Streptococcus suis is a zoonotic pathogen causing substantial economic losses to the pig industry, as well as being a human health burden due to infections worldwide, especially in Southeast Asia. In Thailand, there was high cumulative incidence in humans during 1987–2021, mostly in males. At least five large outbreaks have been documented after the largest outbreak in China in 2005, which was related to the consumption of raw pork or dishes containing pig’s blood. The major clinical features are sepsis or meningitis, with hearing loss a major complication of S. suis disease. Thai human S. suis isolates have shown diversity in serotypes and sequence types (STs), with serotype 2 and STs 1 and 104 being major genotypes. β-Lactam antibiotics can be used in empirical treatment for human S. suis infections; however, intermediate resistance to penicillin has been reported. Reducing S. suis incidence in Thailand requires a multidimensional approach, with combined efforts from the government and public health sectors through policy, regulations, education, and active surveillance.
- Streptococcus suis
- serotype
- sequence type
- Thailand
1. Introduction
2. Epidemiology of Human S. suis Infections in Thailand
In Thailand, S. suis infection was first described in 1987 in Bangkok, with two cases of meningitis [6]. Before the largest outbreak of human S. suis infection occurred in Sichuan province, China in 2005 [7], sporadic human cases had been reported in several provinces in Thailand, especially in the north [8,9,10,11,12,13,14][8][9][10][11][12][13][14]. One outbreak with 10 fatal cases due to septic shock was documented in 2000 in Lamphun province, northern Thailand, well before the largest outbreak occurred in China [14]. That study demonstrated that all cases were healthy men aged 40–49 years who were clustered during the same period and geographic area [14]. All cases had a history of chronic alcohol use and the consumption of raw pork or pig’s blood dishes prior to their illness [14]. National guidelines for human S. suis infections are not yet available in Thailand. However, the practice of S. suis recruitment in the public health system is conducted using the R506 system (a daily case report of communicable diseases) of the Ministry of Public Health that was initiated after the first large outbreak in 2007. As shown in Figure 1, human cases reported in the system showed an increasing trend during 2011–2021. Although the number of cases dropped in 2022, annual data were only up until September. Notably, these reported cases were submitted by the hospital network where they could identify this bacterium. Thus, misidentification of S. suis as other bacteria might have occurred, and this would not have been reported in that system [19,20,21,22][15][16][17][18]. Therefore, the reported human S. suis cases registered in the R506 system may be lower than the real situation.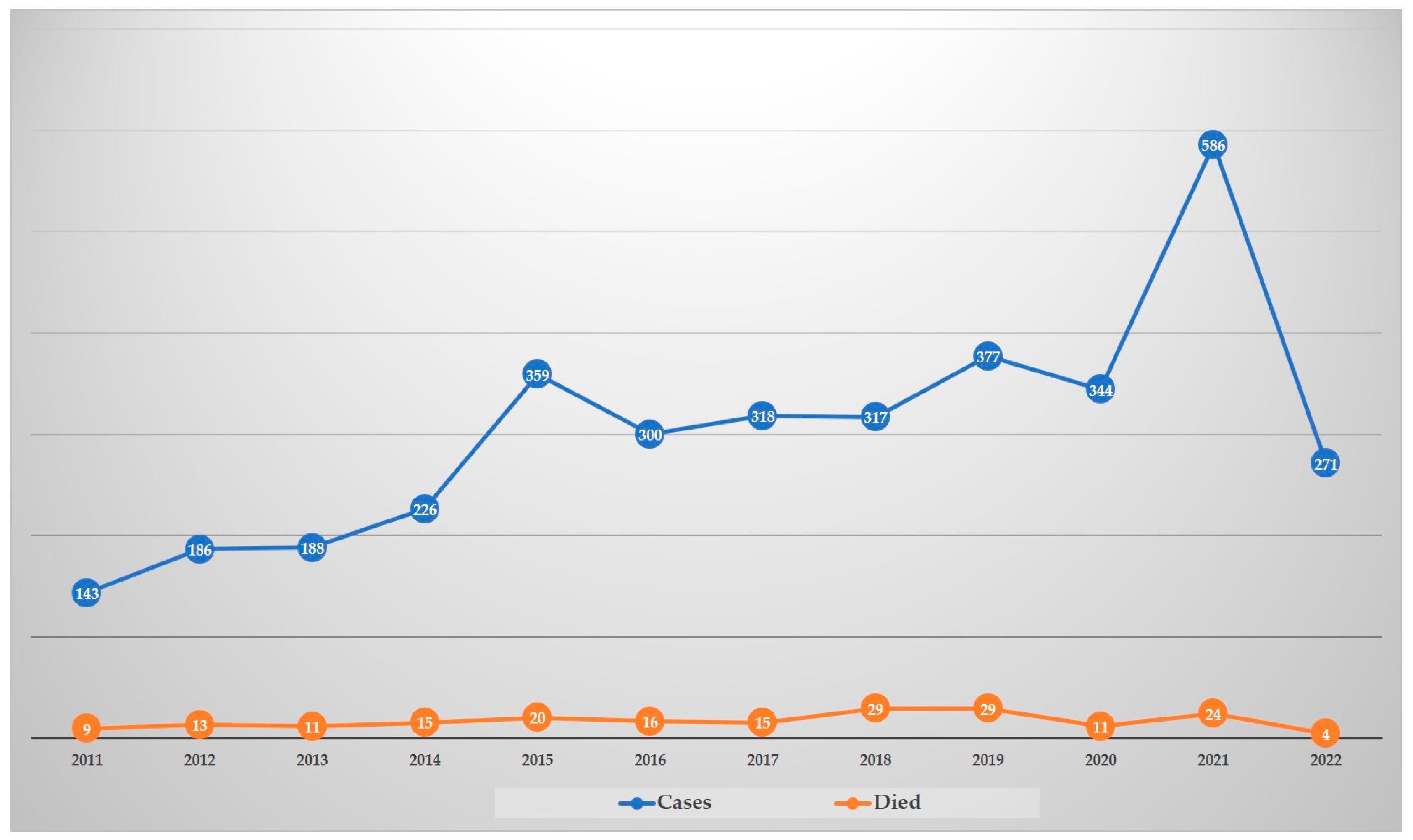
