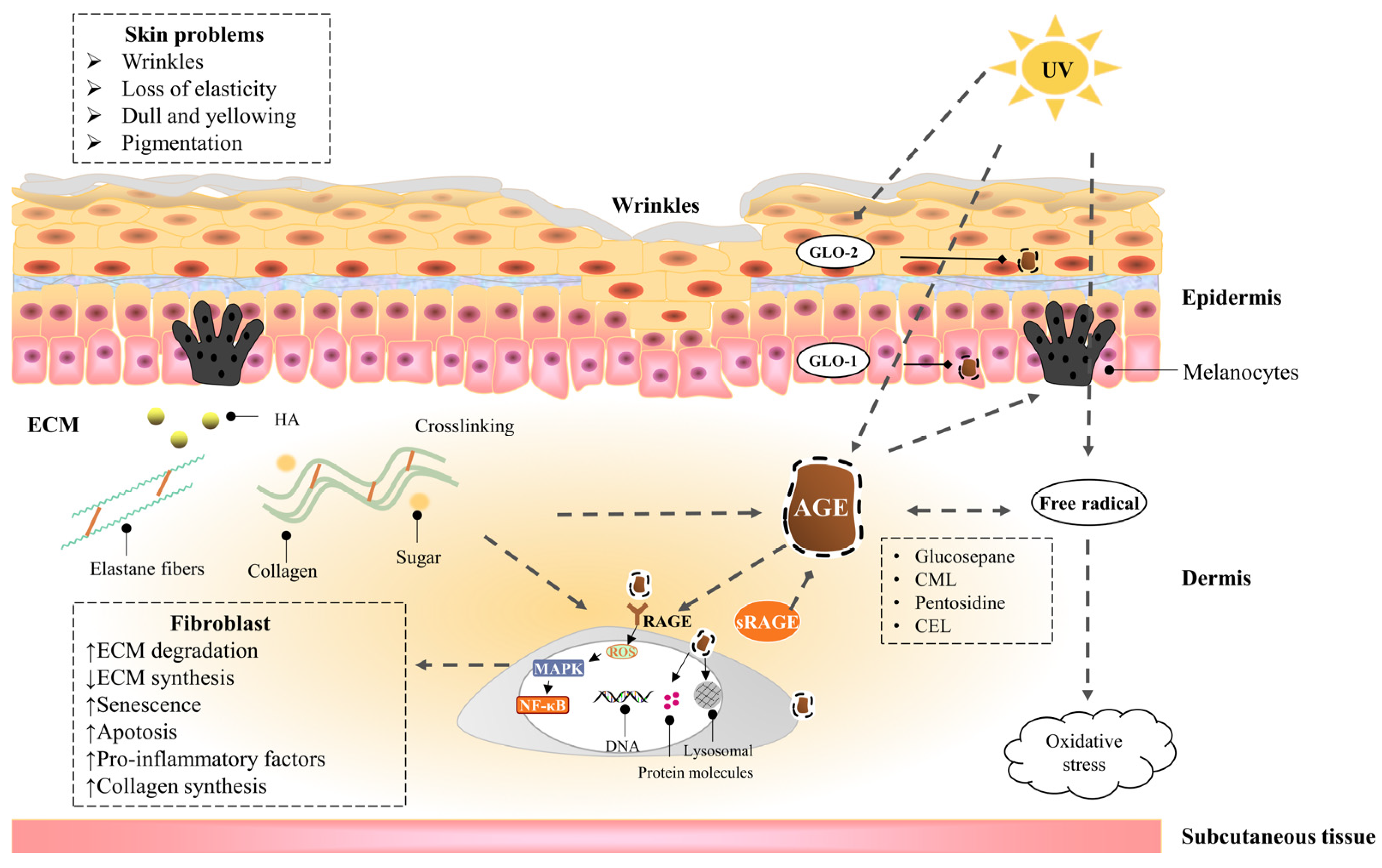
Skin saccharification, a non-enzymatic reaction between proteins, e.g., dermal collagen and naturally occurring reducing sugars, is one of the basic root causes of endogenous skin aging. During the reaction, a series of complicated glycation products produced at different reaction stages and pathways are usually collectively referred to as advanced glycation end products (AGEs). AGEs cause cellular dysfunction through the modification of intracellular molecules and accumulate in tissues with aging. AGEs are also associated with a variety of age-related diseases, such as diabetes, cardiovascular disease, renal failure (uremia), and Alzheimer’s disease. AGEs accumulate in the skin with age and are amplified through exogenous factors, e.g., ultraviolet radiation, resulting in wrinkles, loss of elasticity, dull yellowing, and other skin problems.
