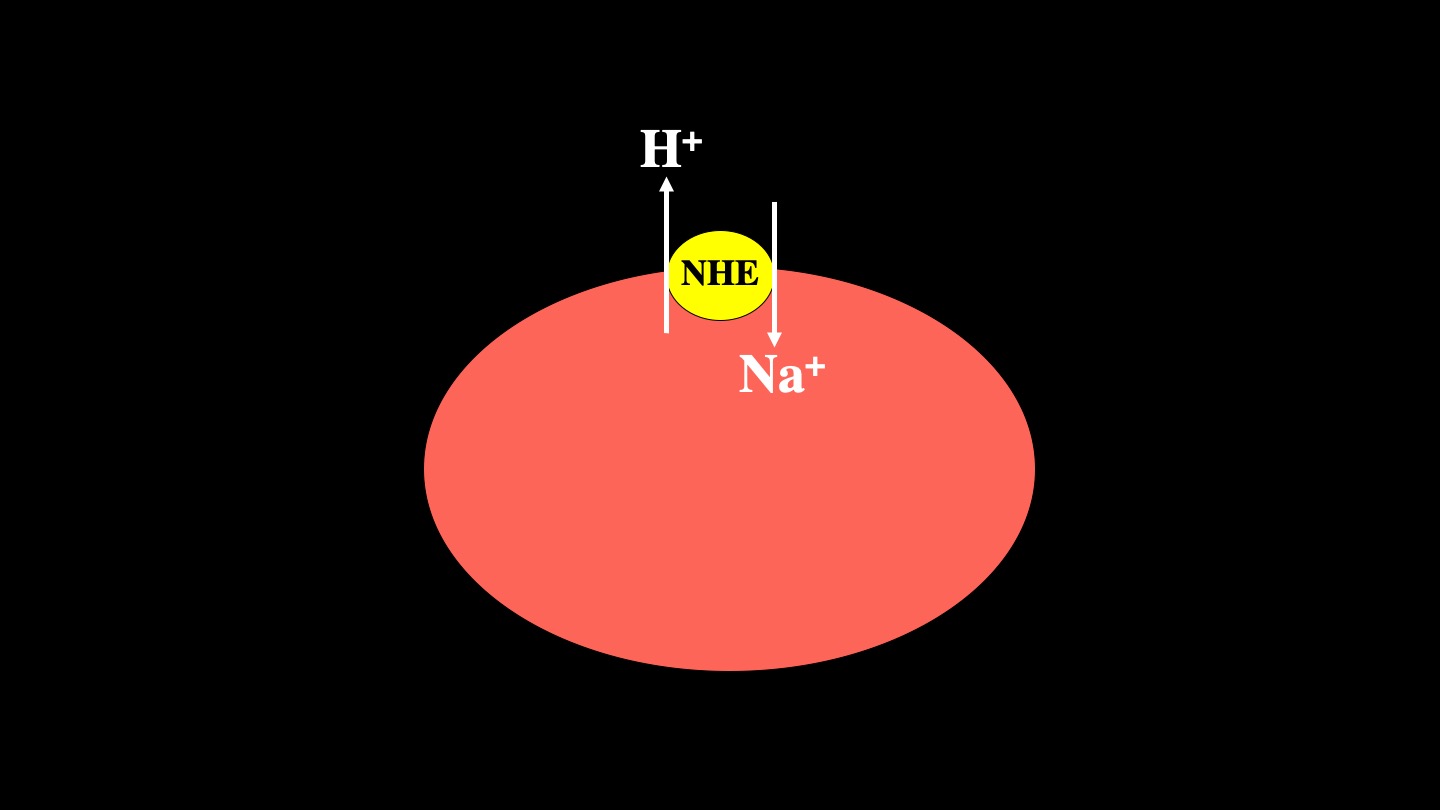
The mammalian Na+/H+ exchanger (NHE1, isoform 1) is a ubiquitously expressed membrane protein of mammalian cells. It removes one intracellular proton in exchange for a single extracellular sodium ion. NHE1 has several important physiological functions. It maintains intracellular pH (pHi) while protecting cells from acidification as a result of metabolism. It also regulates cell volume in response to osmotic challenge. There are ten isoforms of NHE that are different gene products. In human pathology, NHE1 is critical in several diseases, including myocardial heart hypertrophy and ischemia reperfusion damage . NHE1 is also important in breast cancer, where it acts as a trigger for metastasis.
