You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Lindsay Dong and Version 1 by Guofeng Liu.
Dynamic covalent polymers, composed of dynamic covalent bonds (DCBs), have received increasing attention in the last decade due to their adaptive and reversible nature compared with common covalent linked polymers. Incorporating the DCBs into the polymeric material endows it with advanced performance including self-healing, shape memory property, and so forth.
- dynamic covalent chemistry
- dynamic covalent bond
- polymeric emissive material
1. Introduction
Polymers, such as plastic, rubber, fiber, and paint coating, are essential materials in our daily life [1,2,3][1][2][3]. Classical polymer materials that are mainly derived from covalent bonds are chemically inert, non-degradable, and insensitive to the surroundings, thus resulting in a fixed molecular make-up for these polymers, even under invasive stimulus, and limiting their dynamic properties. In addition, such polymeric materials suffer from both a low recyclability rate and unrepairable damage, which lead to an accompanying huge pollution of the environment. To address these problems, incorporating dynamic interaction into the polymer system becomes a good candidate. Dynamic interactions, including supramolecular interactions and dynamic covalent bonds (DCBs), can reversibly break down and build up, and well respond to external stimuli. Recently, dynamic interactions have been introduced into the polymer and endow the polymer with various advanced properties, such as self-healing, shape memory, adaptability, and stimuli-responsiveness [4,5,6][4][5][6]. Generally, there is a compromise between mechanical performance and dynamic behavior when dynamic interactions are introduced into the polymeric materials. Supramolecular interaction, possessing a low bonding energy (1–5 kJ/mol) [7], exhibits rapid exchanging character, thus endowing the polymeric materials with reversible regulation under ambient conditions. However, the mechanical properties of such polymeric materials are sacrificed, impeding the polymeric materials from being used in the engineering materials field. Compared to the weak supramolecular interactions, DCBs are types of bonding interactions with higher bonding energy. Once the DCBs being integrated into polymeric materials, the mechanical performance of such materials can be obviously improved and the dynamic behaviors of such materials can be feasibly regulated. Overall, DCBs combine the stability of covalent bonds and the adaptability of non-covalent bonds, thus enabling polymeric materials to be active when exposed to certain environment conditions.
Dynamic covalent chemistry, first proposed by Rowan et al., is related to chemical reactions carried out reversibly under conditions of equilibrium control [8]. In theory, all the chemical reactions are reversible to some extent and the reversible degree of the chemical bonds can be quantified by the thermodynamic equilibrium constant, Kθ [9]. When the Kθ of the chemical reaction is in the range from 10−7–107, the reaction can be considered an irreversible one. Otherwise, the chemical reaction can be seen as reversible. DCBs, as the vital part of dynamic covalent chemistry, are usually stable in ambient conditions while also becoming reversible under external stimulus [10,11][10][11]. For constructing DCBs, the rational regulation of bond energy and modest reversibility of bonding reaction are feasible methods. For example, by introducing a large steric hindrance group, the reaction activation energy can be reduced and it promotes the covalent bond to be effectively reversible [12]. As a special type of covalent bond, DCBs can serve as chemical/covalent linkage to connect some structure elements to synthesize polymeric materials. Such polymeric materials can be divided into two parts: (1) amorphous polymer or gel, and (2) highly crystalline covalent organic frameworks (COFs). The DCBs in the polymeric materials can be easily tuned by endowing the polymeric materials with unique properties.
Polymeric emissive materials (PEM) have aroused increasing attention owing to their potential applications in chemical sensing, information encryption, bioimaging, and organic light-emitting diodes [13,14,15,16][13][14][15][16]. Compared to the small organic molecules, the superior processability as well as mechanical properties of polymers make them more feasible to fabricate devices. Moreover, polymer-based emissive materials can display signal amplification in contrast with the small molecule system [17]. Therefore, PEMs show various superior luminescent performances which the molecular systems do not have. However, polymers are usually robust and their structures are hard to regulate, thus greatly hampering the regulation of their emission behaviors. It is helpful to solve this problem by introducing DCBs into the PEMs since the DCBs are reversible under specific condition and can further modulate the inner structure of PEMs, which makes the obtained PEMs more tunable. Although the research on DCBs-based PEMs is relatively recent, increasing attention has been devoted to this field and considerable progress has been made. To ourthe best knowledge, there are no corresponding reviewpapers about such fascinating PEMs. Hence, we summarized the progress in this field to allow readers to gain a deeper understanding of the DCBs-based PEMs. To construct the PEMs derived from DCBs, two major strategies can be demonstrated: (1) The luminescent components (aggregation-induced emission luminogens, carbon dots, and quantum dots, etc.) as the fundamental units are merged into the polymer chain via DCBs, and (2) the luminescent elements are physically doped into the dynamic covalent polymers. Because of the dynamic nature of DCBs, the luminescent units can be flexibly incorporated into the polymeric materials and therefore show unique external stimuli responsiveness, making the polymers system display multiple emissive behaviors, including tunable emission wavelength, luminescence switch on/off, emission enhancement, etc.
 The reversible condensation of imine bonds has been widely used to fabricate numerous intrinsic self-healing polymers. Recently, some luminescent self-healing polymers based on the imine bonds have been reported. The Kuo group constructed several emissive composite supramolecular self-healing polymers [27][25], in which aminated polydimethyl siloxane (PDMS) combined with triformaldedhyde benzene and the methylene diphenyl diisocyanate to form a dynamic polymer network showing highly stretchable and effective self-healing performance, and perovskite quantum dots endowed the polymer with excellent emissive behavior. Utilizing boiling water to treat composite film for 2 min, the photoluminescence of the composite polymer only decreased by 16% from its initial intensity. It showed high-quality emission even in bending and twisting state, and the emissive color could be rationally tuned by changing the compositions of the perovskite quantum dots. Thus, highly stretchable performance, emissive stability, as well as tunable fluorescence of the composite polymer, make it an alternative to fabricate wearable emission devices. The Jelinek group also reported a luminescent self-healing polymer gel based on the carbon dots [28][26]. They prepared multicolor gels by reacting carbon dots (G-C-dot, B-C-dot, CoAP-C-dot) derived from different aldehydes with branched polyethlenimine (PEI). These carbon dots in gels maintained sufficient distance to block the aggregation-induced quenching, ensuring the effective emission of gels. The photoluminescent quantum yields of the G-C-dot/PEI gel, B-C-dot/G-C-dot/PEI gel, and CoAP-C-dot/PEI gel were 4%, 2%, and 1.9%, respectively. The Commission Internationale de l’Eclairage (CIE) chromaticity coordinates of the three gels were (0.25, 0.30), (0.34, 0.43), and (0.44, 0.46), respectively, which corresponds to cyan, green, and yellow emission. Stacking the B-C-dots/G-C-dots/PEI gel and CoAP-C-dots/PEI gel together, a white emissive gel could be obtained due to the self-healing process undergone between these two gels. Utilizing the multicolor feature of quantum dots, these two self-healing polymer systems could be used in optical devices, strain sensing, etc.