3. Genotypes of Thai Human S. suis Strains
As shown in Table 21, for S. suis isolated from patients in Thailand, serotype 2 (93.4%) was dominant, followed by serotypes 14 (5.2%), 24 (0.6%), 5 (0.4%), 4 (0.1%), 9 (0.1%), 31 (0.1%), and unencapsulated (0.1%), respectively [21,24,25,32,69][17][20][21][22][23]. MLST classified serotype 2 into five CCs: CC1, CC25, CC28, CC104, and CC233/379. Of these, CC1 is a major CC of human S. suis infection in this country and ST1 is the main ST in CC1 [25][21], while serotype 14 was classified to only CC1, with ST105 predominant [25,69][21][23]. For serotype 2, ST104, ST25, ST28, and ST233 were the main STs in CC104, CC25, CC28, and CC233/379, respectively. Notably, STs 1 and 104 for serotype 2 are the predominant STs in Thai human infections, and CC104, CC233/379, and CC221/234 are found exclusively in Thailand [24,25,70][20][21][24].
| Serotype | Clonal Complex | Sequence Type | Reference |
|---|---|---|---|
| 2 | 1 | 1, 11, 105, 126, 144, 298, 337 | [18,21,38,69][1722,][1823,][1924,][2025,][2129,]32,36,[37,22][23][25][26][27][28][29] https://pubmlst.org/organisms/streptococcus-suis (accessed on 5 October 2022) |
| 25 | 25, 102, 103, 380, 381, 395, 515, 516 | ||
| 28 | 28, 382 | ||
| 104 | 101, 104, 391, 392, 393, 512, 513, 514 | ||
| 233/379 | 233, 379, 1656, 1713 | ||
| 1687/1688 | 1687, 1688 | ||
| Singleton | 232, 236 | ||
| 4 | 94 | 94 | |
| 5 | 221/234 | 221 | |
| Singleton | 181, 235 | ||
| 9 | 16 | 16 | |
| 14 | 1 | 11, 105, 127 | |
| 24 | 221/234 | 221, 234 | |
| 31 (Unencapsulated) |
221/234 | 221 | |
| Unencapsulated serotype 2 or 1/2 |
28 | 28 |
4. Antimicrobial Susceptibility
Other studies have revealed that Thai S. suis isolates were susceptible to penicillin [13]. This contrasted with Nakaranurack et al. (2017), who reported that 6 out of 11 Thai S. suis isolates were intermediately resistance to penicillin, whereas cefotaxime and vancomycin were completely susceptible [35][33]. However, a study in 2021 demonstrated that 448 S. suis isolates recovered from human infections in Thailand had 8.2% intermediate resistance to penicillin, while they were all susceptible to cefepime and ceftriaxone [76][34].