- skin glycation
- anti-glycation
- AGEs inhibitors
1. The Hazards of Skin Glycation
1.1. The Harm of High Glucose to the Skin
1.2. Advanced Glycaion End Products Induce Skin Aging
1.2.1. Epidermis
1.2.2. Dermis—Fibroblast
1.2.3. Dermis—Extracellular matrix (ECM)
1.3. UVA Induces Advanced Glycation End Products of the Skin
2. Inhibitors of Advanced Glycation End Products
AGEs inhibitors are mainly divided into five categories: (1) carbonyl trapping agents that weaken carbonyl stress; (2) metal-ion chelators or scavenging free radicals, inhibiting sugar and lipid oxidation reaction; (3) crosslinking breakers that reverse AGEs crosslinking; (4) activating the anti-glycation system—many kinds of herbal extracts and natural compounds inhibit glycation by enhancing the anti-glycation system in the body; (5) RAGE antagonists. These include: anti-RAGE antibodies, sRAGE, and RAGE inhibitors; FPSZM1, a specific and potent chemical inhibitor of AGE receptor, which could improve diabetic nephropathy [53] and Aβ-mediated brain disorder [54]; Azeliragon, an oral small molecule antagonist of RAGE in Phase 3 development for mild cognitive impairment [55]. Small molecules are also in development to inhibit Diaphanous.1, the intracellular RAGE adaptor [56]. Inhibiting oxidative stress and inflammation in tissues by blocking the interaction of AGEs with RAGE is another new way to inhibit the process of late glycation. Figure 2 shows the action sites that inhibit the formation of AGEs in vivo.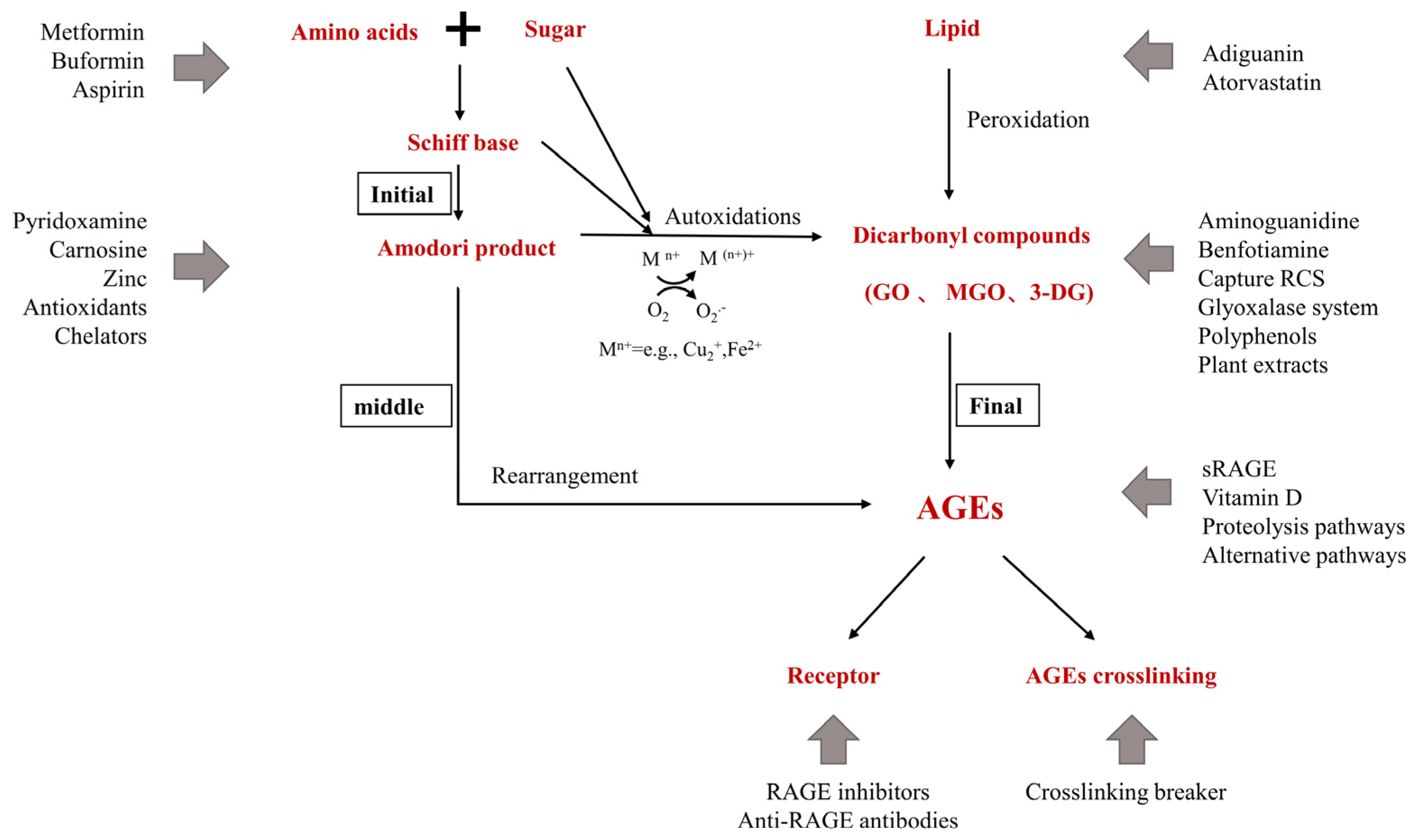
2.1. Pre-Amadori Inhibitors
Aminoguanidine (AG) is an inhibitor of late glycation reactions in vitro found in clinical trials and is an excellent dicarbonyls scavenger that captures reactive carbonyl precursors, such as MGO, GO, and 3-DG. Amadori compounds are important intermediates for AGEs formation in vivo, and CML must be formed primarily by oxidative cleavage of Amadori’s Enediol intermediate between C2-C3 of the ligated sugar. AG was found to have no significant effect on the CML produced during the incubation of Amadori proteins. Therefore, AG is an important pre-Amadori inhibitor. AG is toxic at higher concentrations and has been forbidden in human clinical trials [57]. AG inhibits the development of diabetic complications in animal models of diabetes but does not inhibit the formation of late glycation end products of skin collagen in diabetic rats [58]. Benfotiamine, a synthetic thiamine precursor, activates the enzyme transketolase to accelerate the precursors of AGEs toward the pentose phosphate pathway, thereby reducing the production of AGEs [59].2.2. Post-Amadori Inhibitors
Pyridoxamine (PM), one of the natural forms of vitamin B6, uniquely targets the post-Amadori pathway through metal-ion chelation and blocking oxidative degradation of Amadori intermediates [60]. Good post-Amadori inhibitor compounds should form stable metal-ion complexes with a higher equilibrium constant than the Amadori compound [61]. PM also has the ability to scavenge toxic carbonyl products from sugar and lipid degradation, inhibit reactive oxygen species [62][63], and increase the activation of the detoxifying enzyme GLO-1 [64]. The ilex paraguariensis (IP) extract is also a post-Amadori inhibitor due to its inhibition of the second stage of glycation reaction and conversion of free-radical-mediated Amadori products to AGEs [65].2.3. Crosslinking Breaker
Thiazole salts are AGEs crosslinking breakers, such as OPB-9195 and ALT-711 (alagebrium). OPB-9195 inhibits AGE formation (particularly pentosidine and CML) through the chelation of metal ions and carbonyl trapping [66]. ALT-711 is the first compound in the thiazole class, which has been reported to break down established AGE-related cross-links. Another prototypic AGE cross-link breaker is N-phenacylthiazolium bromide (PTB) which break down protein cross-links by cleaving α-diketone structure. Similar effects have been observed with rosmarinic acid, tannins, and flavonoids [67]. There are also other potent AGEs destroyers, such as curcumin and ALT-946 [68].2.4. Indirect Advanced Glycation End Products Inhibitors
A small number of AGEs inhibitors play a role in the early stages of glycation by disturbing the initial binding between sugars and amino groups and indirectly reducing the formation of AGEs and ALEs. Since AGEs are mostly produced by non-enzymatic glycation of sugars and lipids, hypoglycemic and lipid-lowering drugs can inhibit the production of AGEs in vivo. For example, Atorvastatin (a lipid-lowering drug) inhibits the further formation of Schiff bases and AGEs by interfering with the initial binding between reducing sugars and amino groups [69]; Metformin is used to treat type II diabetes mellitus by inhibiting the production of reactive oxygen species by reducing the expression of the AGEs receptor (RAGE) [70] and capturing MG and other dicarbonyls produced during glycation. Buformin inhibits the formation of AGEs by trapping the carbonyl groups of ammonia and MGO and is a more effective inhibitor of AGEs formation than metformin [71]; Aspirin, or acetylsalicylic acid, inhibits the glycation process by acetylating the proteins’ free amino groups, thereby blocking the attachment of reducing sugars.2.5. Natural Advanced Glycation End Products Inhibitors
Synthetic AGE inhibitors have safety concerns and side effects, so natural products with lower toxicity are the most promising alternatives for developing natural medicines with anti-glycation activity. It has been reported that tea, herbal tea, vegetables, fruits [72], yogurt, and other foods have an inhibitory effect on the saccharification reaction. A large number of experiments in vitro and in vivo have shown that natural compounds have the potential to combat the formation and accumulation of AGEs, including phenols, oligosaccharides and polysaccharides, carotenoids (e.g., β-carotene), saponins [73], and unsaturated fatty acids. Plants have long been used in traditional medicine techniques to treat various diseases and are also a source of new natural medicines discovery. Plant extracts have great anti-aging potential and are rich in a variety of active ingredients, which can inhibit the formation of AGEs by scavenging free radicals, capturing dicarbonyl carbon, etc. [74]. For example, C. ternatea flower extract (CTE) prevents protein glycation by trapping carbonyl groups and scavenging free radicals [75]. The polyphenolic components of peanut peel include gallocatechin, phenolic acids, and resveratrol, which reduce toxicity caused by AGEs and reduce the levels of reactive oxygen species and pro-inflammatory cytokines [76]. Citrus fruit extract significantly reduces the level of protein carbonyl compounds [77]. Akebia quinata fruit extracts (AQFE) can act as an anti-skin aging agent by preventing oxidative stress and other complications associated with AGEs formation [78]. Phenolic components of milk thistle flowers have anti-glycation activity in vitro and on human explants. Polyphenol-rich clove extract, due to its antioxidant properties, is able to inhibit the formation of AGEs and protein glycation [79]. The polyphenol compounds of hazelnut bark extract can reduce the formation of AGEs in vitro [80]. The hydrophobic extract of dunaliella salina, rich in colorless carotene phytoene and phytofluene, has anti-glycation and anti-inflammatory activity and helps reduce the signs of aging (wrinkles) [81]. Cinnamon is a traditional spice, which contains some phenolic components in its aqueous extracts, such as catchin, epicatechin, and procyanidin B2, which inhibit the formation of AGEs through antioxidants and direct capture of active carbonyl substances [82]. Black galangal extract inhibits the formation of fluorescent AGEs, pentosidine, CML, and intermediates 3-DG, GO, and MGO, and it acts on the decomposition of AGEs, thereby reducing the accumulation of AGEs in vivo [67]. Salvia officinalis L. methanol extract, including rosmarinic acid, resveratrol, quercetin, rutin, and luteolin-7-O-glucoside, exerts anti-glycation effects through antioxidation and inhibition of fluorescent substances and carbonyl groups [83]. Pomegranate fruit extract (PE), its phenolic constituents (punicalagin, ellagic acid, and gallic acid), and products of the degradation of ellagitannin (urolithin A and urolithin B) [84] all have effective anti-glycation activities [85].2.6. Polyphenolic Compounds
As major and ubiquitous phytochemicals, including flavonoids, phenolic acids, alfalfa, and lignans, polyphenols exert AGEs inhibition through ROS inhibition, dicarbonyls capture (MGO and GO), and disruption of protein crosslinking [81][86]. Glycation and oxidative stress are closely related, with all steps of sugar oxidation producing oxygen radicals and ultimately resulting in the formation of AGEs. In addition, glycated proteins activate membrane receptors such as RAGE through AGEs and induce intracellular oxidative stress and pro-inflammatory states [87]. Therefore, compounds that scavenge free radicals can effectively inhibit glycation. For example, resveratrol (3,4′,5-Trihydroxystilbene) is a plant polyphenol that reduces oxidative stress [88] and inhibits AGEs-induced proliferation, collagen synthesis, and RAGE receptors [89]. Asiatic acid (AA), a pentacyclic triterpenoid, occurs naturally in many vegetables and fruits. AA pretreatment effectively protects HaCaT cells from subsequent AGE-BSA-induced oxidative and inflammatory stresses, exerting an anti-glycation effect [90]. The natural antioxidant “ellagic acid” (EA) exerts its inhibitory effect on AGEs in diabetic rats by inhibiting glycated ntermediates (including dicarbonyls) and interrupting the auto-oxidation pathway [91]. Flavonoids (flavones, flavanones, isoflavones, and flavonols) are the most common class of polyphenol compounds and have shown significant inhibitory effects on protein glycation and AGEs formation. These mechanisms may involve capturing reactive amino groups, so that they cannot react with glucose or scavenging carbonyl compounds, chelating with trace metal ions that catalyze glycation, scavenging hydroxyl radicals, and inhibiting oxidative degradation of various intermediates [92]. For example, garlic can inhibit protein glycation and dicarbonyls in vitro; quercetin is a phenolic compound found in garlic [93], which has a more effective anti-glycation effect than aminoguanidine [94]. Dietary antioxidants such as quercetin can prevent free radical toxicity [95]. Rutin (flavonoids) is found in fruits and vegetables, making it unable to react with glucose through mechanisms such as capturing reactive amino groups. All five metabolites formed after ingestion effectively inhibit the formation of CML [92]. Anthocyanins are the main flavonoids in blackcurrants that effectively prevent the formation of AGEs by capturing methylglyoxal [96]. Phenolic acids are secondary metabolites that are widely present in plants, including a large distribution of hydroxycinnamic acid (coumalic acid, caffeic acid, ferulic acid, coumarin) and hydroxybenzoic acid (Protocatechuic acid, gallic acid, hydrobenzoic acid, and ellagitannin). These metabolites are also found to have anti-aging potential. For example, ferulic acid inhibits the formation of fluorescent AGEs and CML and reduces fructosamine levels. This leads to the prevention of protein oxidation through the reduction in protein carbonyl formation and protein thiol modification [97]. Isoferulic acid (IFA) is a powerful antioxidant and has an effective inhibitory effect on protein glycation and sugar oxidation [98]. Cinnamic acid and its derivatives reduce the levels of fructosamines, the formation of CML, and the level of amyloid cross-β structures [99].2.7. Other Advanced Glycation End Products Inhibitors
Carnosine is a naturally occurring dipeptide (beta-alanyl-l-histidine), which hinders the formation of protein carbonyl groups and has the ability to chelate transition metal ions, prevent MG-induced glycation, and reduce sugar-induced crosslinking [100], leading to a significantly lower AGEs levels in the epidermis and reticular dermis of human skin explants [101]. Piperazine-2,5-dione reduces the number of late glycation end products accumulated in human dermal fibroblasts with age. Vitamin D therapy may help lower AGEs levels, significantly reduce NF-κB activation, and increase sRAGE levels [102]. Zinc has antioxidant, anti-inflammatory, and anti-apoptotic potential. Zinc deficiency may stimulate the formation of AGEs, while zinc supplementation may inhibit the formation of AGEs and protein carbonyl groups through a variety of signaling pathways and improve AGEs-induced apoptosis and oxidative stress [103].References
- Cao, C.; Xiao, Z.; Wu, Y.; Ge, C. Diet and Skin Aging-From the Perspective of Food Nutrition. Nutrients 2020, 12, 870.
