Clinical manifestation of stroke is characterized by great diversity, ranging from minor disability to considerable neurological impairment interfering with activities of daily living and even death. Prognostic ambiguity has stimulated the interest for implementing stroke recovery biomarkers, including those provided by structural neuroimaging techniques, i.e., diffusion tensor imaging (DTI) and tractography for the study of white matter (WM) integrity. Diffusion tensor imaging (DTI) is an extension of magnetic resonance imaging (MRI) for in vivo mapping of white matter (WM) directionality and organization, allowing the qualitative and quantitative evaluation of major WM tracts and their microstructural integrity. DTI is based on the random diffusion of water molecules. Research on DTI metrics as stroke outcome biomarker is not limited to the acute and subacute phases, as it is also implemented on chronic stroke patients [13,21]. Direct visualization of long tracts and their potential disruption provides insight into pathogenesis of functional deficits in stroke survivors as well as compensatory mechanisms on a microstructural level. Such knowledge may elucidate which group of patients is most likely to benefit from rehabilitation, and even help personalize treatment plans after the acute stroke phase according to each individual’s needs.
- diffusion tensor imaging
- tractography
- acute stroke
- hyperacute stroke
- stroke prognosis
1. Introduction
Clinical manifestation of stroke is characterized by great diversity, ranging from minor disability to considerable neurological impairment interfering with activities of daily living (ADL) and even causing death. Prognostic ambiguity has stimulated an interest in implementing stroke recovery biomarkers. Ideally, prognostic markers hold high sensitivity and specificity, enabling appropriate management of healthcare resources and individualization of rehabilitation treatments. Efficiency of the selected biomarker is also based on its capacity to accurately depict underlying mechanisms of disease. Moreover, it should be non-invasive, readily accessible to patients and clinicians, easily interpreted by physicians, reproducible, and cost-effective [4,5][1][2].
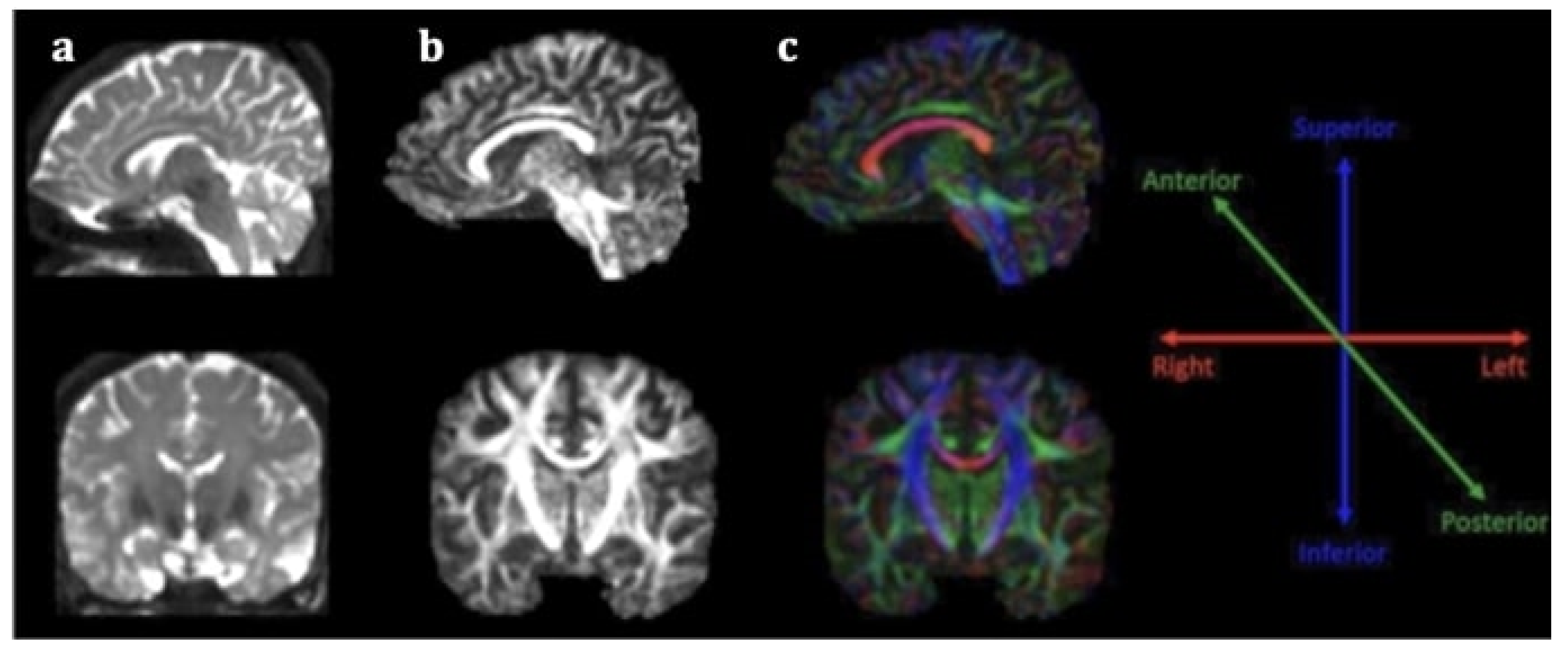
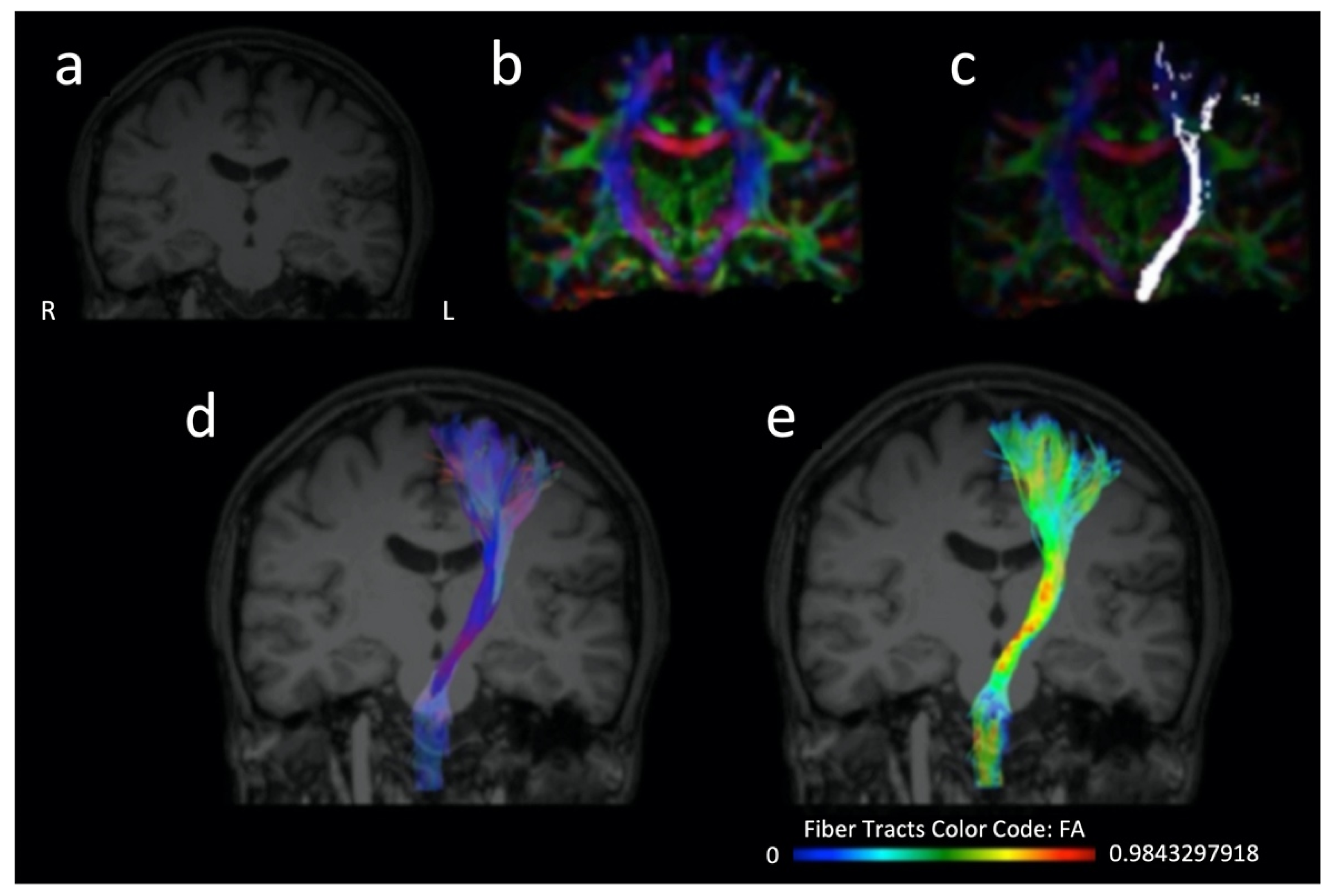
2. Diffusion Tensor Imaging as a Prognostic Tool for Recovery in Acute and Hyperacute Stroke
2.1. Prediction of Recovery Using Different DTI Parameters in Studies with Ischemic Cohorts: The Role of FA
