Probiotics support the host organism’s immune system and/or inactivate toxins produced by pathogens [51,52][51][52]. Probiotics compete with pathogens for nutrients and adhesion sites [53].
Bacterial probiotics play a key role both in the immune responses of the host animal and in the interaction between these responses and intestinal bacterial communities [54]. Montalban-Arques et al. [55] extensively describe the potential microbial strategies that improve the gut mucosal immunity in fish. According to them, the therapeutic approach mechanisms include a competitive exclusion for binding sites and translocation, enhanced barrier function by reversing the increased intestinal permeability, enhanced mucosal immunoglobulin IgT/Z response to enteral antigens, reduction in secretion of inflammatory mediators, stimulation of innate immune functions, stimulation of the release of antimicrobial peptides (AMPs) at the mucosal layer, enhancement of the availability of anti-inflammatory mediators by regulatory immune cells, production of metabolic health-enhancers such as SCFAs by non-digestible prebiotics, and diffusion of SCFAs through the enterocytes to improve mucosa barrier functions.
2.3. Probiotics for Enhancing Feed Utilization
Probiotic supplementation enhances feed utilization and weight gain in aquatic animals and stimulates the host’s appetite and feed palatability by breaking down indigestible components, producing vitamins, and detoxifying poisonous compounds in the diet. Probiotics increase aquatic animals’ resistance to stress caused by environmental and technological hazards [12,29][12][29]. Wang et al. [56] mention another important application of beneficial bacteria, namely serving alternative aquaculture feeds that provide micronutrients such as vitamins, fatty acids, and essential amino acids in addition to macronutrients to support the healthy growth of aquatic animals.
2.4. Concept of Synbiotics
Prebiotics play an important role in the effective functioning of probiotics, so it is justified to use the common name “synbiotics”, which is frequently used in the medical and veterinary literature. Montalban-Arques et al. [55] explain the interactions during the preventive and curative probiotic treatments, stressing that adding a diet of exogenous microbial sources may increase fish health through a host–microbe positive loop. Commensal gut microbes might be modulated by dietary administration of target microbes, non-digestible elements, or a mix of both. The expected output should turn into preventive or curative strategies. The use of synbiotics is expected to restore the homeostatic stage, which is illustrated in Figure 1. The assessment of the selected approach might be quantified, modeled, or dissected using omics tools, germ-free models, and microbiome analyses.