- Liu, X.; Yang, C.; Deng, Y.; Liu, P.; Yang, H.; Du, X.; Du, Y. Polygoni Multiflori Radix Preparat Delays Skin Aging by Inducing Mitophagy. BioMed Res. Int. 2021, 2021, 1–12.
- Gasser, P.; Arnold, F.; Peno-Mazzarino, L.; Bouzoud, D.; Luu, M.T.; Lati, E.; Mercier, M. Glycation Induction and Antiglycation Activity of Skin Care Ingredients On Living Human Skin Explants. Int. J. Cosmet. Sci. 2011, 33, 366–370.
- Danby, F.W. Nutrition and Aging Skin: Sugar and Glycation. Clin. Dermatol. 2010, 28, 409–411.
- Cibrian, D.; de la Fuente, H.; Sánchez-Madrid, F. Metabolic Pathways that Control Skin Homeostasis and Inflammation. Trends Mol. Med. 2020, 26, 975–986.
- Devarakonda, K.; Mobbs, C.V. Mechanisms and Significance of Brain Glucose Signaling in Energy Balance, Glucose Homeostasis, and Food-Induced Reward. Mol. Cell. Endocrinol. 2016, 438, 61–69.
- Quondamatteo, F. Skin and Diabetes Mellitus: What Do we Know? Cell Tissue Res. 2014, 355, 1–21.
- Argyropoulos, A.J.; Robichaud, P.; Balimunkwe, R.M.; Fisher, G.J.; Hammerberg, C.; Yan, Y.; Quan, T. Alterations of Dermal Connective Tissue Collagen in Diabetes: Molecular Basis of Aged-Appearing Skin. PLoS ONE 2016, 11, e153806.
- Mentink, C.J.; Hendriks, M.; Levels, A.A.; Wolffenbuttel, B.H. Glucose-Mediated Cross-Linking of Collagen in Rat Tendon and Skin. Clin. Chim. Acta 2002, 321, 69–76.
- Van Putte, L.; De Schrijver, S.; Moortgat, P. The Effects of Advanced Glycation End Products (Ages) On Dermal Wound Healing and Scar Formation: A Systematic Review. Scars Burn. Heal. 2016, 2, 1011166500.
- Shu, F.; Gao, H.; Wu, W.; Yu, S.; Zhang, L.; Liu, H.; Xiao, S.; Xia, Z.; Zheng, Y. Amniotic Epithelial Cells Accelerate Diabetic Wound Healing by Protecting Keratinocytes and Fibroblasts From High-Glucose-Induced Senescence. Cell Biol. Int. 2022, 46, 755–770.
- Sruthi, C.R.; Raghu, K.G. Advanced Glycation End Products and their Adverse Effects: The Role of Autophagy. J. Biochem. Mol. Toxicol. 2021, 35, e22710.
- Khalifah, R.G.; Baynes, J.W.; Hudson, B.G. Amadorins: Novel Post-Amadori Inhibitors of Advanced Glycation Reactions. Biochem. Biophys. Res. Commun. 1999, 257, 251–258.
- Osawa, T.; Kato, Y. Protective Role of Antioxidative Food Factors in Oxidative Stress Caused by Hyperglycemia. Ann. N. Y. Acad. Sci. 2005, 1043, 440–451.
- Yevdokimova, N.Y. High Glucose-Induced Alterations of Extracellular Matrix of Human Skin Fibroblasts are Not Dependent On Tsp-1-Tgfbeta1 Pathway. J. Diabetes Complicat. 2003, 17, 355–364.
- Bian, X.; Li, B.; Yang, J.; Ma, K.; Sun, M.; Zhang, C.; Fu, X. Regenerative and Protective Effects of Dmsc-Sevs On High-Glucose-Induced Senescent Fibroblasts by Suppressing Rage Pathway and Activating Smad Pathway. Stem Cell Res. Ther. 2020, 11, 166.
- Li, B.; Bian, X.; Hu, W.; Wang, X.; Li, Q.; Wang, F.; Sun, M.; Ma, K.; Zhang, C.; Chang, J.; et al. Regenerative and Protective Effects of Calcium Silicate On Senescent Fibroblasts Induced by High Glucose. Wound Repair Regen. 2020, 28, 315–325.
- Buranasin, P.; Mizutani, K.; Iwasaki, K.; Pawaputanon, N.M.C.; Kido, D.; Takeda, K.; Izumi, Y. High Glucose-Induced Oxidative Stress Impairs Proliferation and Migration of Human Gingival Fibroblasts. PLoS ONE 2018, 13, e201855.