Resistance to tetracycline (98.2%), clindamycin (94%), erythromycin (92.4%), and azithromycin (82.6%) with the resistance genes tet(O) and ermB were the predominant determinant genes of tetracycline and erythromycin (also macrolide-lincosamide–streptogramin B (MLSB)) resistance detected in 448 S. suis isolates [76][34]. Resistance to tetracycline appeared common in S. suis from human infections worldwide [78,79,80[35][36][37][38][39],81,82], whereas the resistance rates to erythromycin were low in Poland, Hong Kong, and Vietnam [78,79,83][35][36][40]. Although tet(O) is prevalent in human S. suis in Thailand, the tet(M) gene is the most widespread in human S. suis in China and Vietnam [79,81][36][38]. The ermB gene was predominant in human isolates in Vietnam and Thailand, while mefA was present in Hong Kong [76,79][34][36]. This may indicate differences in the local spread of tetracycline and erythromycin-resistance genes among human S. suis isolates in different countries or geographical regions.
5. Public Health Control
Kerdsin et al. (2022) mentioned that sociocultural factors, including cultural, religious, and societal behaviors and attitudes associated with the consumption of raw pork or pig’s blood play an important role in human infections [3]. Therefore, effecting a reduction in the human S. suis cases in Thailand requires a multidimensional approach involving the government and community sectors. Enforcement is required of meat inspection regulations and hygiene practices at pork processing facilities, as well as conducting food safety campaigns, establishing an educational program on preventing this infection, and reducing behavior regarding the consumption of raw pork or pig’s blood dishes. Another study in Thailand showed the effectiveness of a food safety campaign [42][41]. Overall, this campaign led to a marked decrease in the annualized incidence of human S. suis disease, from 6.4/100,000 persons in 2010 (before the campaign implementation) to 2.7/100,000 persons in 2011, then to 2.0/100,000 persons in 2012, and finally to 3.5/100,000 persons in 2013 [42][41]. Finally, early diagnostics in S. suis suspected patients using rapid alternative methods rather than traditional culture (a gold standard but slower) could facilitate prompt treatment and reduce the mortality rate, as well as prompt epidemiological investigation [84,85,86,87][42][43][44][45].6. Conclusions
Thai human S. suis isolates have diversified in serotypes and STs, with serotype 2 and STs 1 and 104 being the majority in Thailand. In addition, serotype 14 with ST105 is also prevalent in the country. β-Lactam antibiotics can be used in empirical treatment for human S. suis infections; however, intermediate resistance to penicillin has been reported. This should be of concern and should be carefully monitored. Thai S. suis strains have been reported to be highly resistant to macrolide and tetracycline. Reducing human S. suis disease is Thailand requires a multidimensional approach combining government and public health efforts through policy, regulations, and education, and active community involvement to effect behavioral changes that are evidence-based but culturally sensible and acceptable, along with the implementation in health-care systems of more rapid diagnostics and more relevant screening tools.References
- Goyette-Desjardins, G.; Auger, J.P.; Xu, J.; Segura, M.; Gottschalk, M. Streptococcus suis, an important pig pathogen and emerging zoonotic agent-an update on the worldwide distribution based on serotyping and sequence typing. Emerg. Microbes. Infect. 2014, 3, e45.
- Segura, M.; Aragon, V.; Brockmeier, S.L.; Gebhart, C.; De Greeff, A.; Kerdsin, A.; O’Dea, M.A.; Okura, M.; Saléry, M.; Schultsz, C.; et al. Update on Streptococcus suis Research and Prevention in the Era of Antimicrobial Restriction: 4th International Workshop on S. suis. Pathogens 2020, 9, 374.
- Kerdsin, A.; Segura, M.; Fittipaldi, N.; Gottschalk, M. Sociocultural Factors Influencing Human Streptococcus suis Disease in Southeast Asia. Foods 2022, 11, 1190.
- Thongsawad, S. Burden and Epidemiological Characterisations of Streptococcus suis in Chiang Mai, Thailand. Ph.D. Thesis, The University of Edinburgh, Edinburgh, UK, 2016.
- Rayanakorn, A.; Ademi, Z.; Liew, D.; Lee, L.-H. Burden of disease and productivity impact of Streptococcus suis infection in Thailand. PLoS Negl. Trop. Dis. 2021, 15, e0008985.