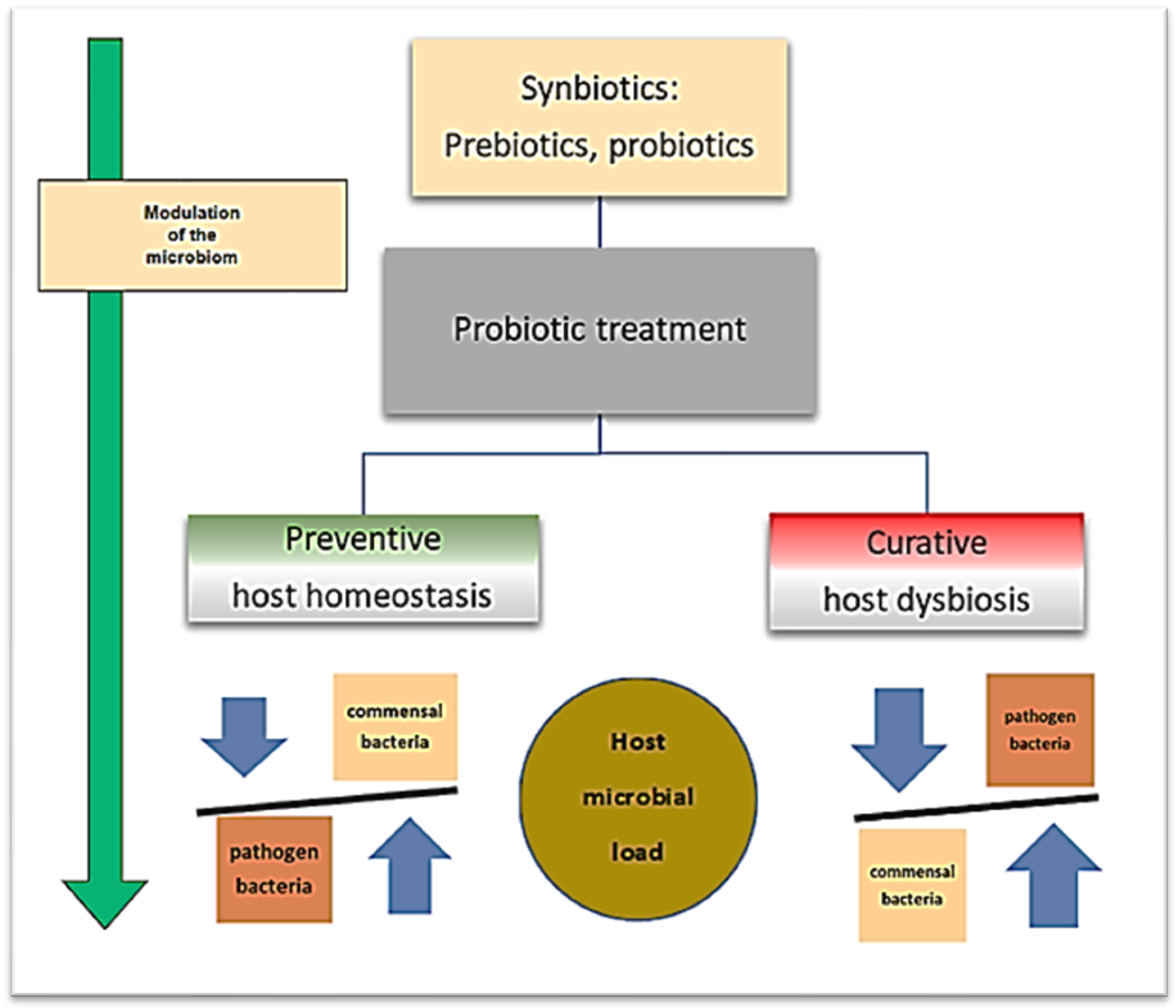
Figure 1.
Wuertz et al. [37] also profoundly discussed the probiotic action modes as illustrated and simplified below (Figure 2). Several reviews discuss in great detail all aspects involved in the beneficial effects of the probiotics mentioned above, providing lists of the microbe species responsible [27,32,37,41,57][27][32][37][41][57].
A recently published excellent review by Mougin and Joyce [38] provides an elucidating summary of disease etiology as a preliminary step toward the development of new prevention methods, underscoring the importance of the early identification of dysbiosis-associated biomarkers prior to any physical signs of the disease.
References
- Wang, A.R.; Ran, C.; Ringø, E.; Zhou, Z.G. Progress in fish gastrointestinal microbiota research. Rev. Aquac. 2018, 10, 626–664.
- FAO. The State of World Fisheries and Aquaculture 2020. In Sustainability in Action; FAO: Rome, Italy, 2020.
- Bartley, D.M. World Aquaculture 2020—A brief overview. In FAO Fisheries and Aquaculture Circular No. 1233; FAO: Rome, Italy, 2022.
- Anonymous. Harness the world’s aquatic ‘blue’ food systems to help end hunger. Nature 2021, 597, 303.
- Golden, C.D.; Koehn, J.Z.; Shepon, A.; Passarelli, S.; Free, C.M.; Viana, D.F.; Thilsted, S.H. Aquatic foods to nourish nations. Nature 2021, 598, 315–320.
- Fry, J.P.; Love, D.C.; MacDonald, G.K.; West, P.C.; Engstrom, P.M.; Nachman, K.E.; Lawrence, R.S. Environmental health impacts of feeding crops to farmed fish. Environ. Int. 2016, 91, 201–214.
- Dossou, S.; Koshio, S.; Ishikawa, M.; Yokoyama, S.; Dawood, M.A.O.; El Basuini, M.F.; Zaineldin Amr, I. Growth performance, blood health, antioxidant status and immune response in red sea bream (Pagrus major) fed Aspergillus oryzae fermented rapeseed meal (RM-Koji). Fish Shellfish Immunol. 2018, 75, 253–262.
- Dossou, S.; Koshio, S.; Ishikawa, M.; Yokoyama, S.; Dawood, M.A.O.; El Basuini, M.F.; El-Hais Abdelaziz Mohammed, A.; Adissin, O. Effect of partial replacement of fish meal by fermented rapeseed meal on growth, immune response and oxidative condition of red sea bream juvenile, Pagrus major. Aquaculture 2018, 490, 228–235.