- Liu, J.; Wu, Y.; Wang, B.; Yuan, X.; Fang, B. High Levels of Glucose Induced the Caspase-3/Parp Signaling Pathway, Leading to Apoptosis in Human Periodontal Ligament Fibroblasts. Cell Biophys. 2013, 66, 229–237.
- Soydas, T.; Sayitoglu, M.; Sarac, E.Y.; Cinar, S.; Solakoglu, S.; Tiryaki, T.; Sultuybek, G.K. Metformin Reverses the Effects of High Glucose On Human Dermal Fibroblasts of Aged Skin via Downregulating Rela/P65 Expression. J. Physiol. Biochem. 2021, 77, 443–450.
- Wang, X.; Jin, H.; Jiang, S.; Xu, Y. Microrna-495 Inhibits the High Glucose-Induced Inflammation, Differentiation and Extracellular Matrix Accumulation of Cardiac Fibroblasts through Downregulation of Nod1. Cell. Mol. Biol. Lett. 2018, 23, 23.
- Yue, E.; Yu, Y.; Wang, X.; Liu, B.; Bai, Y.; Yang, B. Anthocyanin Protects Cardiac Function and Cardiac Fibroblasts From High-Glucose Induced Inflammation and Myocardial Fibrosis by Inhibiting Il-17. Front. Pharmacol. 2020, 11, 593633.
- Chiu, H.C.; Fu, M.M.; Yang, T.S.; Fu, E.; Chiang, C.Y.; Tu, H.P.; Chin, Y.T.; Lin, F.G.; Shih, K.C. Effect of High Glucose, Porphyromonas Gingivalis Lipopolysaccharide and Advanced Glycation End-Products On Production of Interleukin-6/-8 by Gingival Fibroblasts. J. Periodont. Res. 2017, 52, 268–276.
- Lee, J.; Jeong, E.T.; Lim, J.M.; Park, S.G. Development of the Facial Glycation Imaging System for in Situ Human Face Skin Glycation Index Measurement. J. Cosmet. Dermatol. 2021, 20, 2963–2968.
- Nguyen, H.P.; Katta, R. Sugar Sag: Glycation and the Role of Diet in Aging Skin. Ski. Ther. Lett. 2015, 20, 1–5.
- Da, M.S.C.; Webb, M.; Waller, H.; Khunti, K.; Davies, M. Skin Autofluorescence, a Non-Invasive Marker of Advanced Glycation End Products: Clinical Relevance and Limitations. Postgrad. Med. J. 2017, 93, 289–294.
- Smit, A.J.; van de Zande, S.C.; Mulder, D.J. Skin Autofluorescence as Tool for Cardiovascular and Diabetes Risk Prediction. Curr. Opin. Nephrol. Hypertens. 2022, 31, 522–526.
- André, A.; Touré, A.K.; Stien, D.; Eparvier, V. 2,5-Diketopiperazines Mitigate the Amount of Advanced Glycation End Products Accumulated with Age in Human Dermal Fibroblasts. Int. J. Cosmet. Sci. 2020, 42, 596–604.
- Low, E.; Alimohammadiha, G.; Smith, L.A.; Costello, L.F.; Przyborski, S.A.; von Zglinicki, T.; Miwa, S. How Good is the Evidence that Cellular Senescence Causes Skin Ageing? Ageing Res. Rev. 2021, 71, 101456.
- D’Errico, M.; Lemma, T.; Calcagnile, A.; Proietti, D.S.L.; Dogliotti, E. Cell Type and Dna Damage Specific Response of Human Skin Cells to Environmental Agents. Mutat. Res. Mol. Mech. Mutagen. 2007, 614, 37–47.
- Iwamura, M.; Yamamoto, Y.; Kitayama, Y.; Higuchi, K.; Fujimura, T.; Hase, T.; Yamamoto, H. Epidermal Expression of Receptor for Advanced Glycation End Products (Rage) is Related to Inflammation and Apoptosis in Human Skin. Exp. Dermatol. 2016, 25, 235–237.
- Zhu, P.; Ren, M.; Yang, C.; Hu, Y.X.; Ran, J.M.; Yan, L. Involvement of Rage, Mapk and Nf-Kappab Pathways in Ages-Induced Mmp-9 Activation in Hacat Keratinocytes. Exp. Dermatol. 2012, 21, 123–129.
- Tian, M. Effects of Advanced Glycation End-Products (Ages) On Skin Keratinocytes by Nuclear Factor-Kappa B (Nf-Κb) Activation. Afr. J. Biotechnol. 2012, 11, 11132–11142.
- Farrar, M.D. Advanced Glycation End Products in Skin Ageing and Photoageing: What are the Implications for Epidermal Function? Exp. Dermatol. 2016, 25, 947–948.
- Yumnam, S.; Subedi, L.; Kim, S.Y. Glyoxalase System in the Progression of Skin Aging and Skin Malignancies. Int. J. Mol. Sci. 2020, 22, 310.
- Reichert, O.; Fleming, T.; Neufang, G.; Schmelz, M.; Genth, H.; Kaever, V.; Wenck, H.; Stab, F.; Terstegen, L.; Kolbe, L.; et al. Impaired Glyoxalase Activity is Associated with Reduced Expression of Neurotrophic Factors and Pro-Inflammatory Processes in Diabetic Skin Cells. Exp. Dermatol. 2017, 26, 44–50.
- Pageon, H.; Bakala, H.; Monnier, V.M.; Asselineau, D. Collagen Glycation Triggers the Formation of Aged Skin in Vitro. Eur. J. Dermatol. 2007, 17, 12–20.
- Okano, Y.; Masaki, H.; Sakurai, H. Dysfunction of Dermal Fibroblasts Induced by Advanced Glycation End-Products (Ages) and the Contribution of a Nonspecific Interaction with Cell Membrane and Ages. J. Dermatol. Sci. 2002, 29, 171–180.
- Guarneri, F.; Custurone, P.; Papaianni, V.; Gangemi, S. Involvement of Rage and Oxidative Stress in Inflammatory and Infectious Skin Diseases. Antioxidants 2021, 10, 82.
