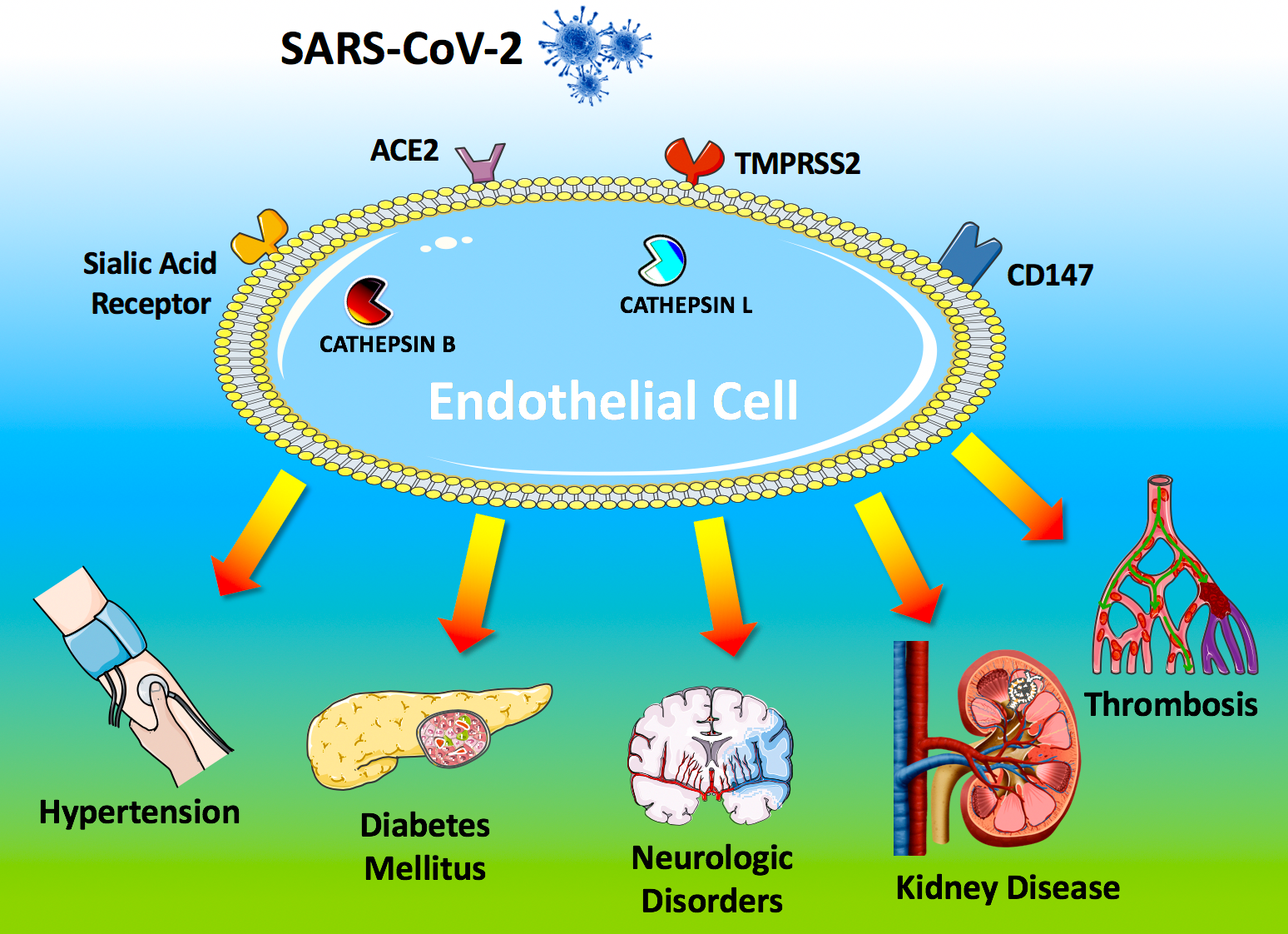
The systemic manifestations commonly observed in COVID-19 patients include hypertension, arterial and venous thromboembolism, kidney disease, cerebrovascular disorders, and diabetes mellitus). These clinical findings strongly suggest that the virus is targeting the endothelium. Here we report a systematic and comprehensive evaluation of the evidence showing that the endothelium is a key target organ in COVID-19, playing a fundamental role in its pathogenesis.
