Skin injury is a common occurrence and mechanical forces are known to significantly impact the biological processes of skin regeneration and wound healing. Immediately following the disruption of the skin, the process of wound healing begins, bringing together numerous cell types to collaborate in several sequential phases. These cells produce a multitude of molecules and initiate multiple signaling pathways that are associated with skin disorders and abnormal wound healing, including hypertrophic scars, keloids, and chronic wounds. Studies have shown that mechanical forces can alter the microenvironment of a healing wound, causing changes in cellular function, motility, and signaling. A better understanding of the mechanobiology of cells in the skin is essential in the development of efficacious therapeutics to reduce skin disorders, normalize abnormal wound healing, and minimize scar formation.
- mechanotransduction
- fibrosis
- wound healing
1. Introduction
Human skin constantly deals with intrinsic and extrinsic forces throughout life. The effect of mechanical force is dependent upon the stiffness and biomechanical properties of the skin, which vary between anatomical locations [6][1].
2. Mechanical Force
Mechanical stimuli contribute to alterations in the wound healing process and underlie the increased susceptibility to excessive scar formation found in particular regions of the body [7,8][2][3]. Modern plastic surgery already employs several mechanomodulatory procedures in order to counter these effects, including z-plasty and the use of steristrips [9,10][4][5]. Both relax skin tension at the site of the wound, effectively reducing scar development, although there remains room for improvement [11–13][6][7][8]. As our understanding of mechanotransduction grows, we are focusing on “next generation” biomolecular approaches to further reduce scarring and fibrosis.
It is important to first grasp how mechanotransduction works at the cellular and tissue levels to better understand how the signaling pathways involved in wound healing and skin fibrosis are affected by mechanical force. As physical force is applied to the skin, mechanical signals are conveyed into chemical information by molecules that transmit this information to the cell (model of cellular tensegrity) [14–17][9][10][11][12]. The extracellular matrix (ECM) and the extracellular fluid (ECF) are essential to the transduction of mechanical forces into cells and control the constant remodeling of the skin. Transmembrane structures play an integral role in this process, as the membrane itself is fluid and requires specialized structures to transduce forces. Components of the cell membrane and cytoskeleton (e.g., actin and RhoA), ion channels, catenin complexes, cell adhesion molecules (e.g., focal adhesions and integrins), and several signaling pathways (e.g., Wnt, FAK-ERK, MAPK/ERK) are known to act as mechanosensors [18–20][13][14][15]. These sensors transmit mechanical signals to cells that bind to the extracellular matrix (ECM) and trigger a further signaling cascade of responses[16][17][18] [21–23] (Figure 1). For instance, the destruction of the ECM in aged skin leads to the further breakdown of fibroblasts within the dermis, as these cells thereby cease to receive mechanical information [24][19]. As a result, collagen production is decreased, while that of collagen-degrading enzymes, such as matrix metalloproteinases (MMPs), is increased [25,26][20][21].
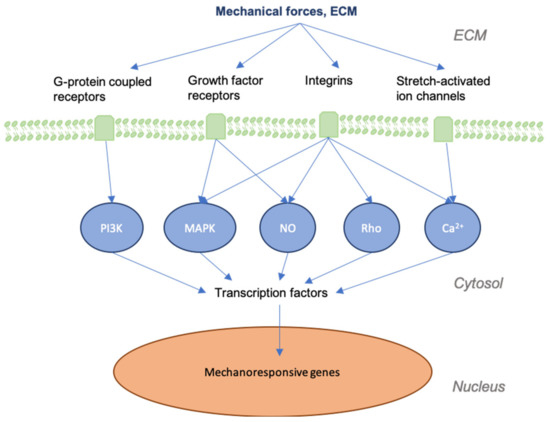
Figure 1. Graphic depicting mechanical forces applied to skin and the associated effects at cellular levels. As skin is stretched, the dermal matrix expands, opening ion channels, receptors, and other mechanotransductors, altering their accessibility to their respective ligands. This alters many of the signaling cascades that are involved in regeneration and the final state of the repaired wound.