- Xu, X.; Zheng, Y.; Huang, Y.; Chen, J.; Gong, Z.; Li, Y.; Lu, C.; Lai, W.; Xu, Q. Cathepsin D Contributes to the Accumulation of Advanced Glycation End Products During Photoaging. J. Dermatol. Sci. 2018, 90, 263–275.
- Arseni, L.; Lombardi, A.; Orioli, D. From Structure to Phenotype: Impact of Collagen Alterations On Human Health. Int. J. Mol. Sci. 2018, 19, 1407.
- Fournet, M.; Bonté, F.; Desmoulière, A. Glycation Damage: A Possible Hub for Major Pathophysiological Disorders and Aging. Aging Dis. 2018, 9, 880.
- Pageon, H. Reaction of Glycation and Human Skin: The Effects On the Skin and its Components, Reconstructed Skin as a Model. Pathol. Biol. 2010, 58, 226–231.
- Jaisson, S.; Gillery, P. Methods to Assess Advanced Glycation End-Products. Curr. Opin. Clin. Nutr. Metab. Care 2021, 24, 411–415.
- Sjoberg, J.S.; Bulterijs, S. Characteristics, Formation, and Pathophysiology of Glucosepane: A Major Protein Cross-Link. Rejuvenation Res. 2009, 12, 137–148.
- Pennacchi, P.C.; de Almeida, M.E.; Gomes, O.L.; Faiao-Flores, F.; de Araujo, C.M.; Dos, S.M.; de Moraes, B.S.; Maria-Engler, S.S. Glycated Reconstructed Human Skin as a Platform to Study the Pathogenesis of Skin Aging. Tissue Eng. Part A 2015, 21, 2417–2425.
- Han, A.R.; Nam, M.H.; Lee, K.W. Plantamajoside Inhibits Uvb and Advanced Glycation End Products-Induced Mmp-1 Expression by Suppressing the Mapk and Nf-Kappab Pathways in Hacat Cells. Photochem. Photobiol. 2016, 92, 708–719.
- Crisan, M.; Taulescu, M.; Crisan, D.; Cosgarea, R.; Parvu, A.; Catoi, C.; Drugan, T. Expression of Advanced Glycation End-Products On Sun-Exposed and Non-Exposed Cutaneous Sites During the Ageing Process in Humans. PLoS ONE 2013, 8, e75003.
- Okano, Y.; Masaki, H.; Sakurai, H. Pentosidine in Advanced Glycation End-Products (Ages) During Uva Irradiation Generates Active Oxygen Species and Impairs Human Dermal Fibroblasts. J. Dermatol. Sci. 2001, 27 (Suppl. 1), S11–S18.
- Zhang, S.; Duan, E. Fighting Against Skin Aging: The Way From Bench to Bedside. Cell Transplant. 2018, 27, 729–738.
- Pageon, H.; Zucchi, H.; Ricois, S.; Bastien, P.; Asselineau, D. Uva Exposure Combined with Glycation of the Dermis are Two Catalysts for Skin Aging and Promotes a Favorable Environment to the Appearance of Elastosis. J. Aging Res. 2021, 2021, 1–13.
- Lee, E.J.; Kim, J.Y.; Oh, S.H. Advanced Glycation End Products (Ages) Promote Melanogenesis through Receptor for Ages. Sci. Rep. 2016, 6, 27848.
- Sanajou, D.; Ghorbani, H.A.; Argani, H.; Aslani, S. Age-Rage Axis Blockade in Diabetic Nephropathy: Current Status and Future Directions. Eur. J. Pharmacol. 2018, 833, 158–164.
- Deane, R.; Singh, I.; Sagare, A.P.; Bell, R.D.; Ross, N.T.; LaRue, B.; Love, R.; Perry, S.; Paquette, N.; Deane, R.J.; et al. A Multimodal Rage-Specific Inhibitor Reduces Amyloid Beta-Mediated Brain Disorder in a Mouse Model of Alzheimer Disease. J. Clin. Investig. 2012, 122, 1377–1392.
- Burstein, A.H.; Sabbagh, M.; Andrews, R.; Valcarce, C.; Dunn, I.; Altstiel, L. Development of Azeliragon, an Oral Small Molecule Antagonist of the Receptor for Advanced Glycation Endproducts, for the Potential Slowing of Loss of Cognition in Mild Alzheimer’s Disease. J. Prev. Alzheimer′s Dis. 2018, 5, 149–154.
- Manigrasso, M.B.; Rabbani, P.; Egana-Gorrono, L.; Quadri, N.; Frye, L.; Zhou, B.; Reverdatto, S.; Ramirez, L.S.; Dansereau, S.; Pan, J.; et al. Small-Molecule Antagonism of the Interaction of the Rage Cytoplasmic Domain with Diaph1 Reduces Diabetic Complications in Mice. Sci. Transl. Med. 2021, 13, f7084.
- Ahmad, S.; Khan, M.S.; Alouffi, S.; Khan, S.; Khan, M.; Akashah, R.; Faisal, M.; Shahab, U. Gold Nanoparticle-Bioconjugated Aminoguanidine Inhibits Glycation Reaction: An in Vivo Study in a Diabetic Animal Model. BioMed Res. Int. 2021, 2021, 5591851.
- Degenhardt, T.P.; Fu, M.X.; Voss, E.; Reiff, K.; Neidlein, R.; Strein, K.; Thorpe, S.R.; Baynes, J.W.; Reiter, R. Aminoguanidine Inhibits Albuminuria, but Not the Formation of Advanced Glycation End-Products in Skin Collagen of Diabetic Rats. Diabetes Res. Clin. Pr. 1999, 43, 81–89.
- Gibson, G.E.; Luchsinger, J.A.; Cirio, R.; Chen, H.; Franchino-Elder, J.; Hirsch, J.A.; Bettendorff, L.; Chen, Z.; Flowers, S.A.; Gerber, L.M.; et al. Benfotiamine and Cognitive Decline in Alzheimer’s Disease: Results of a Randomized Placebo-Controlled Phase Iia Clinical Trial. J. Alzheimer′s Dis. 2020, 78, 989–1010.
- Khalifah, R.G.; Chen, Y.; Wassenberg, J.J. Post-Amadori Age Inhibition as a Therapeutic Target for Diabetic Complications: A Rational Approach to Second-Generation Amadorin Design. Ann. N. Y. Acad. Sci. 2005, 1043, 793–806.