- Na+/H+ exchanger
- membrane protein
- protein degradation
- heart disease
- breast cancer
- intracellular pH
1. Introduction
Mammalian Na+/H+ exchangers are a family of membrane proteins. There are up to 10 isoforms of this family of proteins that are different gene products. Isoform 1, mammalian Na+/H+ exchanger (NHE1, isoform 1) is a ubiquitously expressed in the plasma membrane of mammalian cells. It removes one intracellular proton in exchange for a single extracellular sodium ion (1)[1] (Figure 1).
2. NHE1's Physiological Functions
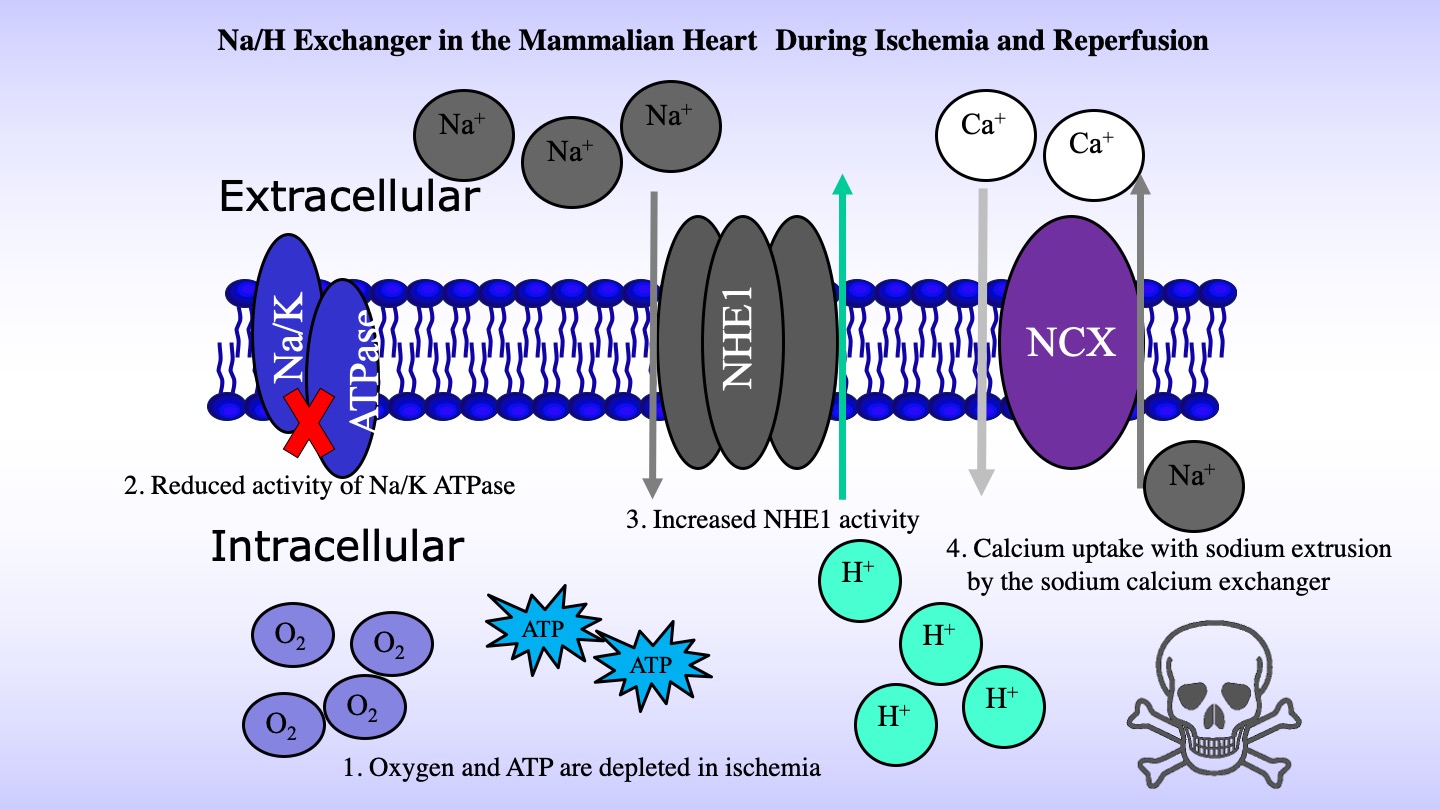
NHE1 has numerous important physiological functions. It maintains intracellular pH (pHi) while protecting cells from acidification as a result of metabolism. It also regulates cell volume in response to osmotic