The imine bonds can form under mild conditions and undergo reversible reactions under external stimuli. Owing to these properties, many dynamic materials have been fabricated via the imine DCBs. The Lehn group reported a dynamic self-sensing system by adding Zn2+ to the polyiminofluorenes [32][27].
The reversible condensation of imine bonds has been widely used to fabricate numerous intrinsic self-healing polymers. Recently, some luminescent self-healing polymers based on the imine bonds have been reported. The Kuo group constructed several emissive composite supramolecular self-healing polymers [27][25], in which aminated polydimethyl siloxane (PDMS) combined with triformaldedhyde benzene and the methylene diphenyl diisocyanate to form a dynamic polymer network showing highly stretchable and effective self-healing performance, and perovskite quantum dots endowed the polymer with excellent emissive behavior. Utilizing boiling water to treat composite film for 2 min, the photoluminescence of the composite polymer only decreased by 16% from its initial intensity. It showed high-quality emission even in bending and twisting state, and the emissive color could be rationally tuned by changing the compositions of the perovskite quantum dots. Thus, highly stretchable performance, emissive stability, as well as tunable fluorescence of the composite polymer, make it an alternative to fabricate wearable emission devices. The Jelinek group also reported a luminescent self-healing polymer gel based on the carbon dots [28][26]. They prepared multicolor gels by reacting carbon dots (G-C-dot, B-C-dot, CoAP-C-dot) derived from different aldehydes with branched polyethlenimine (PEI). These carbon dots in gels maintained sufficient distance to block the aggregation-induced quenching, ensuring the effective emission of gels. The photoluminescent quantum yields of the G-C-dot/PEI gel, B-C-dot/G-C-dot/PEI gel, and CoAP-C-dot/PEI gel were 4%, 2%, and 1.9%, respectively. The Commission Internationale de l’Eclairage (CIE) chromaticity coordinates of the three gels were (0.25, 0.30), (0.34, 0.43), and (0.44, 0.46), respectively, which corresponds to cyan, green, and yellow emission. Stacking the B-C-dots/G-C-dots/PEI gel and CoAP-C-dots/PEI gel together, a white emissive gel could be obtained due to the self-healing process undergone between these two gels. Utilizing the multicolor feature of quantum dots, these two self-healing polymer systems could be used in optical devices, strain sensing, etc.
The imine bonds can form under mild conditions and undergo reversible reactions under external stimuli. Owing to these properties, many dynamic materials have been fabricated via the imine DCBs. The Lehn group reported a dynamic self-sensing system by adding Zn2+ to the polyiminofluorenes [32][27].
 The acylhydrazone bonds can undergo reversible bond exchange reaction, resulting in the structural diversity of the polymers linked by acylhydrazone bonds. The first example of acylhdrazone component exchange within polymers was reported by Skene and Lehn [41][30]. Additionally, the luminescent properties of polymers containing acylhydrazone bonds can be regulated under different chemical stimuli. The Hirsch group reported the pH-dependent morphology and optical property of a lysine-derived polymer [45][31]. Acylhydrazone bond is one of most important dynamic linkers to construct COFs. Compared to the rotatable imine bonds, the relatively rigid acylhydrazone bonds are more feasible to form luminescent COFs. In 2016, the Wang group utilized the 2,5-bis(3-(ethylthio)propoxy)terephthalo hydrazide and 1,3,5-triformylbenzene as the fundamental units to synthesize a luminescent COF, which showed blue emission both in solution and solid state [51][32].
The acylhydrazone bonds can undergo reversible bond exchange reaction, resulting in the structural diversity of the polymers linked by acylhydrazone bonds. The first example of acylhdrazone component exchange within polymers was reported by Skene and Lehn [41][30]. Additionally, the luminescent properties of polymers containing acylhydrazone bonds can be regulated under different chemical stimuli. The Hirsch group reported the pH-dependent morphology and optical property of a lysine-derived polymer [45][31]. Acylhydrazone bond is one of most important dynamic linkers to construct COFs. Compared to the rotatable imine bonds, the relatively rigid acylhydrazone bonds are more feasible to form luminescent COFs. In 2016, the Wang group utilized the 2,5-bis(3-(ethylthio)propoxy)terephthalo hydrazide and 1,3,5-triformylbenzene as the fundamental units to synthesize a luminescent COF, which showed blue emission both in solution and solid state [51][32].