- Carbone, D.; Faggio, C. Importance of prebiotics in aquaculture as immunostimulants. Effects on immune system of Sparus aurata and Dicentrarchus labrax. Fish Shellfish Immunol. 2016, 54, 172–178.
- Guardiola, F.A.; Porcino, C.; Cerezuela, R.; Cuesta, A.; Faggio, C.; Esteban, M.A. Impact of date palm fruits extracts and probiotic enriched diet on antioxidant status, innate immune response and immune-related gene expression of European seabass (Dicentrarchus labrax). Fish Shellfish Immunol. 2016, 52, 298–308.
- Aliko, V.; Qirjo, M.; Sula, E.; Morina, V.; Faggio, C. Antioxidant defense system, immune response and erythron profile modulation in goldfish, Carassius auratus, after acute manganese treatment. Fish Shellfish Immunol. 2018, 76, 101–109.
- Dawood, M.A.O.; Koshio, S.; Abdel-Daim, M.M.; Van Doan, H. Probiotic application for sustainable aquaculture. Rev. Aquac. 2019, 11, 907–924.
- Avadí, A.; Pelletier, N.; Aubin, J.; Ralite, S.; Núñez, J.; Fréon, P. Comparative environmental performance of artisanal and commercial feed use in Peruvian freshwater aquaculture. Aquaculture 2015, 435, 52–66.
- Burić, M.; Bavčević, L.; Grgurić, S.; Vresnik, F.; Križan, J.; Antonić, O. Modelling the environmental footprint of sea bream cage aquaculture in relation to spatial stocking design. J. Environ. Manag. 2020, 270, 110811.
- European Commission. Directorate-General for Maritime Affairs and Fisheries, The EU Blue Economy Report 2019; Publications Office: Luxembourg, 2019; Available online: https://data.europa.eu/doi/10.2771/21854 (accessed on 16 November 2022).
- FAO/WHO. Report of A Joint FAO/WHO Expert Consultation On the Evaluation of Health and Nutritional Properties of Probiotics in Food including Powder Milk with Live Lactic Acid Bacteria; FAO/WHO: Cordoba, Argentina, 2001; Available online: https://www.iqb.es/digestivo/pdfs/probioticos.pdf (accessed on 15 September 2022).
- Verschuere, L.; Rombaut, G.; Sorgeloos, P.; Verstraete, W. Probiotic bacteria as biological control agents in aquaculture. Microbiol. Mol. Biol. Rev. 2000, 64, 655–671.
- El-Saadony, M.T.; Alagawany, M.; Patra, A.K.; Kar, I.; Tiwari, R.; Dawood, M.A.O.; Dhama, K.; Abdel-Latif, H.M.R. The functionality of probiotics in aquaculture: An overview. Fish Shellfish Immunol. 2021, 117, 36–52.
- Felis, G.E.; Dellaglio, F.; Torriani, S. Taxonomy of Probiotic Microorganisms. In Prebiotics and Probiotics Science and Technology, Dimitris Charalampopoulos; Rastall, R.A., Ed.; Springer: Berlin/Heidelberg, Germany, 2009; Volume 15, pp. 591–639.
- Ran, C.; Huang, L.; Liu, Z.; Xu, L.; Yang, Y.; Tacon, P.; Auclair, E.; Zhou, Z. A Comparison of the Beneficial Effects of Live and Heat-Inactivated Baker’s Yeast on Nile Tilapia: Suggestions on the Role and Function of the Secretory Metabolites Released from the Yeast. PLoS ONE 2015, 10, e0145448.
- Abdalkareem, S.; Walid, J.; Abdelbasset, K.; Shichiyakh, R.A.; Al-Shawi, S.G.; Yasin, G.; Jalil, A.T.; Karim, Y.S.; Mustafa, Y.F.; Norbakhsh, M. Probiotic effects of the fungi, Aspergillus niger on growth, immunity, haematology, intestine fungal load and digestive enzymes of the common carp. Cyprinus Carpio. Aquac. Res. 2022, 53, 3828–3840.
- Elsayed, A.; Abada, A.; ElWakeli, A.S.K.; Mohamed, R.; Fadl, A.E.A. Effects of probiotic (Sanolife PRO-W) application on benthic meiofauna and Nile tilapia growth performance in earthen ponds. Aquac. Res. 2022, 53, 2708–2723.