2.2. Prediction of Recovery Using Different DTI Parameters in Studies with Ischemic Cohorts: The Role of the FA Ratio
With regards to the FA ratio (rFA), Ali and colleagues [46][19] investigated 21 patients and demonstrated that an FA ratio between the affected and unaffected side under 0.8 at admission was associated with deficient motor recovery at discharge. Additionally, they visualized WM tracts using DTI tractography and found that patients with whole involvement of pyramidal tract exhibited higher NIHSS at discharge compared with the groups with intact and partial involvement.
2.3. Prediction of Recovery Using Different DTI Parameters in Studies with Ischemic Cohorts: The Role of MD
Regarding MD in stroke outcome prognosis, in the study of Liu and colleagues [32][20] 33 patients with acute subcortical stroke were investigated with DTI focused on the integrity of the inferior cerebellar peduncles (ICP) and lower limb FM assessment within 1 week, 4 weeks and 12 weeks post-stroke. Both MD and FA in contralesional ICP showed association with lower-limb FM score changes. Etherton and colleagues [61][21] studied a cohort of 42 patients to assess the role of DTI in early neurological improvement. Normal-appearing white matter (NAWM) MD was significantly lower in the group with early neurological improvement, and in multivariable logistic regression it was an independent predictor for early neurological improvement.2.4. Prediction of Recovery Using Different DTI Parameters in Studies with Ischemic Cohorts: The Role of AD
With regards to the AD, Moulton and colleagues [52][22] examined 28 thrombolysed patients and found that the strongest independent predictor of clinical outcome was the corona radiata AD ratio (rAD), correlating with motor NIHSS scores on day 7 and with mRS at 3 months. Interestingly, FA values could not be correlated with clinical recovery. In another study of 45 patients under thrombolysis, Moulton and colleagues [18][23] reported that rAD in CST predicted long-term motor recovery, whereas rAD in AF independently predicted a 3-month aphasia outcome, thus highlighting its potential as an efficient biomarker in the acute phase of stroke.2.5. Prediction of Recovery Using Different DTI Parameters in Studies with Ischemic Cohorts: The Role of Fiber Number Ratio