2. Polymeric Emissive Materials Based on Imine Bond
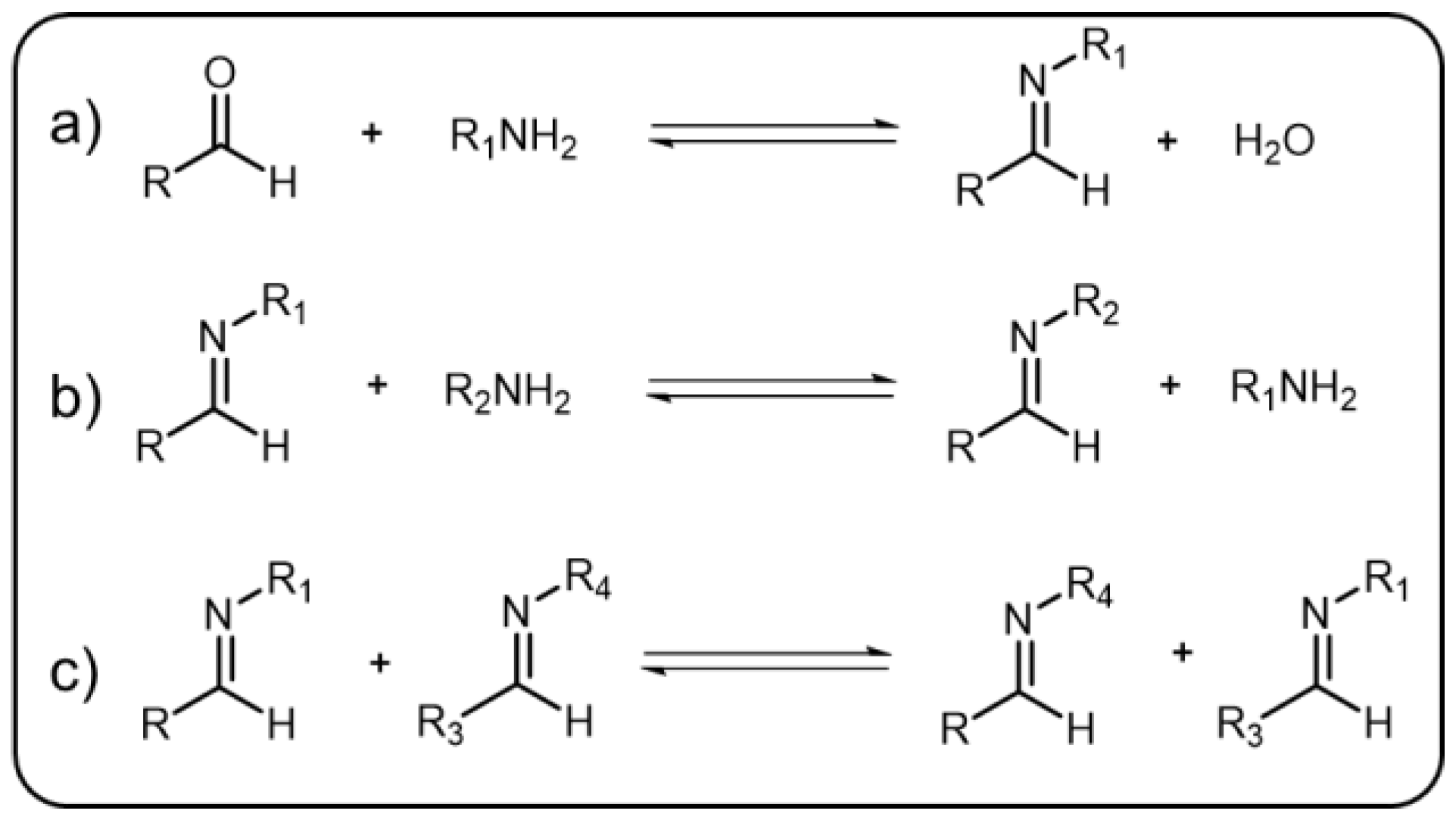
The condensation reaction, which takes place between amines and aldehydes to form imine along with a by-product of water, is a well-known organic reaction [20][18]. This reaction was named after Hugo Schiff, a well-known German chemist, as the Schiff-base reaction [21][19]. Generally, the by-product water formed in this reaction should be continuously removed since the enrichment of water would result in the equilibrium of the reaction being preferred to the opposite direction and facilitate the hydrolysis of imine. The pH value, similar to water, plays a key role for controlling the equilibrium of the reaction. In other words, intense acid pH can significantly affect the formation and break of the imine bonds, which are relatively stable under alkaline or neutral condition but hydrolyze under acid environment. Additionally, there are many other factors that can impel the reaction to go forward or backward. The equilibrium-controlled imine reactions have been divided into three types (Scheme 2Scheme 1) [20,22][18][20]: (1) Hydrolysis, in which the imine hydrolyze back to the precursor materials; (2) Exchange, in which another amine is introduced resulting in the two amines exchanging; and (3) Metathesis, in which upon introduction of a second imine, the two imines can undergo an exchange reaction of the amine to give a new imine. As a result, imine bond is considered as a representative DCB that can break and reform efficiently in a reversible manner. Due to the efficient reversible transformation, imine bond has been used as an ideal linkage as well as a stimuli-responsive element in dynamic polymers [23,24][21][22]. Other than being applied to polymers, imine-based systems are vital in supramolecular chemistry and materials science [25,26][23][24].
Scheme 21.
The three types of imine reactions: (
a
) imine condensation, (
b
) exchange, and (
c
) metathesis.
3. Polymeric Emissive Materials Based on Acylhydrazone Bond
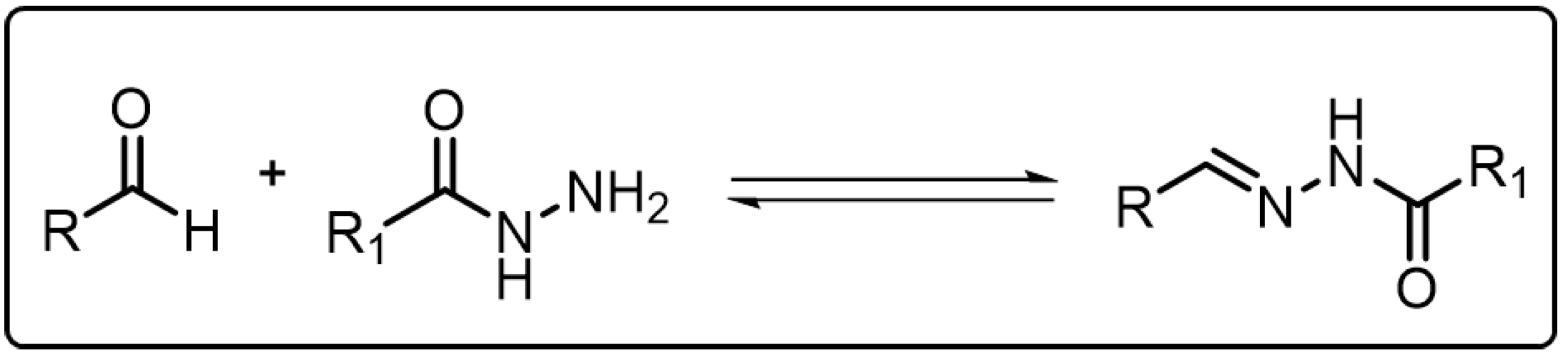
Acylhydrazone bonds, which are structurally similar to the imine bonds, are generally formed from the condensation reaction of an acylhydrazine group and a carbonyl group as shown in Scheme 3Scheme 2 [20][18]. For acylhydrazone bonds, there are acylaminos adjacent to the C=N bonds, which decrease the electrophilicity of the C=N bonds, enabling the acylhydrazone bonds to be more stable than imine bonds even in the presence of water [39][28]. However, acylhydrazone bonds can also show reversible behavior as the imine bonds do. Under neutral and basic condition, acylhydrazone bonds are robust enough. When only catalytic amount of acid is added, the acylhydrazone bond can undergo a rapid bond exchange reaction similar to the imine exchange reaction. Besides acid, anilines and their derivatives can be also used as catalysts to accelerate the exchange or metathesis reaction of acylhydrazone bond even in neutral condition. By virtue of the adaptive nature of the acylhydrazone bond, it has been widely adopted in developing versatile dynamic covalent polymers [40][29].
Scheme 32.
The formation of acylhydrazone via reversible condensation reaction.