- Phuapradit, P.; Boongird, P.; Boonyakarnkul, S.; Niramarnsakul, S.; Ponglikitmongkol, S.; Vorachit, M. Meningitis caused by Streptococcus suis in humans. Intern. Med. 1987, 3, 120–122.
- Yu, H.; Jing, H.; Chen, Z.; Zheng, H.; Zhu, X.; Wang, H.; Wang, S.; Liu, L.; Zu, R.; Luo, L.; et al. Streptococcus suis study groups. Human Streptococcus suis outbreak, Sichuan, China. Emerg. Infect. Dis. 2006, 12, 914–920.
- Teekakirikul, P.; Wiwanitkit, V. Streptococcus suis infection: Overview of case reports in Thailand. S. Asian J. Trop. Med. Public Health 2003, 34 (Suppl. 2), 178–183.
- Vilaichone, R.K.; Vilaichone, W.; Nunthapisud, P.; Wilde, H. Streptococcus suis infection in Thailand. J. Med. Assoc. Thai. 2002, 85 (Suppl. 1), S109–S117.
- Donsakul, K.; Dejthevaporn, C.; Witoonpanich, R. Streptococcus suis infection: Clinical features and diagnostic pitfalls. S. Asian J. Trop. Med. Public Health 2003, 34, 154–158.
- Suankratay, C.; Intalapaporn, P.; Nunthapisud, P.; Arunyingmongkol, K.; Wilde, H. Streptococcus suis meningitis in Thailand. S. Asian J. Trop. Med. Public Health 2004, 35, 868–876.
- Rusmeechan, S.; Sribusara, P. Streptococcus suis meningitis: The newest serious infectious disease. J. Med. Assoc. Thail. 2008, 91, 654–658.
- Wangkaew, S.; Chaiwarith, R.; Tharavichitkul, P.; Supparatpinyo, K. Streptococcus suis infection: A series of 41 cases from Chiang Mai University Hospital. J. Infect. 2006, 52, 455–460.
- Fongcom, A.; Pruksakorn, S.; Mongkol, R.; Tharavichitkul, P.; Yoonim, N. Streptococcus suis infection in northern Thailand. J. Med. Assoc. Thail. 2001, 84, 1502–1508.
- Le, H.T.T.; Sugiyama, N.; Duangsonk, K.; Tharavichitkul, P.; Osawa, R. Phenotypic and PCR-based identification of bacterial strains isolated from patients with suspected Streptococcus suis infection in northern Thailand. Jpn. J. Infect. Dis. 2012, 65, 171–174.
- Tarini, N.M.A.; Setiabudy, M.; Susilawathi, N.; Fatmawati, N.; Mayura, I.; Darwinata, E.; Sudiariani, N. Misidentification of S. suis as a Zoonotic Agent. Open Access Maced. J. Med. Sci. 2019, 7, 2309–2312.
- Hatrongjit, R.; Kerdsin, A.; Gottschalk, M.; Takeuchi, D.; Hamada, S.; Oishi, K.; Akeda, Y. First human case report of sepsis due to infection with Streptococcus suis serotype 31 in Thailand. BMC Infect. Dis. 2015, 15, 392.
- Prapasiri, P.; Owusu, J.T.; Thammathitiwat, S.; Ditsungnoen, D.; Boonmongkon, P.; Sangwichian, O.; Prasert, K.; Srihapanya, S.; Sornwong, K.; Kerdsin, A.; et al. Streptococcus suis Infection in Hospitalized Patients, Nakhon Phanom Province, Thailand. Emerg. Infect. Dis. 2015, 21, 345–348.
- Takeuchi, D.; Kerdsin, A.; Pienpringam, A.; Loetthong, P.; Samerchea, S.; Luangsuk, P.; Khamisara, K.; Wongwan, N.; Areeratana, P.; Chiranairadul, P.; et al. Population-Based Study of Streptococcus suis Infection in Humans in Phayao Province in Northern Thailand. PLoS ONE 2012, 7, e31265.
- Kerdsin, A.; Dejsirilert, S.; Puangpatra, P.; Sripakdee, S.; Chumla, K.; Boonkerd, N.; Polwichai, P.; Tanimura, S.; Takeuchi, D.; Nakayama, T.; et al. Genotypic Profile of Streptococcus suis Serotype 2 and Clinical Features of Infection in Humans, Thailand. Emerg. Infect. Dis. 2011, 17, 835–842.