As for the rFN, Jang and colleagues [55][24] studied 31 pontine infarct patients using DTI at days 7–28 and clinical evaluation at 6 months using MI, a modified Brunnstrom classification (MBC), and functional ambulation category (FAC). The rFN and the CST area ratio significantly correlated with all 6-month motor outcomes.2.6. Prediction of Recovery Using Different DTI Parameters in Studies with Hemorrhagic Cohorts: The Role of FA
Regarding DTI in prediction of recovery in hemorrhagic stroke, the majority of studies focus on FA in CST. Ma and colleagues [35][25] applied DTI imaging in a cohort of 23 hemorrhagic stroke patients at different time points, including day 0. They evaluated outcome with motor function score (MFS) 90 days post-stroke. Significant association was noted between initial FA of the affected cerebral peduncle and MFS of day 90. Additionally, the initial FA value over 0.45 was a motor outcome predictor with high sensitivity and specificity.2.7. Prediction of Recovery Using Different DTI Parameters in Studies with Hemorrhagic Cohorts: The Role of FA Ratio
The rFA of cerebral peduncles (CP) was also investigated in the study of Wang and colleagues [64][26]. They demonstrated that within 3 days the ratio of rFA (affected/unaffected side) exhibited negative correlation with the paresis grading and mRS and positive correlation with the FIM scores at the end of follow-up. On the other hand, rFA at 2 weeks had positive correlation with the FIM and negative correlation with mRS scores and PG at the end of follow-up. Notably, as compared to the DTI within 3 days of ICH onset, the application of DTI at 2 weeks after ICH was superior to DTI within 3 days in terms of accurate prediction of motor outcome and daily living activities.2.8. Prediction of Recovery Using Different DTI Parameters in Studies with Hemorrhagic Cohorts: The Role of Qualitative Assessment of CST Integrity
The role of CST integrity in relation to hand function was explored by Gong and colleagues [45][27] in a study of 75 hypertensive hemorrhage patients receiving DTI in approximately 3 weeks following stroke. It was shown that degree of CST integrity was negatively correlated with the Brunnstrom Recovery Staging-Hand (BRS-H) at 3 weeks and 3 months. In particular, patients with intact or complete disruption of CST failed to present substantial improvement in BRS-H at 3 months. On the contrary, those having partial CST impairment based on DTI were remarkably improved at 3 months in comparison to 3 weeks post-stroke.2.9. Prediction of Recovery Using Different DTI Parameters in Studies with Both Ischemic and Hemorrhagic Cohorts: The Role of FA and the FA Ratio