4. Polymeric Emissive Materials Based on Boronic Ester Bond
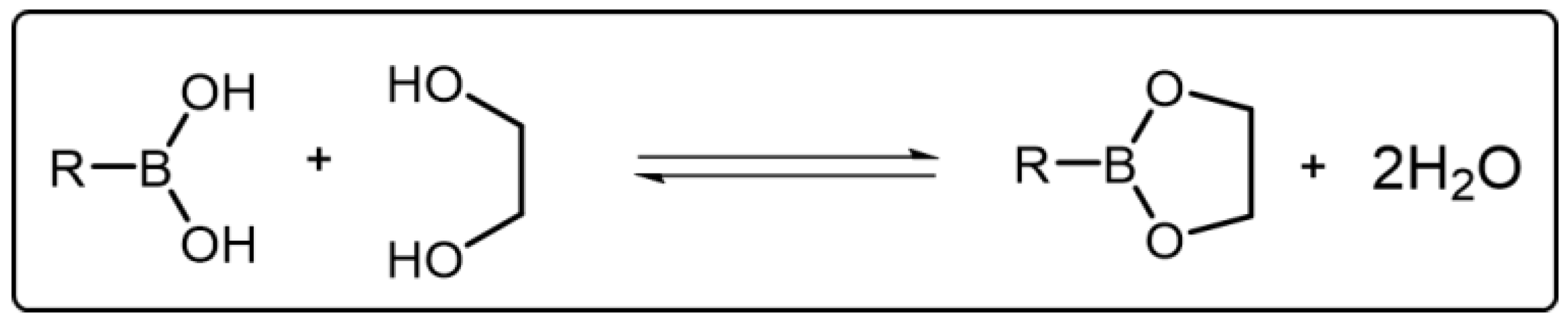
Boronic acid can bind with diol to give a cyclic boronic ester and additional water through a condensation reaction [54,55][33][34] (Scheme 4Scheme 3). In this condensation reaction, boronic acids act as Lewis acids to receive electrons while diols act as Lewis bases to donate electrons for producing boronic ester. The formation of boronic ester is usually in a reversible manner. For instance, in aqueous solution, when the pH value is above the pKa of boronic acid, the equilibrium of reaction is biased to the formation of boronic ester. On the contrary, the equilibrium of reaction is biased to the hydrolysis of boronic ester [56][35]. Thus, the exchange of the boronic ester bond can occur associatively or dissociatively by tuning the pH of the reaction condition. In other words, the boronic ester bond is reversible in response to pH. Besides pH, the dynamic balance of boronic ester bond can be also regulated by other stimuli. Boronic ester bond, when embedded into polymeric materials, similar to other DCBs, can endow the materials with advanced properties owing to its adaptivity, which extends the application prospects of such polymeric materials.
Scheme 43.
Reversible break and reform of the boronic esters between boronic acids and diols.
Boronic ester, similar to the imine or acylhydrazone bond, has been widely applied to construct COFs. The boronic esters serving as the COFs’ linkers are formed through the condensation of boronic acids and catechol derivatives. Introducing fluorescent units modified with such boronic acids and catechol derivatives, the luminescent COFs can be constructed. In 2008, Wan et al. reported the first example of a belt shaped luminescent COF, consisting of a typical fluorophore pyrene and triphenylene functionalities [62][36]. Utilizing the solvothermal method, the COF was synthesized through a condensation reaction of 2,3,6,7,10,11-hexahydroxytriphenylene and pyrene-2,7-diboronic acid. The COF could harvest a wide range of photons, due to the intramolecular singlet energy transferred from the triphenylene to the pyrene units, and showed bright luminescence. Since then, a series of luminescent COFs linked by boronic ester bonds have been reported. For example, the McGrier group constructed three luminescent COFs containing a homogeneous and heterogeneous distribution of dehydrobenzoannulene (DBA) vertex units [63][37]. These COFs were synthesized by condensation reaction of the pyrene-2,7-diboronic acid and different ratio of catechol modified DBA. The three COFs were named after Py-DBA-COF 1, Py-DBA-COF 2, and Py-MV-DBA-COF, respectively. Py-DBA-COF 1 and Py-DBA-COF 2 were formed via linking PDBA with DBA [12] and DBA [18][38], respectively, while Py-MV-DBA-COF was obtained by condensing PDBA with DBA [12] and DBA [18][38], in which the molar ratios of PDBA, DBA [12], and DBA [18][38] were 2:1:1. The Py-DBA-COF 2 showed a blue-greenish luminescent color and Py-DBA-COF 1 and Py-MV-DBA-COF were yellow emissive. Compared to the DBA [18][38]-based COFs, the lowest-energy transitions of the DBA [12]-based COFs were symmetry-forbidden in the ground state, which could be the reason why the emissive colors of these COFs were different. Besides polycyclic aromatic hydrocarbons like pyrene, AIEgens are also charming fluorophores with which to construct boronic-ester-bond-linked luminescent COFs. The Jiang group reported the first AIE-based COF which was formed by condensation of TPE-cored boronic acid and 1,2,4,5-tetrahydroxybenzene [64][39]. Incorporating TPE units into such COFs which possessed rigid environment could reduce the excitation-energy dissipation of the TPE units, resulting in high luminescence of the COF with the absolute fluorescence quantum yield up to 32%. In this regard, boronic ester bond can be considered a feasible linkage to binding more emissive elements for fabricating a series of luminescent COFs.
5. Polymeric Emissive Materials Based on Transesterification
Transesterification is a typical reversible reaction where an ester can transform into another through an associative and dissociative process of the alkoxy moiety and it has been widely used in laboratories and industries [65][40] (Scheme 5Scheme 4). In order to facilitate the transesterification reaction, elevated temperature as well as a catalyst are both necessary factors. In 2011, Leibler and coworkers developed a new kind of polymeric material which was later named vitrimer [66][41]. The vitrimer, composed of epoxy networks, could rearrange its topology and show viscosity variation through transesterification mechanism. After that, increasing attention has been attached to transesterification for exploiting more polymeric materials with ideal characters [67][42]. Nevertheless, emissive dynamic polymers based on transesterification reactions have rarely been reported. Recently, a blue emissive self-healing polymer composed of polyglycerol and itaconic anhydride was synthesized by Shan et al. and the fluorescence intensity of corresponding polymers could be selectively quenched by Fe3+ [68][43]. However, in this system there are no regular relationships between the transesterification and the fluorescence behavior of the polymer. As mentioned above, the transesterification reaction could significantly affect the mechanical performance of the polymers. Therefore, it is meaningful to realize the visualization of the transesterification in the polymer system. The Cui group reported a dynamic polymer based on transesterification which showed fluorescent color exchange as the transesterification reaction took place [69][44].