challenge. In human pathology, NHE1 is critical in several diseases, including myocardial heart hypertrophy and ischemia reperfusion damage. There it is thought to contribute to calcium overload. Loss of cellular ATP production due ischemia, contributes to decreased sodium extrusion by the Na+/K+ ATPase. Accumulation of intracellular sodium NHE1 leads to reversal of the action of the Na+/Ca2+exchanger and accumulation of detrimental intracellular calcium. Blockage of activity of NHE1 has been proposed as a therapy of ischemia reperfusion damage, but further development and use of inhibitors is still required (2)[2] (Figure 2).
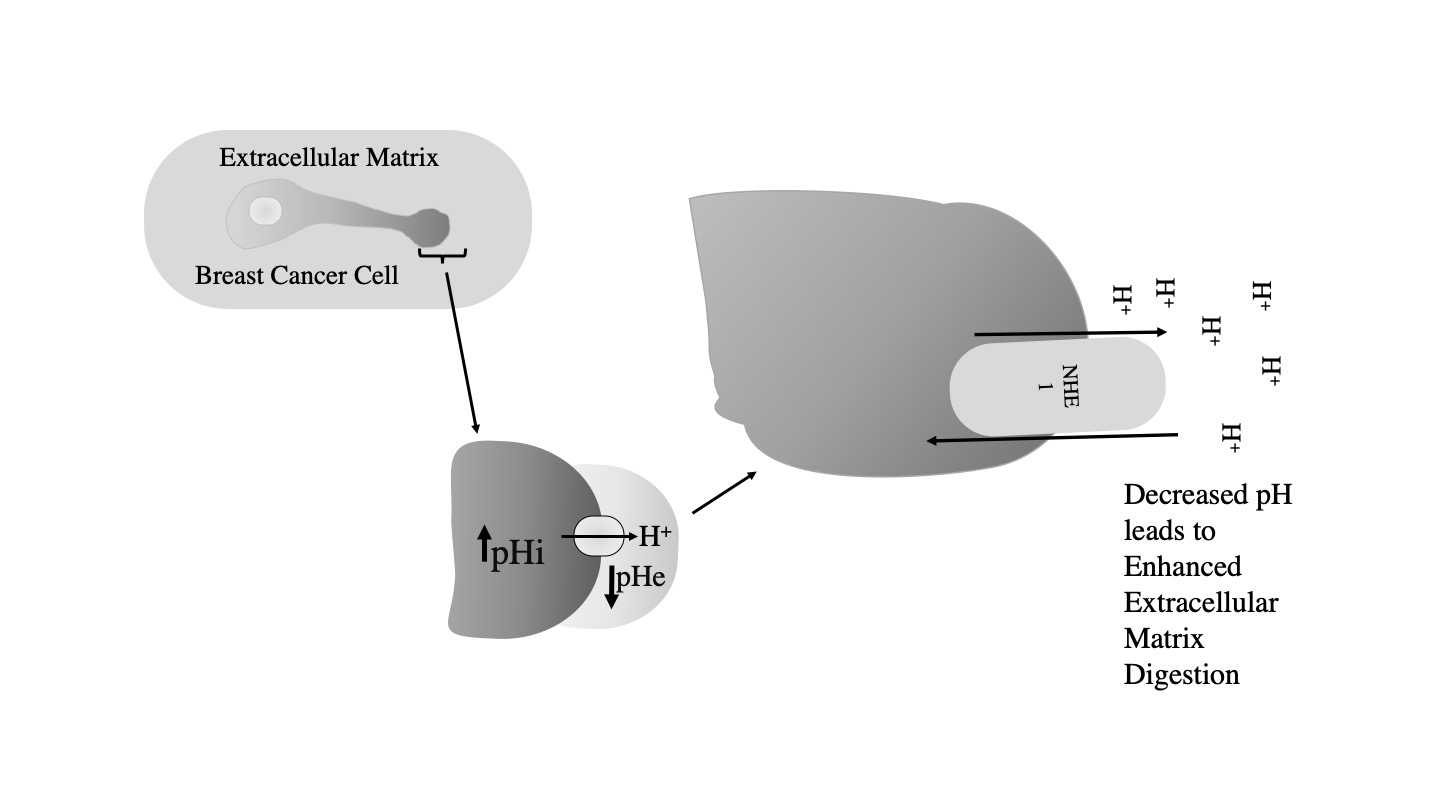
NHE1 is also important in breast cancer, where it acts as a trigger for metastasis. It is thought that elevated NHE1 