In a study including both hemorrhagic and ischemic stroke patients, Imura and colleagues [47][28] evaluated the most efficient DTI parameters in predicting motor outcomes and activities of daily living function. They employed FA, FN, and ADC within 10 days post-stroke. Clinical outcome was re-assessed at 1 month. Only FA of the affected CST exhibited significant correlation with the motor outcome and activities of daily living function within 10 days and at 1 month post-onset. In a similar cohort, Nakashima and colleagues [39][29] examined 17 patients with DTI and voxel-based morphometry and assessed clinical outcome at 3 months using FMA and the Motor Activity Log (MAL). In patients with incomplete CST disruption in tractography, rFA of the bilateral CP showed significant correlation with FMA, amount of use, and quality of movement. The rFA on CP was also examined in the study of Koyama and colleagues [40][30] who included 80 patients with hemorrhagic and ischemic stroke and DTI on days 14–21. Both the hemorrhagic and the infarct groups showed similar patterns of statistically significant correlations between rFA and outcome measures.3. Conclusions
In conclusion, the utility of DTI and specific parameters (e.g., FA, FA ratio, diffusivity values, and fiber number ratio) for tracking longitudinal changes and identifying prognostic correlates in acute and hyperacute stroke patients. However, it is worth mentioning that additional efforts are needed to translate the insights gained from DTI studies in stroke patients into practical applications in clinical settings and routine clinical practice. Of note, ROC curves and various machine-learning frameworks in structural imaging in other neurological and psychiatric groups suggest that DTI may have a role in predicting patients’ outcomes with adequate accuracy at a single-patient level, thus providing valuable information for patients’ therapeutic management and both short- and long-term outcomes.References
- Karatzetzou, S.; Tsiptsios, D.; Terzoudi, A.; Aggeloussis, N.; Vadikolias, K. Transcranial magnetic stimulation implementation on stroke prognosis. Neurol. Sci. 2022, 43, 873–888.
- Gkantzios, A.; Tsiptsios, D.; Karatzetzou, S.; Kitmeridou, S.; Karapepera, V.; Giannakou, E.; Vlotinou, P.; Aggelousis, N.; Vadikolias, K. Stroke and Emerging Blood Biomarkers: A Clinical Prospective. Neurol. Int. 2022, 14, 65.