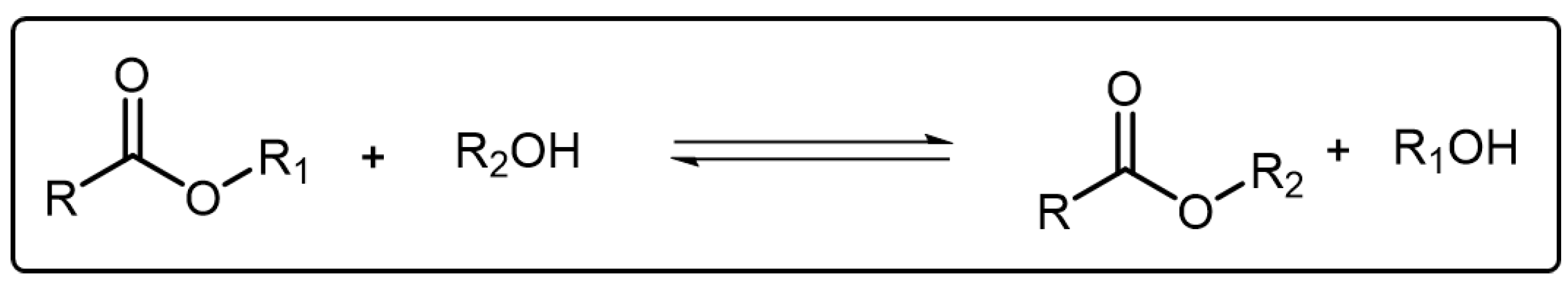

Scheme 54.
The representative reaction formula of transesterification.
6. Polymeric Emissive Materials Based on Diels–Alder Reaction
The Diels–Alder (DA) reaction, named after Diels and Alder, was first discovered in 1928 [70][45]. It refers to a [4+2] cycloaddition reaction where an electron rich diene and an electron deficient dieniphile as the reactants form a cyclohexene product (Scheme 6Scheme 5a). The DA reactions are generally reversible in nature and the retro DA reactions usually require elevated temperature to take place. The cycloaddition between funans and maleimides (Scheme 6Scheme 5b) is one of the most representative DA reactions, in which the equilibrium moves forward at room temperature but backward at about 110 °C [71][46]. Besides funans and maleimides, the DA reaction can also take place between π-extended anthracene and maleimide derivatives to form five-membered ring products which are more stable under elevated temperature compared to the cycloaddition products of funans–maleimides [72][47]. To cleave the DA bonds of anthracene–maleimide derivatives, or in other words, to trigger the retro DA reaction in anthracene–maleimide derivatives, the temperature usually needs to be heated to 200 °C. External force, similar to elevated temperature, can also trigger the retro DA reaction of anthracene–maleimide derivatives in a polymer system (Scheme 6Scheme 5c). The DA bond, as a fundamental linkage, was used to construct polymer networks dating back to 1979 [73][48]. Since then, numerous polymers based on DA bonds have been reported. The reversibility of the DA reaction has made it the earliest chemical method to fabricate dynamic covalent polymers with a self-healing property [74][49]. By embedding fluorescence moiety into the DA-based polymer, stimuli-responsive emissive materials can be developed.
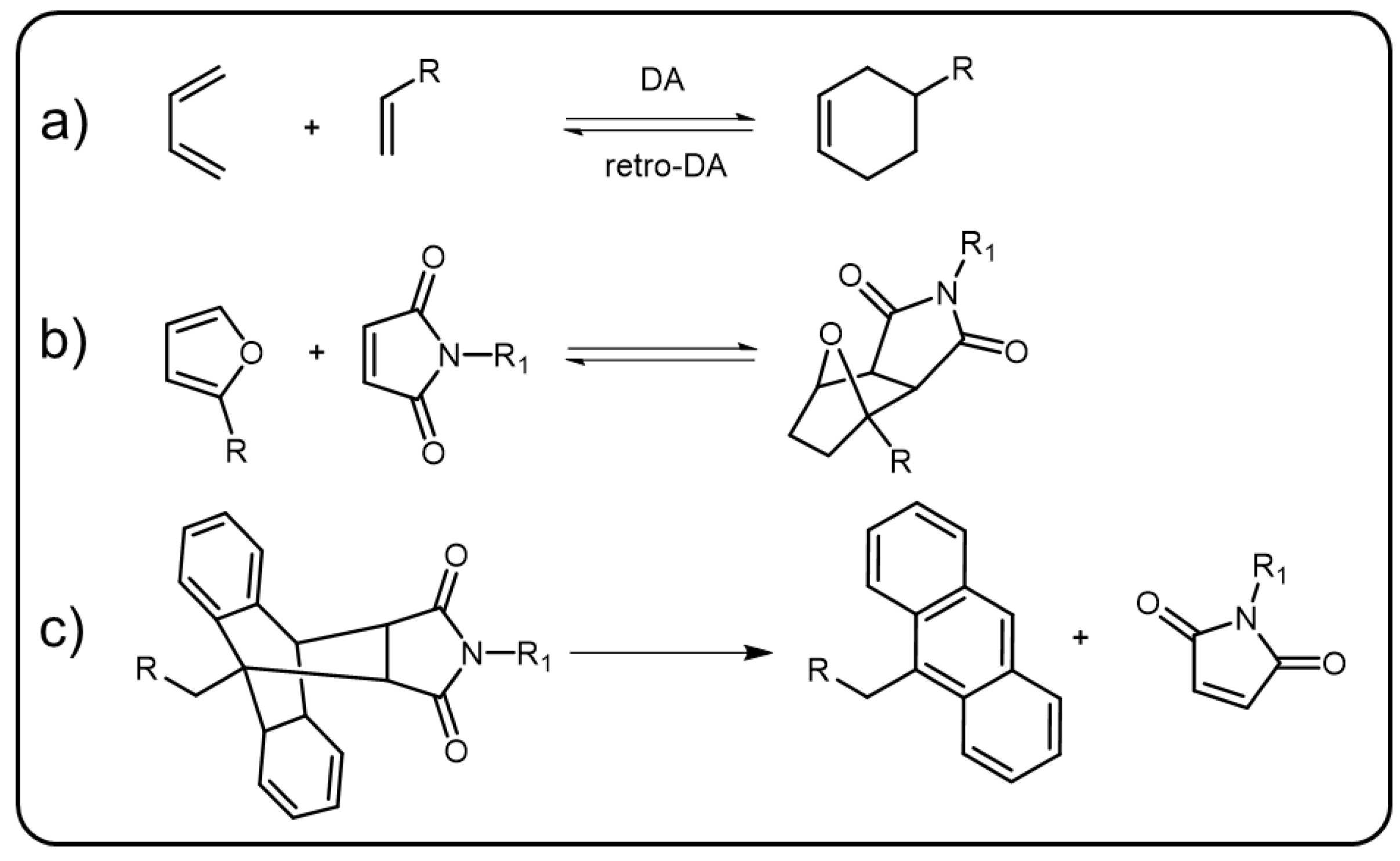

Scheme 65. (a) The general formula of reversible Diels–Alder reaction. (b) Elevated temperature-induced reversible DA reaction between furan and maleimide derivatives. (c) Force-induced retro DA reaction between π-extended anthracene and maleimide derivatives.