- COVID-19
- coronavirus
- SARS-CoV-2
- hypertension
- thrombosis
- Kawasaki
- PMIS
1. Introduction
To access host cells, SARS-CoV-2 uses a surface glycoprotein (peplomer) known as spike; ACE2 has been shown to be a co-receptor for coronavirus entry [1]. Therefore, the density of ACE2 in each tissue may correlate with the severity of the disease in that tissue. Other factors on the surface of human cells have been suggested to mediate the entry of SARS-CoV-2, including transmembrane serine protease 2 (TMPRSS2), sialic acid receptors, and extracellular matrix metalloproteinase inducer (CD147, also known as basigin). Additionally, cathepsin B and L have been shown to be critical entry factors in the pathogenesis of COVID-19.
Intriguingly, all of these factors involved in the entry of SARS-CoV-2 in the host cell have been shown [2] to be expressed by endothelial cells (Figure 1).
ACE2 is the most studied of these receptors: its genetic inactivation has been demonstrated to cause severe lung injury in H5N1-challenged mice, whereas administration of recombinant human ACE2 ameliorates H5N1 virus-induced lung injury in mice [3].
Figure 1. Endothelial dysfunction is a major determinant of COVID-19.
2. Systemic Manifestations
The SARS-CoV-2 coronavirus accesses host cells via the binding of its spike glycoprotein to angiotensin-converting enzyme 2 (ACE2), sialic acid receptor, transmembrane serine protease 2 (TMPRSS2), and extracellular matrix metalloproteinase inducer (CD147); cathepsin B and L also participate in virus entry. All of these factors are expressed in endothelial cells. Endothelial dysfunction is a common feature of the clinical manifestations commonly observed in COVID-19 patients. All of the drugs proposed as a potential therapeutic strategy to treat COVID-19 patients have been shown to improve endothelial function, including tocilizumab, colchicine, chloroquine/hydroxychloroquine, and azithromycin, and famotidine (see text for details and references).
ACE2 is currently at the center of a heated debate among physicians, and there are concerns that medical management of hypertension, including the use of inhibitors of the renin-angiotensin-aldosterone system (RAAS), may contribute to the adverse health outcomes observed [4]. TMPRSS2 binds the viral spike glycoprotein [5]; recent structural assays have suggested that coronaviruses can bind sialic acid receptors; CD147 has been shown to be essential for the entry of cytomegalovirus into endothelial cells; both cathepsin B and L are present in endothelial cells (Figure 1) [6].
The endothelium prevents blood clotting by providing an antithrombotic surface, maintained by heparan sulphate present in the matrix surrounding the cells, by the expression of tissue factor inhibitor, thrombomodulin, and by the production of tissue-type plasminogen activator that promotes fibrinolysis [7].
Endothelial dysfunction refers to a systemic condition in which the endothelium loses its physiological properties, including the tendency to promote vasodilation, fibrinolysis, and anti-aggregation [8]; moreover, endothelial dysfunction appears to be a consistent finding in patients with diabetes [9].
The cases of Kawasaki disease and pediatric multisystem inflammatory syndrome (PMIS) reported in young COVID-19 patients support our view of a systemic vasculitis caused by SARS-CoV-2 [10].
COVID-19 patients often exhibit clotting disorders, with organ dysfunction and coagulopathy, resulting in higher mortality [11]. Critical data came from the analysis of coagulation tests including prothrombin time (PT), activated partial thromboplastin time (APTT), antithrombin activity (AT), fibrinogen, fibrin degradation product (FDP), and D-dimer, in samples collected on admission and during the hospital stay of COVID-19 patients. Non-survivors had significantly higher D-dimer and FDP levels, and longer PT vs survivors on admission [12]. Moreover, significant reduction and lowering of fibrinogen and AT levels were observed in non-survivors during late stages of hospitalization, which is compatible with a clinical diagnosis of disseminated intravascular coagulation (DIC). Specifically, among 191 COVID-19 patients seen at two hospitals in Wuhan, D-dimer levels over 1 μg/L at admission predicted an 18-fold increase in odds of dying before discharge [13]. When DIC is caused by a systemic infection, it features an acute systemic over-inflammatory response, strictly linked to endothelial dysfunction [14]. Most recently a case of a COVID-19 patient with an increase of Factor VIII clotting activity and a massive elevation of von Willebrand Factor (vWF) has been reported [15], further supporting our theory: indeed, vWF can be seen as a marker of endothelial damage, since it is normally stored in Weibel-Palade bodies within endothelial cells. Equally important, angiotensin II levels in the plasma of COVID-19 patients were markedly elevated and linearly associated to viral load and lung injury [16].
References
- Hypertension, Thrombosis, Kidney Failure, and Diabetes: Is COVID-19 an Endothelial Disease? A Comprehensive Evaluation of Clinical and Basic Evidence . JCM. Retrieved 2020-5-20
- Allegra Battistoni; Massimo Volpe; Might renin–angiotensin system blockers play a role in the COVID-19 pandemic?. European Heart Journal - Cardiovascular Pharmacotherapy 2020, In press, -, 10.1093/ehjcvp/pvaa030.