- Moura, L.M.; Luccas, R.; de Paiva, J.P.Q.; Amaro, E., Jr.; Leemans, A.; Leite, C.D.C.; Otaduy, M.C.G.; Conforto, A.B. Diffusion Tensor Imaging Biomarkers to Predict Motor Outcomes in Stroke: A Narrative Review. Front. Neurol. 2019, 10, 445.
- Mori, S.; Zhang, J. Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron 2006, 51, 527–539.
- Chung, H.W.; Chou, M.C.; Chen, C.Y. Principles and limitations of computational algorithms in clinical diffusion tensor MR tractography. AJNR Am. J. Neuroradiol. 2011, 32, 3–13.
- Winklewski, P.J.; Sabisz, A.; Naumczyk, P.; Jodzio, K.; Szurowska, E.; Szarmach, A. Understanding the Physiopathology Behind Axial and Radial Diffusivity Changes-What Do We Know? Front. Neurol. 2018, 9, 92.
- Jang, S.H. The corticospinal tract from the viewpoint of brain rehabilitation. J. Rehabil. Med. 2014, 46, 193–199.
- Jang, S.H. The role of the corticospinal tract in motor recovery in patients with a stroke: A review. NeuroRehabilitation 2009, 24, 285–290.
- Puig, J.; Blasco, G.; Schlaug, G.; Stinear, C.M.; Daunis, I.E.P.; Biarnes, C.; Figueras, J.; Serena, J.; Hernandez-Perez, M.; Alberich-Bayarri, A.; et al. Diffusion tensor imaging as a prognostic biomarker for motor recovery and rehabilitation after stroke. Neuroradiology 2017, 59, 343–351.
- Bhasin, A.; Srivastava, P.; Kumaran, S.S. Correlation of DTI-Derived Measures to Therapy-Mediated Recovery after Stroke: Preliminary Findings. Neurol. India 2021, 69, 1210–1216.
- Alegiani, A.C.; MacLean, S.; Braass, H.; Siemonsen, S.; Gerloff, C.; Fiehler, J.; Cho, T.H.; Derex, L.; Hermier, M.; Berthezene, Y.; et al. Comprehensive analysis of early fractional anisotropy changes in acute ischemic stroke. PLoS ONE 2017, 12, e0188318.
- Berndt, M.T.; Purner, D.; Maegerlein, C.; Wunderlich, S.; Friedrich, B.; Zimmer, C.; Sepp, D.; Kaesmacher, J.; Boeckh-Behrens, T. Basal Ganglia versus Peripheral Infarcts: Predictive Value of Early Fiber Alterations. AJNR Am. J. Neuroradiol. 2021, 42, 264–270.
- Xia, Y.; Huang, G.; Quan, X.; Qin, Q.; Li, H.; Xu, C.; Liang, Z. Dynamic Structural and Functional Reorganizations Following Motor Stroke. Med. Sci. Monit. 2021, 27, e929092.
- Mahmoud, B.E.; Mohammad, M.E.; Serour, D.K. What can DTI add in acute ischemic stroke patients? Egypt. J. Radiol. Nucl. Med. 2019, 50, 67.
- Liu, G.; Dang, C.; Chen, X.; Xing, S.; Dani, K.; Xie, C.; Peng, K.; Zhang, J.; Li, J.; Zhang, J.; et al. Structural remodeling of white matter in the contralesional hemisphere is correlated with early motor recovery in patients with subcortical infarction. Restor. Neurol. Neurosci. 2015, 33, 309–319.
- Takenobu, Y.; Hayashi, T.; Moriwaki, H.; Nagatsuka, K.; Naritomi, H.; Fukuyama, H. Motor recovery and microstructural change in rubro-spinal tract in subcortical stroke. Neuroimage Clin. 2014, 4, 201–208.
- Keser, Z.; Sebastian, R.; Hasan, K.M.; Hillis, A.E. Right Hemispheric Homologous Language Pathways Negatively Predicts Poststroke Naming Recovery. Stroke 2020, 51, 1002–1005.
- Forkel, S.J.; de Schotten, M.T.; Dell’Acqua, F.; Kalra, L.; Murphy, D.G.; Williams, S.C.; Catani, M. Anatomical predictors of aphasia recovery: A tractography study of bilateral perisylvian language networks. Brain 2014, 137, 2027–2039.