Due to the easy formation under mild conditions and the reversible property at elevated temperature, DA bonds have been used to develop various kinds of thermal-stimuli-responsive luminescent polymers. In 2017, Mutlu et al. reported a thermally driven self-reporting polymer released system [75][50]. In this system, pyrene units were modified to parent polymers through DA reactions. There were some nitroxide radicals which could quench the luminescence of the pyrene units linked to the polymer chains. Thus, the emission of the polymer was silent under ambient condition. When treated with elevated temperature, the pyrene units were released from the polymer, resulting in the fluorescence enhancement of this system and the fluorescence intensity maxima was located at 397 nm. Additionally, the Martin group prepared several DA based π-conjugated polymers and studied their thermal-triggered optical properties in detail. First, they grafted oligo-(phenylene ethynylene)s (OPEs)-based π-conjugated donor and acceptor to two polymer chains via DA reaction, respectively [76][51]. Mixing these two polymers and heating up to 67 °C for several days, the mixed donor–acceptor grafting polymer was obtained. Owing to the fluorescence resonance energy transfer from the donor to the acceptor, the side chain grafting polymer showed efficient emission of the acceptor even if excited by absorption maximum of the donor. They further introduced the π-conjugated donor and acceptor into the polymer main chain [77][52].
Anthracene can be seen a diene to react with suitable dienophiles (maleimide derivatives), forming [4+2] DA cycloadducts [84][53]. During the formation process of the cycloadducts, the conformation of the anthracene transforms from the planar into twist state. When external force is applied to the adducts, the retro DA reaction will occur and facilitate the anthracene to return to the planar π-extended state which is emissive nature. Due to the mechanoluminescence, anthracene–maleimide, acting as the polymer linker sensitive to mechanical force, has been gradually studied [85,86,87][54][55][56]. In 2016, linear poly(methyl acrylate) (PMA) and crosslinked poly(hexyl methacrylate) containing anthracene–maleimide were developed by the Sijbesma group [88][57]. In thise work, the π-extended anthracenes could be released from the anthracene–maleimide adducts via applying mechanical force to the polymer, both in the solution and solid state, and further showed blue emissive feature. Hence, the fluorescent π-extended anthracene releasing system could be used as a sensitive probe for mechanical force. They further introduced triplet–triplet annihilation photon upconversion (TTA-UC) into the mechanical sensing system [89][58]. 9-phenylethynylanthracene, a known emitter in TTA-UC systems, was modified to PMA and then linked to a maleimide-group-terminated PMA through DA reaction. Adding the triplet sensitizer octaethylporphyrin to the as prepared poly(hexyl methacrylate), a mechanical force induced TTA-UC emission of the 9-phenylethynylanthracene could be detected and the upconversion quantum yield was 0.0823%. Very recently, the Li group adopted the same strategy to prepare a mechanical sensing polymer showing two distinct emissions [90][59]. These two emissions derived from the anthracene and amino-substituted maleimide, respectively. The maleimide fluorophores exhibited fluorescence quenching in protic or polar solvent, enabling dual emission behavior from this single polymer system in different solvents. All these DA-bond-linked polymers demonstrated above showed mechanical trigging emission behavior which might be applied to detect the damage of functional polymers. It is interesting that the dynamic break and reform of the DA bond can adjust the conjugation, even the electron distribution of the polymeric material, which is superior to other dynamic bonds, such as imine and boronic ester bonds, for regulating the optical properties of polymers.
7. Polymeric Emissive Materials Based on Dynamic C-C Bond
C-C bonds are generally regarded as irreversible and stable. However, the triphenylmethyl dimer itself was found that could undergo break and reform reaction via reversible recombination and homolytic cleavage of a C-C bond to form triphenylmethyl radicals [91][60], which, facilitating the dynamic C-C bond, gradually come into our sight. Actually, the dynamic C-C bonds refer to reversible δ(C-C) bonds (Scheme 7Scheme 6a) which can undergo association and dissociation reaction under mild condition without catalysts. For achieving the dynamic C-C bond, the stabilization of the radical state through promoting spin-delocalization is significant. Introducing bulky substituents to protect the spin center and extending the π-systems to participate in the spin delocalization have proved to be effective methods [92,93][61][62]. As most DCBs, dynamic C-C bonds have been used as the reversible linkers of the polymers to build versatile dynamic covalent polymers. Diarylbibenzofuranone (DABBF), a typical molecular containing central dynamic C-C bond (Scheme 7Scheme 6b), has been widely incorporated into the polymer structures [94][63]. When the C-C bonds homogeneously cleave under stress, the blue radical derived from diarylbibenzofuranone can be obtained. There are some similar molecules that can also show color change behavior as their dynamic C-C bonds are cut off, such as tetraarylsuccinonitrile (TASN) (Scheme 7Scheme 6c), diarylbibenzothiophenonyl, difluorenylsuccinonitrile and diarylbiindolinone, etc. [95,96,97,98][64][65][66][67]. TASN, the unique one among these mechanochromic molecules, could present a luminescent behavior when transformed into corresponding radicals, which was first reported by the Otsuka group.