Previous studies have demonstrated the significance of mechanical force in modulating hypertrophic scar (HTS) formation in a mechanically stress-induced murine scar model [27,28][22][23]. Sustained mechanical load applied to incisions resulted in fibrotic responses and HTS formation, demonstrating that mechanomodulation is linked to scarring through the stimulation of an inflammatory response [29][24]. Clinical evidence also supports the importance of mechanical stress on the development of scarring, as the use of a compression device on human patients postoperatively led to reductions in scar size through mechanical offloading in multiple human randomized clinical trials (RCTs) [30,31][25][26]. Specifically, a contracting elastomeric silicone dressing device has been shown to significantly improve scars through application of compressive forces to the incision by minimizing impact on the wound through the off-loading of tension [30,32][25][27]. Similarly, multiple RCTs of skin taping to improve scar appearance have shown clinical benefit in the appearance of scars [33–36][28][29][30][31].
References
- Victor W. Wong; Kemal Levi; Satoshi Akaishi; Geoffrey Schultz; Reinhold H. Dauskardt; Scar Zones. Plastic and Reconstructive Surgery 2012, 129, 1272-1276, 10.1097/prs.0b013e31824eca79.
- Bayat, A.; McGrouther, D.A.; Ferguson, M.W. Skin scarring. BMJ 2003, 326, 88–92.
- Barnes, L.A.; Marshall, C.D.; Leavitt, T.; Hu, M.S.; Moore, A.L.; Gonzalez, J.G.; Longaker, M.T.; Gurtner, G.C. Mechanical Forces in Cutaneous Wound Healing: Emerging Therapies to Minimize Scar Formation. Adv. Wound Care 2018, 7, 47–56.
- Sharma, M.; Wakure, A. Scar revision. Indian J. Plast Surg. 2013, 46, 408–418.
- Block, L.; Gosain, A.; King, T.W. Emerging Therapies for Scar Prevention. Adv. Wound Care (New Rochelle) 2015, 4, 607–614.
- Matsumoto, E.A.; Liang, H.; Mahadevan, L. Topology, Geometry, and Mechanics of Z-Plasty. Phys. Rev. Lett. 2018, 120, 068101.
- Kerrigan, C.L.; Homa, K. Evaluation of a new wound closure device for linear surgical incisions: 3M Steri-Strip S Surgical Skin Closure versus subcuticular closure. Plast Reconstr. Surg. 2010, 125, 186–194.
- Nast, A.; Eming, S.; Fluhr, J.; Fritz, K.; Gauglitz, G.; Hohenleutner, S.; Panizzon, R.G.; Sebastian, G.; Sporbeck, B.; Koller, J. German S2k guidelines for the therapy of pathological scars (hypertrophic scars and keloids). J. Dtsch. Dermatol. Ges. 2012, 10, 747–762
- Ingber, D.E. Control of capillary growth and differentiation by extracellular matrix. Use of a tensegrity (tensional integrity) mechanism for signal processing. Chest 1991, 99, 34–40.
- Ingber, D.E. Cellular mechanotransduction: Putting all the pieces together again. FASEB J. 2006, 20, 811–827.
- Ingber, D.E. Tensegrity I. Cell structure and hierarchical systems biology. J. Cell Sci. 2003, 116, 1157–1173.
- Ingber, D.E. Tensegrity II. How structural networks influence cellular information processing networks. J. Cell Sci. 2003, 116, 1397–1408.
- Knapik, D.M.; Perera, P.; Nam, J.; Blazek, A.D.; Rath, B.; Leblebicioglu, B.; Das, H.; ChuWu, L.; Hewett, T.E.; Agarwal, S.K.J.; et al. Mechanosignaling in bone health, trauma and inflammation. Antioxid. Redox Signal. 2014, 20, 970–985.
- Young, S.R.; Gerard-O’Riley, R.; Kim, J.B.; Pavalko, F.M. Focal adhesion kinase is important for fluid shear stress-induced mechanotransduction in osteoblasts. J. Bone Miner. Res. 2009, 24, 411–424.
- Marjoram, R.J.; Lessey, E.C.; Burridge, K. Regulation of RhoA activity by adhesion molecules and mechanotransduction. Curr. Mol. Med. 2014, 14, 199–208.
- Alenghat, F.J.; Ingber, D.E. Mechanotransduction: All signals point to cytoskeleton, matrix, and integrins. Sci. STKE 2002, 119, pe6.
- Sukharev, S.; Betanzos, M.; Chiang, C.S.; Guy, H.R. The gating mechanism of the large mechanosensitive channel MscL. Nature 2001, 409, 720–724.