- Limbu, S.M.; Chen, L.-Q.; Zhang, M.-L.; Du, Z.-Y. A global analysis on the systemic effects of antibiotics in cultured fish and their potential human health risk: A review. Rev. Aquac. 2021, 13, 1015–1059.
- Infante-Villamil, S.; Huerlimann, R.; Jerry, D.R. Microbiome diversity and dysbiosis in aquaculture. Rev. Aquac. 2021, 13, 1077–1096.
- Markets and Markets. Probiotic Markets. Available online: https://www.marketsandmarkets.com/Market-Reports/probiotic-market-advanced-technologies-and-global-market-69.html (accessed on 16 November 2022).
- Balcázar, J.L.; de Blas, I.; Ruiz-Zarzuela, I.; Cunningham, D.; Vendrell, D.; Múzquiz, J.L. The role of probiotics in aquaculture. Vet. Microbiol. 2006, 114, 173–186.
- Oliva-Teles, A. Nutrition and health of aquaculture fish. J. Fish. Dis. 2012, 35, 83–108.
- Zhou, X.; Wang, Y. Probiotics in Aquaculture - Benefits to the Health, Technological Applications and Safety. In Health and Environment in Aquaculture; Carvalho, E.D., David, G.S., Silva, R.J., Eds.; IntechOpen: London, UK, 2012.
- Mohapatra, S.; Chakraborty, T.; Kumar, V.; DeBoeck, G.; Mohanta, K.N. Aquaculture and stress management: A review of probiotic intervention. J. Anim. Physiol. Anim. Nutr. 2013, 97, 405–430.
- Pandiyan, P.; Balaraman, D.; Thirunavukkarasu, R.; George, E.G.J.; Subaramaniyan, K.; Manikkam, S.; Balamurugan Sadayappan, B. Probiotics in aquaculture. Drug Invent. Today 2013, 5, 55–59.
- Bidhan, C.D.E.; Meena, D.K.; Behera, B.K.; Das, P.; Das Mohapatra, P.K.; Sharma, A.P. Probiotics in fish and shellfish culture: Immunomodulatory and ecophysiological responses. Fish Physiol. Biochem. 2014, 40, 921–971.
- Pérez-Sánchez, T.; Ruiz-Zarzuela, I.; de Blas, I.; Balcázar, J.I. Probiotics in aquaculture: A current assessment. Rev. Aquac. 2014, 6, 133–146.
- Hai, N.V. The use of probiotics in aquaculture. J. Appl. Microbiol. 2015, 119, 917–935.
- Das, S.; Mondal, K.; Haque, S. A review on application of probiotic, prebiotic and synbiotic for sustainable development of aquaculture. J. Entomol. Zool. Stud. 2017, 5, 422–429.
- Chauhan, A.; Singh, R. Probiotics in aquaculture: A promising emerging alternative approach. Symbiosis 2019, 77, 99–113.
- Diwan, A.D.; Sanjay, N.; Harke, S.N.; Gopalkrishna Panache, A.N. Aquaculture industry prospective from gut microbiome of fish and shellfish: An overview. J. Anim. Physiol. Anim. Nutr. 2022, 106, 441–469.
- Wuertz, S.; Schroeder, A.; Wanka, K.M. Probiotics in Fish Nutrition—Long-Standing Household Remedy or Native Nutraceuticals? Water 2021, 13, 1348.
- Mougin, J. Joyce A Fish disease prevention via microbial dysbiosis-associated biomarkers in aquaculture. Rev. Aquac. 2022, 2022, 1–16.
- Kuebutornye, F.K.A.; Wang, Z.W.; Lu, Y.S.; Abarike, E.D.; Sakyi, M.E.; Li, Y.; Xie, C.X. Hlordzi Effects of three host-associated Bacillus species on mucosal immunity and gut health of Nile tilapia, Oreochromis niloticus and its resistance against Aeromonas hydrophila infection. Fish Shellfish Immunol. 2020, 97, 83–95.
- Li, X.; Ringø, E.; Hoseinifar, S.H.; Lauzon Hélene, L.; Birkbeck, H.; Yang, D. The adherence and colonization of microorganisms in the fish gastrointestinal tract. Rev. Aquac. 2019, 11, 603–618.