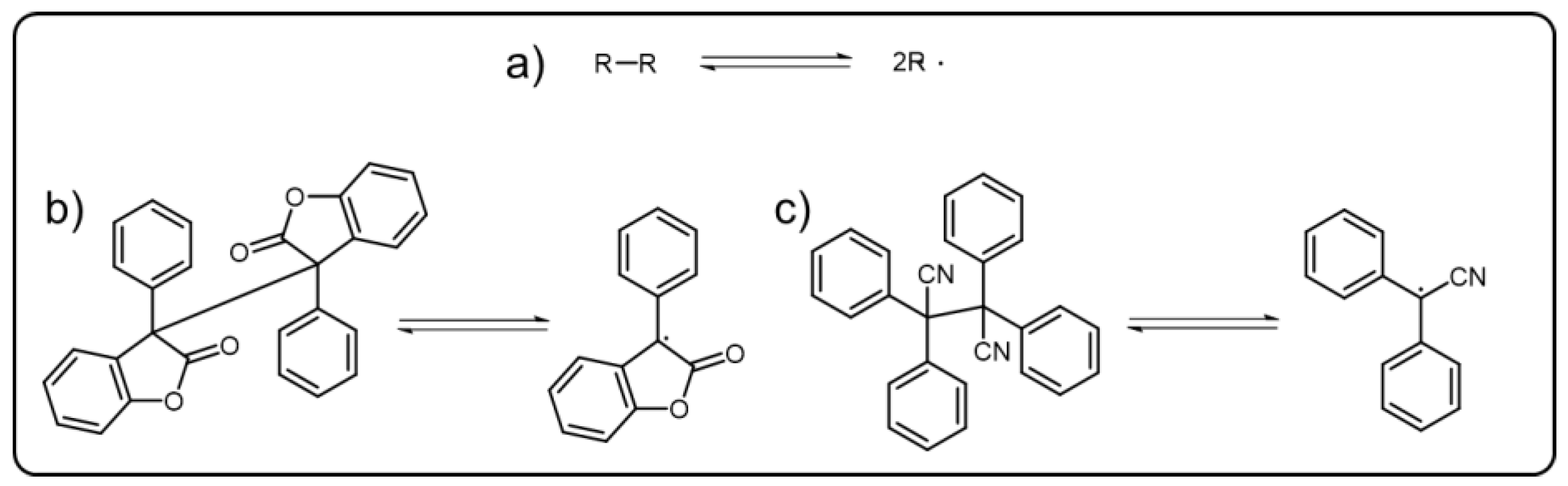
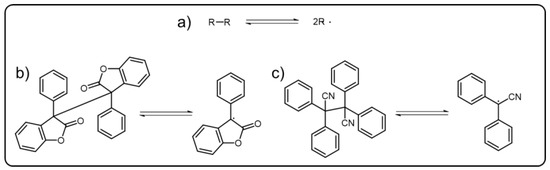


Scheme 76. (a) Schematic representation of reversible dynamic C-C bond. The typical central radicals derived from (b) DABBF and (c) TASN.
References
- Zhang, W.; Li, J.-X.; Tang, R.-C.; Zhai, A.-D. Hydrophilic and antibacterial surface functionalization of polyamide fabric by coating with polylysine biomolecule. Prog. Org. Coat. 2020, 142, 105571.
- Rai, P.; Mehrotra, S.; Priya, S.; Gnansounou, E.; Sharma, S.K. Recent advances in the sustainable design and applications of biodegradable polymers. Bioresour. Technol. 2021, 325, 124739.
- Kazmi, S.M.S.; Munir, M.J.; Wu, Y.-F. Application of waste tire rubber and recycled aggregates in concrete products: A new compression casting approach. Resour. Conserv. Recycl. 2021, 167, 105353.
- Roy, N.; Bruchmann, B.; Lehn, J.-M. Dynamers: Dynamic polymers as self-healing materials. Chem. Soc. Rev. 2015, 44, 3786–3807.
- Bei, Y.; Ma, Y.; Song, F.; Kou, Z.; Hu, L.; Bo, C.; Jia, P.; Zhou, Y. Recent progress of biomass based self-healing polymers. J. Appl. Polym. Sci. 2022, 139, 51977.
- Sattar, M.A.; Patnaik, A. Design principles of interfacial dynamic bonds in self-healing materials: What are the parameters? Chem.-Asian J. 2020, 15, 4215–4240.
- Krishnakumar, B.; Sanka, R.V.S.P.; Binder, W.H.; Parthasarthy, V.; Rana, S.; Karak, N. Vitrimers: Associative dynamic covalent adaptive networks in thermoset polymers. Chem. Eng. J. 2020, 385, 123820.
- Rowan, S.J.; Cantrill, S.J.; Cousins, G.R.L.; Sanders, J.K.M.; Stoddart, J.F. Dynamic covalent chemistry. Angew. Chem. Int. Ed. 2002, 41, 898–952.
- Zhang, Z.P.; Rong, M.Z.; Zhang, M.Q. Polymer engineering based on reversible covalent chemistry: A promising innovative pathway towards new materials and new functionalities. Prog. Polym. Sci. 2018, 80, 39–93.
- Li, Z.; Yu, R.; Guo, B. Shape-memory and self-healing polymers based on dynamic covalent bonds and dynamic noncovalent interactions: Synthesis, mechanism, and application. ACS Appl. Bio Mater. 2021, 4, 5926–5943.
- Winne, J.M.; Leibler, L.; Du Prez, F.E. Dynamic covalent chemistry in polymer networks: A mechanistic perspective. Polym. Chem. 2019, 10, 6091–6108.
- Huang, S.; Kong, X.; Xiong, Y.; Zhang, X.; Chen, H.; Jiang, W.; Niu, Y.; Xu, W.; Ren, C. An overview of dynamic covalent bonds in polymer material and their applications. Eur. Polym. J. 2020, 141, 110094.
- Wang, H.; Ji, X.; Page, Z.A.; Sessler, J.L. Fluorescent materials-based information storage. Mat. Chem. Front. 2020, 4, 1024–1039.
- Liu, Y.; Li, C.; Ren, Z.; Yan, S.; Bryce, M.R. All-organic thermally activated delayed fluorescence materials for organic light-emitting diodes. Nat. Rev. Mater. 2018, 3, 18020.
- Qian, J.; Tang, B.Z. AIE luminogens for bioimaging and theranostics: From organelles to animals. Chem 2017, 3, 56–91.
- Hu, Z.; Deibert, B.J.; Li, J. Luminescent metal-organic frameworks for chemical sensing and explosive detection. Chem. Soc. Rev. 2014, 43, 5815–5840.
- Kim, H.N.; Guo, Z.; Zhu, W.; Yoon, J.; Tian, H. Recent progress on polymer-based fluorescent and colorimetric chemosensors. Chem. Soc. Rev. 2011, 40, 79–93.
- Belowich, M.E.; Stoddart, J.F. Dynamic imine chemistry. Chem. Soc. Rev. 2012, 41, 2003–2024.
- Schiff, H. Mittheilungen aus dem universitätslaboratorium in pisa: Eine neue reihe organischer basen. Justus Liebigs Ann. Chem. 1864, 131, 118–119.
- Janica, I.; Patroniak, V.; Samori, P.; Ciesielski, A. Imine-based architectures at surfaces and interfaces: From self-assembly to dynamic covalent chemistry in 2D. Chem.-Asian J. 2018, 13, 465–481.