- Ali, G.G.; Elhameed, A.M.A. Prediction of motor outcome in ischemic stroke involving the pyramidal tract using diffusion tensor imaging. Egypt. J. Radiol. Nucl. Med. 2012, 43, 25–31.
- Liu, G.; Guo, Y.; Dang, C.; Peng, K.; Tan, S.; Xie, C.; Xing, S.; Zeng, J. Longitudinal changes in the inferior cerebellar peduncle and lower limb motor recovery following subcortical infarction. BMC Neurol. 2021, 21, 320.
- Etherton, M.R.; Wu, O.; Giese, A.K.; Lauer, A.; Boulouis, G.; Mills, B.; Cloonan, L.; Donahue, K.L.; Copen, W.; Schaefer, P.; et al. White Matter Integrity and Early Outcomes After Acute Ischemic Stroke. Transl. Stroke Res. 2019, 10, 630–638.
- Moulton, E.; Amor-Sahli, M.; Perlbarg, V.; Pires, C.; Crozier, S.; Galanaud, D.; Valabregue, R.; Yger, M.; Baronnet-Chauvet, F.; Samson, Y.; et al. Axial Diffusivity of the Corona Radiata at 24 Hours Post-Stroke: A New Biomarker for Motor and Global Outcome. PLoS ONE 2015, 10, e0142910.
- Moulton, E.; Magno, S.; Valabregue, R.; Amor-Sahli, M.; Pires, C.; Lehericy, S.; Leger, A.; Samson, Y.; Rosso, C. Acute Diffusivity Biomarkers for Prediction of Motor and Language Outcome in Mild-to-Severe Stroke Patients. Stroke 2019, 50, 2050–2056.
- Jang, S.H.; Lee, J.; Lee, M.Y.; Park, S.M.; Choi, W.H.; Do, K.H. Prediction of motor outcome using remaining corticospinal tract in patients with pontine infarct: Diffusion tensor imaging study. Somatosens. Mot. Res. 2016, 33, 99–103.
- Ma, C.; Liu, A.; Li, Z.; Zhou, X.; Zhou, S. Longitudinal study of diffusion tensor imaging properties of affected cortical spinal tracts in acute and chronic hemorrhagic stroke. J. Clin. Neurosci. 2014, 21, 1388–1392.
- Wang, D.M.; Li, J.; Liu, J.R.; Hu, H.Y. Diffusion tensor imaging predicts long-term motor functional outcome in patients with acute supratentorial intracranial hemorrhage. Cerebrovasc. Dis. 2012, 34, 199–205.
- Gong, Z.; Zhang, R.; Jiang, W.; Fu, Z. Integrity of The Hand Fibers of The Corticospinal Tract Shown by Diffusion Tensor Imaging Predicts Hand Function Recovery After Hemorrhagic Stroke. J. Stroke Cerebrovasc. Dis. 2021, 30, 105447.
- Imura, T.; Nagasawa, Y.; Inagawa, T.; Imada, N.; Izumi, H.; Emoto, K.; Tani, I.; Yamasaki, H.; Ota, Y.; Oki, S.; et al. Prediction of motor outcomes and activities of daily living function using diffusion tensor tractography in acute hemiparetic stroke patients. J. Phys. Ther. Sci. 2015, 27, 1383–1386.
- Nakashima, A.; Moriuchi, T.; Mitsunaga, W.; Yonezawa, T.; Kataoka, H.; Nakashima, R.; Koizumi, T.; Shimizu, T.; Ryu, N.; Higashi, T. Prediction of prognosis of upper-extremity function following stroke-related paralysis using brain imaging. J. Phys. Ther. Sci. 2017, 29, 1438–1443.
- Koyama, T.; Koumo, M.; Uchiyama, Y.; Domen, K. Utility of Fractional Anisotropy in Cerebral Peduncle for Stroke Outcome Prediction: Comparison of Hemorrhagic and Ischemic Strokes. J. Stroke Cerebrovasc. Dis. 2018, 27, 878–885.