activity contributes to extracellular acidification and increased extracellular proteolysis. This contributes to extracellular matrix degradation and increased cell mobility (3-5)[3][4][5] (Figurre 3).
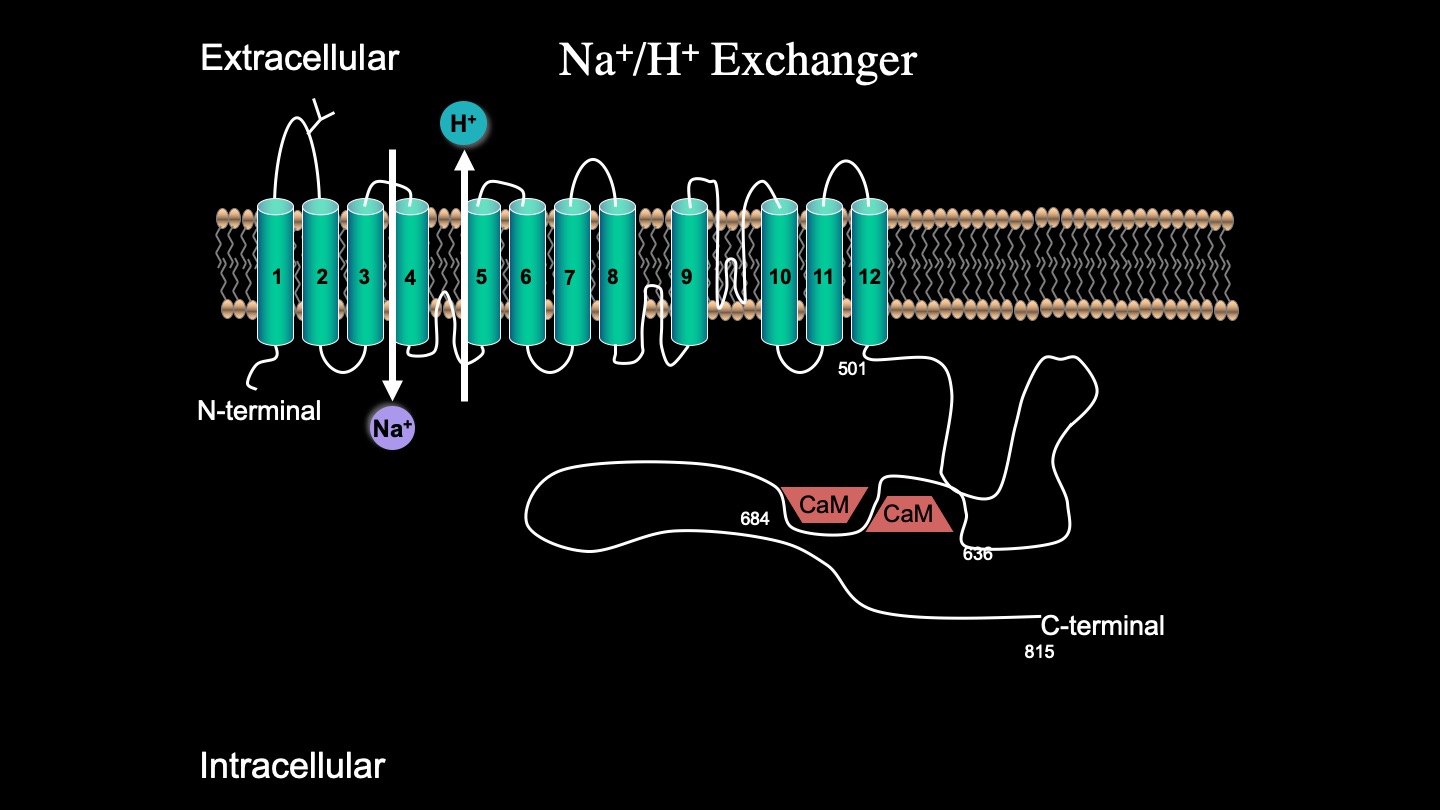
While the complete 3D structure of NHE1 is unknown, NHE1 is known to consist of two domains: a membrane domain that transports ions and a C-terminal cytosolic regulatory domain. The N-terminal membrane domain is comprised of 500 amino acids, and the C-terminal cytosolic regulatory domain is 315 amino acids long and functions to regulate the membrane domain (6)[6] (Figure 4).
Genetic mutations in NHE1 have been previously documented. A severe mutation in the human NHE1 gene SLC9A1 causes ataxia and deafness in a disease called Lichtenstein–Knorr syndrome (7)[7]. Another genetic mutation in the transmembrane region of NHE1 also abolishes activity (8)[8]. We (8)[8] examined the effects of a number of mutations that result in stop codon polymorphisms in the NHE1 protein; an NHE1 mutant with deleted C-terminal 80 amino acids had only minor defects. Surprisingly, truncating the NHE1 protein much more at amino acid 543 resulted in greatly reduced NHE1 activity. This was caused by a reduced expression and surface localization. The smaller protein showed an enhanced degradation, though RNA stability was unchanged. Recently (9)[9], we demonstrated that amino acids 562-568 of the C-terminus of the protein are important in targeting and activity. They may represent an important new signal that is important in maintenance of NHE1 protein levels.