- Adrover, M.; Vilanova, B.; Frau, J.; Munoz, F.; Donoso, J. The Pyridoxamine Action On Amadori Compounds: A Reexamination of its Scavenging Capacity and Chelating Effect. Bioorganic Med. Chem. 2008, 16, 5557–5569.
- Ramis, R.; Ortega-Castro, J.; Caballero, C.; Casasnovas, R.; Cerrillo, A.; Vilanova, B.; Adrover, M.; Frau, J. How Does Pyridoxamine Inhibit the Formation of Advanced Glycation End Products? The Role of its Primary Antioxidant Activity. Antioxidants 2019, 8, 344.
- Voziyan, P.A.; Hudson, B.G. Pyridoxamine: The Many Virtues of a Maillard Reaction Inhibitor. Ann. New York Acad. Sci. 2005, 1043, 807–816.
- Sourris, K.C.; Watson, A.; Jandeleit-Dahm, K. Inhibitors of Advanced Glycation End Product (Age) Formation and Accumulation. Handb. Exp. Pharmacol. 2021, 264, 395–423.
- Lunceford, N.; Gugliucci, A. Ilex Paraguariensis Extracts Inhibit Age Formation More Efficiently than Green Tea. Fitoterapia 2005, 76, 419–427.
- Reddy, V.P.; Beyaz, A. Inhibitors of the Maillard Reaction and Age Breakers as Therapeutics for Multiple Diseases. Drug Discov. Today 2006, 11, 646–654.
- Yagi, M.; Inoue, K.; Sato, Y.; Ishizaki, K.; Sakiyama, C.; Yonei, Y. Antiglycative Effect of Black Galangal, Kaempferia Parviflora Wall. Ex. Baker (Zingiberaceae). Glycative Stress Res. 2021, 8, 1–7.
- Peng, X.; Ma, J.; Chen, F.; Wang, M. Naturally Occurring Inhibitors Against the Formation of Advanced Glycation End-Products. Food Funct. 2011, 2, 289–301.
- Yamagishi, S.; Nakamura, K.; Matsui, T.; Inoue, H. A Novel Pleiotropic Effect of Atorvastatin On Advanced Glycation End Product (Age)-Related Disorders. Med. Hypotheses 2007, 69, 338–340.
- Ishibashi, Y.; Matsui, T.; Takeuchi, M.; Yamagishi, S. Metformin Inhibits Advanced Glycation End Products (Ages)-Induced Renal Tubular Cell Injury by Suppressing Reactive Oxygen Species Generation Via Reducing Receptor for Ages (Rage) Expression. Horm. Metab. Res. 2012, 44, 891–895.
- Kiho, T.; Kato, M.; Usui, S.; Hirano, K. Effect of Buformin and Metformin On Formation of Advanced Glycation End Products by Methylglyoxal. Clin. Chim. Acta 2005, 358, 139–145.
- Mesias, M.; Navarro, M.; Gokmen, V.; Morales, F.J. Antiglycative Effect of Fruit and Vegetable Seed Extracts: Inhibition of Age Formation and Carbonyl-Trapping Abilities. J. Sci. Food Agric. 2013, 93, 2037–2044.
- Yousof, A.M.; Jannat, S.; Mizanur, R.M. Ginsenoside Derivatives Inhibit Advanced Glycation End-Product Formation and Glucose-Fructose Mediated Protein Glycation in Vitro Via a Specific Structure-Activity Relationship. Bioorganic Chem. 2021, 111, 104844.
- Shin, S.; Lee, J.A.; Kim, M.; Kum, H.; Jung, E.; Park, D. Anti-Glycation Activities of Phenolic Constituents From Silybum Marianum (Milk Thistle) Flower in Vitro and On Human Explants. Molecules 2015, 20, 3549–3564.
- Chayaratanasin, P.; Adisakwattana, S.; Thilavech, T. Protective Role of Clitoria Ternatea L. Flower Extract On Methylglyoxal-Induced Protein Glycation and Oxidative Damage to Dna. Bmc Complement. Med. Ther. 2021, 21, 80.
- Fernandes, A.; Vieira, N.C.; Santana, A.L.; Gandra, R.; Rubia, C.; Castro-Gamboa, I.; Macedo, J.A.; Macedo, G.A. Peanut Skin Polyphenols Inhibit Toxicity Induced by Advanced Glycation End-Products in Raw264.7 Macrophages. Food Chem. Toxicol. 2020, 145, 111619.
- Ramful, D.; Tarnus, E.; Rondeau, P.; Da, S.C.; Bahorun, T.; Bourdon, E. Citrus Fruit Extracts Reduce Advanced Glycation End Products (Ages)- And H2O2-Induced Oxidative Stress in Human Adipocytes. J. Agric. Food Chem. 2010, 58, 11119–11129.
- Shin, S.; Son, D.; Kim, M.; Lee, S.; Roh, K.B.; Ryu, D.; Lee, J.; Jung, E.; Park, D. Ameliorating Effect of Akebia Quinata Fruit Extracts On Skin Aging Induced by Advanced Glycation End Products. Nutrients 2015, 7, 9337–9352.
- Suantawee, T.; Wesarachanon, K.; Anantsuphasak, K.; Daenphetploy, T.; Thien-Ngern, S.; Thilavech, T.; Pasukamonset, P.; Ngamukote, S.; Adisakwattana, S. Protein Glycation Inhibitory Activity and Antioxidant Capacity of Clove Extract. J. Food Sci. Technol. 2015, 52, 3843–3850.
- Spagnuolo, L.; Della, P.S.; Fanali, C.; Dugo, L.; De Gara, L. Antioxidant and Antiglycation Effects of Polyphenol Compounds Extracted From Hazelnut Skin On Advanced Glycation End-Products (Ages) Formation. Antioxidants 2021, 10, 424.
- Khanam, A.; Ahmad, S.; Husain, A.; Rehman, S.; Farooqui, A.; Yusuf, M.A. Glycation and Antioxidants: Hand in the Glove of Antiglycation and Natural Antioxidants. Curr. Protein Pept. Sci. 2020, 21, 899–915.