- Kerdsin, A.; Akeda, Y.; Takeuchi, D.; Dejsirilert, S.; Gottschalk, M.; Oishi, K. Genotypic diversity of Streptococcus suis strains isolated from humans in Thailand. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 917–925.
- Takamatsu, D.; Wongsawan, K.; Osaki, M.; Nishino, H.; Ishiji, T.; Tharavichitkul, P.; Khantawa, B.; Fongcom, A.; Takai, S.; Sekizaki, T. Streptococcus suis in humans, Thailand. Emerg. Infect. Dis. 2008, 14, 181–183.
- Kerdsin, A.; Oishi, K.; Sripakdee, S.; Boonkerd, N.; Polwichai, P.; Nakamura, S.; Uchida, R.; Sawanpanyalert, P.; Dejsirilert, S. Clonal dissemination of human isolates of Streptococcus suis serotype 14 in Thailand. J. Med. Microbiol. 2009, 58, 1508–1513.
- Kerdsin, A.; Hatrongjit, R.; Wongsurawat, T.; Jenjaroenpun, P.; Chopjitt, P.; Boueroy, P.; Fittipaldi, N.; Zheng, H.; Gottschalk, M. Genomic Characterization of Streptococcus suis Serotype 24 Clonal Complex 221/234 From Human Patients. Front. Microbiol. 2021, 12, 812436.
- Brizuela, J.; Kajeekul, R.; Roodsant, T.; Riwload, A.; Boueroy, P.; Thaipadungpanit, J.; Jenjaroenpun, P.; Wongsurawat, T.; Batty, E.; van der Putten, B.; et al. Streptococcus suis outbreak in Thailand caused by an emerging zoonotic strain with acquired multidrug resistance. Microb. Genom. 2022; accepted.
- Kerdsin, A.; Gottschalk, M.; Hatrongjit, R.; Hamada, S.; Akeda, Y.; Oishi, K. Fatal Septic Meningitis in Child Caused by Streptococcus suis Serotype 24. Emerg. Infect. Dis. 2016, 22, 1519–1520.
- Kerdsin, A.; Dejsirilert, S.; Sawanpanyalert, P.; Boonnark, A.; Noithachang, W.; Sriyakum, D.; Simkum, S.; Chokngam, S.; Gottschalk, M.; Akeda, Y.; et al. Sepsis and spontaneous bacterial peritonitis in Thailand. Lancet 2011, 378, 960.
- Kerdsin, A.; Takeuchi, D.; Gottschalk, M.; Hamada, S.; Akeda, Y.; Oishi, K. A human case of Streptococcus suis infection caused by an unencapsulated strain. JMM Case Rep. 2014, 1, e002329.
- Kerdsin, A.; Hatrongjit, R.; Gottschalk, M.; Takeuchi, D.; Hamada, S.; Akeda, Y.; Oishi, K. Emergence of Streptococcus suis serotype 9 infection in humans. J. Microbiol. Immunol. Infect. 2017, 50, 545–546.
- Kerdsin, A.; Takeuchi, D.; Nuangmek, A.; Akeda, Y.; Gottschalk, M.; Oishi, K. Genotypic Comparison between Streptococcus suis Isolated from Pigs and Humans in Thailand. Pathogens 2020, 9, 50.
- Maneerat, K.; Yongkiettrakul, S.; Kramomtong, I.; Tongtawe, P.; Tapchaisri, P.; Luangsuk, P.; Chaicumpa, W.; Gottschalk, M.; Srimanote, P. Virulence Genes and Genetic Diversity of Streptococcus suis Serotype 2 Isolates from Thailand. Transbound. Emerg. Dis. 2013, 60 (Suppl. 2), 69–79.
- Tharavichitkul, P.; Wongsawan, K.; Takenami, N.; Pruksakorn, S.; Fongcom, A.; Gottschalk, M.; Khanthawa, B.; Supajatura, V.; Takai, S. Correlation between PFGE Groups and mrp/epf/sly Genotypes of Human Streptococcus suis Serotype 2 in Northern Thailand. J. Pathog. 2014, 2014, 350416.
- Nakaranurack, C.; Puttilerpong, C.; Suwanpimolkul, G. A Decennium of Etiology and Antimicrobial Susceptibility Patterns in Patients with Infective Endocarditis at a University Hospital, Thailand. Jpn. J. Infect. Dis. 2017, 70, 295–300.