- Geiger, B.; Spatz, J.P.; Bershadsky, A.D. Environmental sensing through focal adhesions. Nat. Rev. Mol. Cell Biol. 2009, 10, 21–33
- James Varani; Michael K. Dame; Laure Rittie; Suzanne E.G. Fligiel; Sewon Kang; Gary J. Fisher; John J. Voorhees; Decreased Collagen Production in Chronologically Aged Skin. The American Journal of Pathology 2006, 168, 1861-1868, 10.2353/ajpath.2006.051302.
- Ogawa, R.; Hsu, C.K. Mechanobiological dysregulation of the epidermis and dermis in skin disorders and in degeneration. J. Cell. Mol. Med. 2013, 17, 817–822.
- Fligiel, S.E.; Varani, J.; Datta, S.C.; Kang, S.; Fisher, G.J.; Voorhees, J.J. Collagen degradation in aged/photodamaged skin in vivo and after exposure to matrix metalloproteinase-1 in vitro. J. Investig. Dermatol. 2003, 120, 842–848.
- Wong, V.W.; Rustad, K.C.; Akaishi, S.; Sorkin, M.; Glotzbach, J.P.; Januszyk, M.; Nelson, E.R.; Levi, K.; Paterno, J.; Vial, I.N.; et al. Focal adhesion kinase links mechanical force to skin fibrosis via inflammatory signaling. Nat. Med. 2011, 18, 148–152.
- Aarabi, S.; Bhatt, K.A.; Shi, Y.; Paterno, J.; Chang, E.I.; Loh, S.A.; Holmes, J.W.; Longaker, M.T.; Yee, H.; Gurtner, G.C. Mechanical load initiates hypertrophic scar formation through decreased cellular apoptosis. FASEB J. 2007, 21, 3250–3261.
- Michael T. Longaker; Rod J. Rohrich; Lauren Greenberg; Heather Furnas; Robert Wald; Vivek Bansal; Hisham Seify; Anthony Tran; Jane Weston; Joshua M. Korman; et al.Rodney ChanDavid KaufmanVipul R. DevJoseph A. MeleMichael JanuszykChristy CowleyPeggy McLaughlinBill BeasleyGeoffrey C. Gurtner A Randomized Controlled Trial of the embrace Advanced Scar Therapy Device to Reduce Incisional Scar Formation. Plastic and Reconstructive Surgery 2014, 134, 536-546, 10.1097/prs.0000000000000417.
- Lim, A.F.; Weintraub, J.; Kaplan, E.N.; Januszyk, M.; Cowley, C.; McLaughlin, P.; Beasley, B.; Gurtner, G.C.; Longaker, M.T. The embrace device significantly decreases scarring following scar revision surgery in a randomized controlled trial. Plast Reconstr. Surg. 2014, 133, 398–405.
- Orgill, D.P.; Ogawa, R. Discussion: The embrace device significantly decreases scarring following scar revision surgery in a randomized controlled trial. Plast Reconstr. Surg. 2014, 133, 406–407.
- Geoffrey C. Gurtner; Reinhold H. Dauskardt; Victor W. Wong; Kirit A. Bhatt; Kenneth Wu; Ivan N. Vial; Karine Padois; Joshua M. Korman; Michael T. Longaker; Improving Cutaneous Scar Formation by Controlling the Mechanical Environment. Annals of Surgery 2011, 254, 217-225, 10.1097/sla.0b013e318220b159.
- Lionelli, G.T.; Lawrence, W.T. Wound dressings. Surg. Clin. N. Am. 2003, 83, 617–638.
- Reiffel, R.S. Prevention of hypertrophic scars by long-term paper tape application. Plast Reconstr. Surg. 1995, 96, 1715–1718.
- Rosengren, H.; Askew, D.A.; Heal, C.; Buettner, P.G.; Humphreys, W.O.; Semmens, L.A. Does taping torso scars following dermatologic surgery improve scar appearance? Derm. Pr. Concept. 2013, 3, 75–83.
- Atkinson, J.A.; McKenna, K.T.; Barnett, A.G.; McGrath, D.J.; Rudd, M. A randomized, controlled trial to determine the efficacy of paper tape in preventing hypertrophic scar formation in surgical incisions that traverse Langer’s skin tension lines. Plast Reconstr. Surg. 2005, 116, 1648–1656.