- Celestino Sardu; Jessica Gambardella; Marco Morelli; Xujun Wang; Raffaele Marfella; Gaetano Santulli; Hypertension, Thrombosis, Kidney Failure, and Diabetes: Is COVID-19 an Endothelial Disease? A Comprehensive Evaluation of Clinical and Basic Evidence. Journal of Clinical Medicine 2020, 9, 1417, 10.3390/jcm9051417.
- Angiotensin-converting enzyme 2 protects from lethal avian influenza A H5N1 infections . https://www.nature.com/articles/ncomms4594. Retrieved 2020-5-20
- Shutoku Matsuyama; Naganori Nao; Kazuya Shirato; Miyuki Kawase; Shinji Saito; Ikuyo Takayama; Noriyo Nagata; Tsuyoshi Sekizuka; Hiroshi Katoh; Fumihiro Kato; et al.Masafumi SakataMaino TaharaSatoshi KutsunaNorio OhmagariMakoto KurodaTadaki SuzukiTsutomu KageyamaMakoto Takeda Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells. Proceedings of the National Academy of Sciences 2020, 117, 7001-7003, 10.1073/pnas.2002589117.
- Sardu, C. et al; Is COVID-19 an endothelial disease? Clinical and basic evidence. Preprint 2020, -, -, 10.13140/RG.2.2.17295.74407.
- Santulli et al.; Is COVID-19 an Endothelial Disease? Clinical and Basic Evidence. Preprints 2020, 10.20944/preprints202004.0204.v1, -, 10.20944/preprints202004.0204.v1.
- Gaetano Santulli; MicroRNAs and Endothelial (Dys) Function. Journal of Cellular Physiology 2015, 231, 1638-44, 10.1002/jcp.25276.
- Zhenqi Liu; The vascular endothelium in diabetes and its potential as a therapeutic target. Reviews in Endocrine and Metabolic Disorders 2013, 14, 1-3, 10.1007/s11154-013-9238-8.
- Shelley Riphagen; Xabier Gomez; Carmen Gonzalez-Martinez; Nick Wilkinson; Paraskevi Theocharis; Hyperinflammatory shock in children during COVID-19 pandemic.. The Lancet 2020, In press, -, 10.1016/s0140-6736(20)31094-1.
- Yan Zhang; Meng Xiao; Shulan Zhang; Peng Xia; Wei Cao; Wei Jiang; Huan Chen; Xin Ding; Hua Zhao; Hongmin Zhang; et al.Chunyao WangJing ZhaoXuefeng SunRan TianWei WuDong WuJie MaYu ChenDong ZhangJing XieXiaowei YanXiang ZhouZhengyin LiuJinglan WangBin DuYan QinPeng GaoXuzhen QinYingchun XuWen ZhangTaisheng LiFengchun ZhangYongqiang ZhaoYongzhe LiShuyang Zhang Coagulopathy and Antiphospholipid Antibodies in Patients with Covid-19. New England Journal of Medicine 2020, 382, e38, 10.1056/nejmc2007575.
- Deepa Rj Arachchillage; Mike Laffan; Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. Journal of Thrombosis and Haemostasis 2020, 18, 1233-1234, 10.1111/jth.14820.
- Fei Zhou; Ting Yu; Ronghui Du; Guohui Fan; Ying Liu; Zhibo Liu; Jie Xiang; Yeming Wang; Bin Song; Xiaoying Gu; et al.Lulu GuanYuan WeiHui LiXudong WuJiuyang XuShengJin TuYi ZhangHua ChenBin Cao Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The Lancet 2020, 395, 1054-1062, 10.1016/s0140-6736(20)30566-3.
- Santulli, G et al.; Is Endothelial Dysfunction the Concealed Cornerstone of COVID-19?. BMJ 2020, ., In press, ..
- Robert Escher; Neal Breakey; Bernhard Lämmle; Severe COVID-19 infection associated with endothelial activation.. Thrombosis Research 2020, 190, 62, 10.1016/j.thromres.2020.04.014.
- Yuhao Qin; Jun Xu; Zheng Zhang; Lifei Wang; Jinxiu Li; Chengyu Jiang; Zhaoqin Wang; Li Chen; Yingxia Liu; Lu Yin; et al.Shuguang TanFengming HuangLing PengJing YuanDandan ZhaoYang YangCheng FengCong ZhangCongzhao ZhouJianming LiFuxiang WangLei Liu Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. SCIENTIA SINICA Vitae 2020, 50, 258-269, 10.1360/ssv-2020-0037.