- Peng, X.; Cheng, K.W.; Ma, J.; Chen, B.; Ho, C.T.; Lo, C.; Chen, F.; Wang, M. Cinnamon Bark Proanthocyanidins as Reactive Carbonyl Scavengers to Prevent the Formation of Advanced Glycation Endproducts. J. Agric. Food Chem. 2008, 56, 1907–1911.
- Khedher, M.R.B.; Hafsa, J.; Haddad, M.; Hammami, M. Inhibition of Protein Glycation by Combined Antioxidant and Antiglycation Constituents from a Phenolic Fraction of Sage (Salvia officinalis L.). Plant Foods Hum. Nutr. 2020, 75, 505–511.
- Verzelloni, E.; Pellacani, C.; Tagliazucchi, D.; Tagliaferri, S.; Calani, L.; Costa, L.G.; Brighenti, F.; Borges, G.; Crozier, A.; Conte, A.; et al. Antiglycative and Neuroprotective Activity of Colon-Derived Polyphenol Catabolites. Mol. Nutr. Food Res. 2011, 55 (Suppl. 1), S35–S43.
- Liu, W.; Ma, H.; Frost, L.; Yuan, T.; Dain, J.A.; Seeram, N.P. Pomegranate Phenolics Inhibit Formation of Advanced Glycation Endproducts by Scavenging Reactive Carbonyl Species. Food Funct. 2014, 5, 2996–3004.
- Khan, M.; Liu, H.; Wang, J.; Sun, B. Inhibitory Effect of Phenolic Compounds and Plant Extracts On the Formation of Advance Glycation End Products: A Comprehensive Review. Food Res. Int. 2020, 130, 108933.
- Edeas, M.; Attaf, D.; Mailfert, A.S.; Nasu, M.; Joubet, R. Maillard Reaction, Mitochondria and Oxidative Stress: Potential Role of Antioxidants. Pathol. Biol. 2010, 58, 220–225.
- Hajizadeh-Sharafabad, F.; Sahebkar, A.; Zabetian-Targhi, F.; Maleki, V. The Impact of Resveratrol On Toxicity and Related Complications of Advanced Glycation End Products: A Systematic Review. BioFactors 2019, 45, 651–665.
- Khangholi, S.; Majid, F.A.; Berwary, N.J.; Ahmad, F.; Aziz, R.B. The Mechanisms of Inhibition of Advanced Glycation End Products Formation through Polyphenols in Hyperglycemic Condition. Planta Med. 2016, 82, 32–45.
- Wang, Z.H. Anti-Glycative Effects of Asiatic Acid in Human Keratinocyte Cells. BioMedicine 2014, 4, 19.
- Ahmad, S.; Alouffi, S.; Khan, S.; Khan, M.; Akasha, R.; Ashraf, J.M.; Farhan, M.; Shahab, U.; Khan, M.Y. Physicochemical Characterization of in Vitro Ldl Glycation and its Inhibition by Ellagic Acid (Ea): An in Vivo Approach to Inhibit Diabetes in Experimental Animals. BioMed Res. Int. 2022, 2022, 5583298.
- Abbas, G.; Al-Harrasi, A.S.; Hussain, H.; Hussain, J.; Rashid, R.; Choudhary, M.I. Antiglycation Therapy: Discovery of Promising Antiglycation Agents for the Management of Diabetic Complications. Pharm. Biol. 2016, 54, 198–206.
- Khan, M.; Otaibi, A.A.; Alsukaibi, A.; Alshammari, E.M.; Al-Zahrani, S.A.; Sherwani, S.; Khan, W.A.; Saha, R.; Verma, S.R.; Ahmed, N. Biophysical, Biochemical, and Molecular Docking Investigations of Anti-Glycating, Antioxidant, and Protein Structural Stability Potential of Garlic. Molecules 2022, 27, 1868.
- Ashraf, J.M.; Shahab, U.; Tabrez, S.; Lee, E.J.; Choi, I.; Ahmad, S. Quercetin as a Finer Substitute to Aminoguanidine in the Inhibition of Glycation Products. Int. J. Biol. Macromol. 2015, 77, 188–192.
- Alam, M.M.; Ahmad, I.; Naseem, I. Inhibitory Effect of Quercetin in the Formation of Advance Glycation End Products of Human Serum Albumin: An in Vitro and Molecular Interaction Study. Int. J. Biol. Macromol. 2015, 79, 336–343.
- Chen, X.Y.; Huang, I.M.; Hwang, L.S.; Ho, C.T.; Lo, C.Y. Anthocyanins in Blackcurrant Effectively Prevent the Formation of Advanced Glycation End Products by Trapping Methylglyoxal. J. Funct. Foods 2014, 8, 259–268.
- Meeprom, A.; Sompong, W.; Chan, C.B.; Adisakwattana, S. Isoferulic Acid, a New Anti-Glycation Agent, Inhibits Fructose- and Glucose-Mediated Protein Glycation in Vitro. Molecules 2013, 18, 6439–6454.
- Arfin, S.; Siddiqui, G.A.; Naeem, A.; Moin, S. Inhibition of Advanced Glycation End Products by Isoferulic Acid and its Free Radical Scavenging Capacity: An in Vitro and Molecular Docking Study. Int. J. Biol. Macromol. 2018, 118, 1479–1487.
- Adisakwattana, S.; Sompong, W.; Meeprom, A.; Ngamukote, S.; Yibchok-Anun, S. Cinnamic Acid and its Derivatives Inhibit Fructose-Mediated Protein Glycation. Int. J. Mol. Sci. 2012, 13, 1778–1789.
- Ghodsi, R.; Kheirouri, S. Carnosine and Advanced Glycation End Products: A Systematic Review. Amino Acids 2018, 50, 1177–1186.
- Narda, M.; Peno-Mazzarino, L.; Krutmann, J.; Trullas, C.; Granger, C. Novel Facial Cream Containing Carnosine Inhibits Formation of Advanced Glycation End-Products in Human Skin. Ski. Pharmacol. Physiol. 2018, 31, 324–331.
- Kheirouri, S.; Alizadeh, M. Vitamin D and Advanced Glycation End Products and their Receptors. Pharmacol. Res. 2020, 158, 104879.
- Kheirouri, S.; Alizadeh, M.; Maleki, V. Zinc Against Advanced Glycation End Products. Clin. Exp. Pharmacol. Physiol. 2018, 45, 491–498.