- Etyemez, M.; Balcázar, J.L. Bacterial community structure in the intestinal ecosystem of rainbow trout (Oncorhynchus mykiss) as revealed by pyrosequencing-based analysis of 16S rRNA genes. Res. Veter Sci. 2015, 100, 8–11.
- Ringø, E.; Song, S.K. Application of dietary supplements (synbiotics and probiotics in combination with plant products and b-glucans) in aquaculture. Aquac. Nutr. 2016, 22, 4–24.
- Dulski, T.; Kozłowski, K.; Ciesielski, S. Habitat and seasonality shape the structure of tench (Tinca tinca L.) gut microbiome. Sci. Rep. 2020, 10, 4460 .
- Liu, H.; Guo, X.; Gooneratne, S.R.; Lai, R.; Zeng, C.; Zhan, F.; Wang, W. The gut microbiome and degradation enzyme activity of wild freshwater fishes influenced by their trophic levels. Sci. Rep. 2016, 6, 24340.
- Newaj-Fyzul, A.; Al-Harbi, A.H.; Austin, B. Review: Developments in the use of probiotics for disease control in aquaculture. Aquaculture 2014, 431, 1–11.
- Servin, A. Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens. Microbiol. Rev. 2004, 28, 405–440.
- Sahoo, T.K.; Kumar, T.; Prasant Kumar, J.; Patel Kumar, A.; Sriram, S. Bacteriocins and their applications for the treatment of bacterial diseases in aquaculture: A review. Aquac. Res. Aquac. Res. 2016, 47, 1013–1027.
- Nayak, A.; Karunasagar, I.; Chakraborty, A.; Maiti, B. Potential application of bacteriocins for sustainable aquaculture. Rev. Aquac. 2022, 14, 1234–1248.
- Pereira, W.A.; Mendonça, C.M.N.; Urquiza, A.V.; Þór Marteinsson, V.; LeBlanc, J.G.; Cotter, P.D.; Figueroa Villalobos, E.; Romero, J.; Oliveira, R.P.S. Use of Probiotic Bacteria and Bacteriocins as an Alternative to Antibiotics in Aquaculture. Microorganisms 2022, 10, 1705.
- Mukherjee, A.; Dutta, D.; Banerjee, S.; Ringø, E.; Breines, E.M.; Hareide, E.; Chandra, G.; Ghosh, K. Potential probiotics from Indian major carp, Cirrhinus mrigala. Characterization, pathogen inhibitory activity, partial characterization of bacteriocin and production of exoenzymes. Res. Vet. Sci. 2016, 108, 76–84.
- Brandão, R.L.; Castro, I.M.; Bambirra, E.A.; Amaral, S.C.; Fietto, L.G.; Tropia, M.J.M.; Nicoli, J.R. Intracellular signal triggered by cholera toxin in Saccharomyces boulardii and Saccharomyces cerevisiae. Appl. Environ. Microbiol. 1998, 64, 564–568.
- Ezendam, J.; van Loveren, H. Probiotics: Immunomodulation and evaluation of safety and efficacy. Nutr. Rev. 2006, 64, 1–14.
- Servin, L.; Coconnier, M. Adhesion of probiotic strains to the intestinal mucosa and interaction with pathogens. Best Pract. Res. Clin. Gastroenterol. 2003, 17, 741–754.
- Dawood, M.A.O.; Koshio, S. Recent advances in the role of probiotics and prebiotics in carp aquaculture: A review. Aquaculture 2016, 454, 243–251.
- Montalban-Arques, A.; De Schryver, P.; Bossier, P.; Gorkiewicz, G.; Mulero, V.; Gatlin, D.M., III; Galindo-Villegas, J. Selective manipulation of the gut microbiota improves immune status in vertebrates. Front. Immunol. 2015, 6, 512.
- Wang, C.; Chuprom, J.; Wang, Y.; Fu, L. Beneficial bacteria for aquaculture: Nutrition, bacteriostasis and immunoregulation. J. Appl. Microbiol. 2020, 128, 28–40.
- Ringø, E.; Van Doan, H.; Lee, S.H.; Soltani, M.; Hoseinifar, S.H.; Harikrishnan, R.; Song, S.K. Probiotics, lactic acid bacteria, and bacilli: Interesting supplementation for aquaculture. J. Appl. Microbiol. 2020, 129, 116–136.
More