- Bamphensin, N.; Chopjitt, P.; Hatrongjit, R.; Boueroy, P.; Fittipaldi, N.; Gottschalk, M.; Kerdsin, A. Non-Penicillin-Susceptible Streptococcus suis Isolated from Humans. Pathogens 2021, 10, 1178.
- Bojarska, A.; Molska, E.; Janas, K.; Skoczynska, A.; Stefaniuk, E.; Hryniewicz, W.; Sadowy, E. Streptococcus suis in invasive human infections in Poland: Clonality and determinants of virulence and antimicrobial resistance. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 917–925.
- Hoa, N.T.; Chieu, T.T.; Nghia, H.D.; Mai, N.T.; Anh, P.H.; Wolbers, M.; Baker, S.; Campbell, J.I.; Chau, N.V.; Hien, T.T.; et al. The antimicrobial resistance patterns and associated determinants in Streptococcus suis isolated from humans in southern Vietnam, 1997–2008. BMC Infect. Dis. 2011, 11, 6.
- Marie, J.; Morvan, H.; Berthelot-Hérault, F.; Sanders, P.; Kempf, I.; Gautier-Bouchardon, A.V.; Jouy, E.; Kobisch, M. Antimicrobial susceptibility of Streptococcus suis isolated from swine in France and from humans in different countries between 1996 and 2000. J. Antimicrob. Chemother. 2002, 50, 201–209.
- Ye, C.; Bai, X.; Zhang, J.; Jing, H.; Zheng, H.; Du, H.; Cui, Z.; Zhang, S.; Jin, D.; Xu, Y.; et al. Spread of Streptococcus suis sequence type 7, China. Emerg. Infect. Dis. 2008, 14, 787–791.
- Chang, B.; Wada, A.; Ikebe, T.; Ohnishi, M.; Mita, K.; Endo, M.; Matsuo, H.; Asatuma, Y.; Kuramoto, S.; Sekiguchi, H.; et al. Characteristics of Streptococcus suis isolated from patients in Japan. Jpn. J. Infect. Dis. 2006, 59, 397–399.
- Chu, Y.W.; Cheung, T.K.; Chu, M.Y.; Tsang, V.Y.; Fung, J.T.; Kam, K.M.; Lo, J.Y. Resistance to tetracycline, erythromycin and clindamycin in Streptococcus suis serotype 2 in Hong Kong. Int. J. Antimicrob. Agents 2009, 34, 181–182.
- Takeuchi, D.; Kerdsin, A.; Akeda, Y.; Chiranairadul, P.; Loetthong, P.; Tanburawong, N.; Areeratana, P.; Puangmali, P.; Nakayama, T.; Yamamoto, K.; et al. Impact of a Food Safety Campaign on Streptococcus suis Infection in Humans in Thailand. Am. J. Trop. Med. Hyg. 2017, 96, 1370–1377.
- Nga, T.V.T.; Nghia, H.D.T.; Tu, L.T.P.; Diep, T.S.; Mai, N.T.H.; Chau, T.T.H.; Sinh, D.X.; Phu, N.H.; Chau, N.V.V.; Campbell, J.; et al. Real-time PCR for detection of Streptococcus suis serotype 2 in cerebrospinal fluid of human patients with meningitis. Diagn. Microbiol. Infect. Dis. 2011, 70, 461–467.
- Nakayama, T.; Zhao, J.; Takeuchi, D.; Kerdsin, A.; Chiranairadul, P.; Areeratana, P.; Loetthong, P.; Pienpringam, A.; Akeda, Y.; Oishi, K. Colloidal gold-based immunochromatographic strip test compromising optimised combinations of anti-S. suis capsular polysaccharide polyclonal antibodies for detection of Streptococcus suis. Biosens. Bioelectron. 2014, 60, 175–179.
- Zhang, X.; Wu, Z.; Wang, K. Diagnosis of Streptococcus suis Meningoencephalitis with metagenomic next-generation sequencing of the cerebrospinal fluid: A case report with literature review. BMC Infect. Dis. 2020, 20, 884.
- Thu, I.; Tragoolpua, K.; Intorasoot, S.; Anukool, U.; Khamnoi, P.; Kerdsin, A.; Tharinjaroen, C.S. Direct detection of Streptococcus suis from cerebrospinal fluid, positive hemoculture, and simultaneous differentiation of serotypes 1, 1/2, 2, and 14 within single reaction. Pathogens 2021, 10, 996.