Literature Cited
- Malo, M. E., and Fliegel, L. (2006) Physiological role and regulation of the Na+/H+ exchanger. Canadian journal of physiology and pharmacology 84, 1081-1095
- Karmazyn, M. (2013) NHE-1: still a viable therapeutic target. J Mol Cell Cardiol 61, 77-82
- Amith, S. R., and Fliegel, L. (2017) Na(+)/H(+) exchanger-mediated hydrogen ion extrusion as a carcinogenic signal in triple-negative breast cancer etiopathogenesis and prospects for its inhibition in therapeutics. Semin Cancer Biol 43, 35-41
- Amith, S. R., Wilkinson, J. M., and Fliegel, L. (2016) Na+/H+ exchanger NHE1 regulation modulates metastatic potential and epithelial-mesenchymal transition of triple-negative breast cancer cells. Oncotarget 7, 21091-21113
- Greco, M. R., Antelmi, E., Busco, G., Guerra, L., Rubino, R., Casavola, V., Reshkin, S. J., and Cardone, R. A. (2014) Protease activity at invadopodial focal digestive areas is dependent on NHE1-driven acidic pHe. Oncol Rep 31, 940-946
- Dutta, D., and Fliegel, L. (2019) Molecular modeling and inhibitor docking analysis of the Na(+)/H(+) exchanger isoform one (1). Biochemistry and cell biology = Biochimie et biologie cellulaire 97, 333-343
- Guissart, C., Li, X., Leheup, B., Drouot, N., Montaut-Verient, B., Raffo, E., Jonveaux, P., Roux, A. F., Claustres, M., Fliegel, L., and Koenig, M. (2015) Mutation of SLC9A1, encoding the major Na+/H+ exchanger, causes ataxia-deafness Lichtenstein-Knorr syndrome. Human molecular genetics 24, 463-470
- Li, X., and Fliegel, L. (2015) A novel human mutation in the SLC9A1 gene results in abolition of Na+/H+ exchanger activity. PLoS One 10, e0119453
- Li, X., Dutta, D., Jung, M., Zimmermann, R., and Fliegel, L. (2020) Amino Acids 563-566 of the Na(+)/H(+) Exchanger Isoform 1 C-Terminal Cytosolic Tail Prevent Protein Degradation and Stabilize Protein Expression and Activity. International journal of molecular sciences 21
References
- Malo, M. E., and Fliegel, L. (2006) Physiological role and regulation of the Na+/H+ exchanger. Canadian journal of physiology and pharmacology 84, 1081-1095
- Karmazyn, M. (2013) NHE-1: still a viable therapeutic target. J Mol Cell Cardiol 61, 77-82
- Amith, S. R., and Fliegel, L. (2017) Na(+)/H(+) exchanger-mediated hydrogen ion extrusion as a carcinogenic signal in triple-negative breast cancer etiopathogenesis and prospects for its inhibition in therapeutics. Semin Cancer Biol 43, 35-41
- Amith, S. R., Wilkinson, J. M., and Fliegel, L. (2016) Na+/H+ exchanger NHE1 regulation modulates metastatic potential and epithelial-mesenchymal transition of triple-negative breast cancer cells. Oncotarget 7, 21091-21113
- Greco, M. R., Antelmi, E., Busco, G., Guerra, L., Rubino, R., Casavola, V., Reshkin, S. J., and Cardone, R. A. (2014) Protease activity at invadopodial focal digestive areas is dependent on NHE1-driven acidic pHe. Oncol Rep 31, 940-946
- Dutta, D., and Fliegel, L. (2019) Molecular modeling and inhibitor docking analysis of the Na(+)/H(+) exchanger isoform one (1). Biochemistry and cell biology = Biochimie et biologie cellulaire 97, 333-343
- Guissart, C., Li, X., Leheup, B., Drouot, N., Montaut-Verient, B., Raffo, E., Jonveaux, P., Roux, A. F., Claustres, M., Fliegel, L., and Koenig, M. (2015) Mutation of SLC9A1, encoding the major Na+/H+ exchanger, causes ataxia-deafness Lichtenstein-Knorr syndrome. Human molecular genetics 24, 463-470
- Li, X., and Fliegel, L. (2015) A novel human mutation in the SLC9A1 gene results in abolition of Na+/H+ exchanger activity. PLoS One 10, e0119453
- Li, X., Dutta, D., Jung, M., Zimmermann, R., and Fliegel, L. (2020) Amino Acids 563-566 of the Na(+)/H(+) Exchanger Isoform 1 C-Terminal Cytosolic Tail Prevent Protein Degradation and Stabilize Protein Expression and Activity. International journal of molecular sciences 21