- Bui, R.; Brook, M.A. Dynamic covalent schiff-base silicone polymers and elastomers. Polymer 2019, 160, 282–290.
- Lee, S.-H.; Shin, S.-R.; Lee, D.-S. Self-healing of cross-linked PU via dual-dynamic covalent bonds of a Schiff base from cystine and vanillin. Mater. Des. 2019, 172, 107774.
- Liu, G.; Fu, K.; Wang, X.; Qan, C.; Liu, J.; Wang, D.; Wang, H.; Zhu, L.; Zhao, Y. One-dimensional helical aggregates organized from achiral imine-based polymers. ACS Mater. Lett. 2022, 4, 715–723.
- Wang, Y.; Liu, C.; Fu, K.; Liang, J.; Pang, S.; Liu, G. Multiple chirality inversion of pyridine Schiff-base cholesterol-based metal-organic supramolecular polymers. Chem. Commun. 2022, 58, 9520–9523.
- He, C.-L.; Liang, F.-C.; Veeramuthu, L.; Cho, C.-J.; Benas, J.-S.; Tzeng, Y.-R.; Tseng, Y.-L.; Chen, W.-C.; Rwei, A.; Kuo, C.-C. Super tough and spontaneous water-assisted autonomous self-healing elastomer for underwater wearable electronics. Adv. Sci. 2021, 8, 2102275.
- Bhattacharya, S.; Phatake, R.S.; Barnea, S.N.; Zerby, N.; Zhu, J.-J.; Shikler, R.; Lemcoff, N.G.; Jelinek, R. Fluorescent self-healing carbon dot/polymer gels. ACS Nano 2019, 13, 1433–1442.
- Giuseppone, N.; Lehn, J.M. Constitutional dynamic self-sensing in a zinc(II)/polyiminofluorenes system. J. Am. Chem. Soc. 2004, 126, 11448–11449.
- Garcia, F.; Smulders, M.M.J. Dynamic covalent polymers. J. Polym. Sci. Pol. Chem. 2016, 54, 3551–3577.
- Apostolides, D.E.; Patrickios, C.S. Dynamic covalent polymer hydrogels and organogels crosslinked through acylhydrazone bonds: Synthesis, characterization and applications. Polym. Int. 2018, 67, 627–649.
- Skene, W.G.; Lehn, J.M.P. Dynamers: Polyacylhydrazone reversible covalent polymers, component exchange, and constitutional diversity. Proc. Natl. Acad. Sci. USA 2004, 101, 8270–8275.
- Lee, S.; Kaya, C.; Jang, H.; Koch, M.; Loretz, B.; Buhler, E.; Lehr, C.-M.; Hirsch, A.K.H. pH-dependent morphology and optical properties of lysine-derived molecular biodynamers. Mat. Chem. Front. 2020, 4, 905–909.
- Ding, S.-Y.; Dong, M.; Wang, Y.-W.; Chen, Y.-T.; Wang, H.-Z.; Su, C.-Y.; Wang, W. Thioether-based fluorescent covalent organic framework for selective detection and facile removal of mercury(II). J. Am. Chem. Soc. 2016, 138, 3031–3037.
- Nishiyabu, R.; Kubo, Y.; James, T.D.; Fossey, J.S. Boronic acid building blocks: Tools for self assembly. Chem. Commun. 2011, 47, 1124–1150.
- Bull, S.D.; Davidson, M.G.; Van den Elsen, J.M.H.; Fossey, J.S.; Jenkins, A.T.A.; Jiang, Y.-B.; Kubo, Y.; Marken, F.; Sakurai, K.; Zhao, J.; et al. Exploiting the reversible covalent bonding of boronic acids: Recognition, sensing, and assembly. Acc. Chem. Res. 2013, 46, 312–326.
- Roy, D.; Cambre, J.N.; Sumerlin, B.S. Triply-responsive boronic acid block copolymers: Solution self-assembly induced by changes in temperature, pH, or sugar concentration. Chem. Commun. 2009, 16, 2106–2108.
- Wan, S.; Guo, J.; Kim, J.; Ihee, H.; Jiang, D. A belt-shaped, blue luminescent, and semiconducting covalent organic framework. Angew. Chem. Int. Ed. 2008, 47, 8826–8830.
- Crowe, J.W.; Baldwin, L.A.; McGrier, P.L. Luminescent covalent organic frameworks containing a homogeneous and heterogeneous distribution of dehydrobenzoannulene vertex units. J. Am. Chem. Soc. 2016, 138, 10120–10123.
- Ma, J.; Shu, T.; Sun, Y.; Zhou, X.; Ren, C.; Su, L.; Zhang, X. Luminescent covalent organic frameworks for biosensing and bioimaging applications. Small 2022, 18, 2103516.
- Dalapati, S.; Jin, E.; Addicoat, M.; Heine, T.; Jiang, D. Highly emissive covalent organic frameworks. J. Am. Chem. Soc. 2016, 138, 5797–5800.
- Otera, J. Transesterification. Chem. Rev. 1993, 93, 1449–1470.
- Montarnal, D.; Capelot, M.; Tournilhac, F.; Leibler, L. Silica-like malleable materials from permanent organic networks. Science 2011, 334, 965–968.
- Wang, S.; Urban, M.W. Self-healing polymers. Nat. Rev. Mater. 2020, 5, 562–583.
- Shan, Y.; Liu, T.; Zhao, B.; Hao, C.; Zhang, S.; Li, Y.; Wu, Y.; Zhang, J. A renewable dynamic covalent network based on itaconic anhydride crosslinked polyglycerol: Adaptability, UV blocking and fluorescence. Chem. Eng. J. 2020, 385, 123960.
- Wang, S.; Wang, H.; Zhang, P.; Xue, L.; Chen, J.; Cui, J. Folding fluorescent probes for self-reporting transesterification in dynamic polymer networks. Mater. Horiz. 2021, 8, 1481–1487.
- Diels, O.; Alder, K. Synthesen in der hydroaromatischen Reihe. Liebigs Ann. Chem. 1928, 460, 98–122.
- Boul, P.J.; Reutenauer, P.; Lehn, J.M. Reversible Diels-Alder reactions for the generation of dynamic combinatorial libraries. Org. Lett. 2005, 7, 15–18.
- Sun, H.; Kabb, C.P.; Dai, Y.; Hill, M.R.; Ghiviriga, I.; Bapat, A.P.; Sumerlin, B.S. Macromolecular metamorphosis via stimulus-induced transformations of polymer architecture. Nat. Chem. 2017, 9, 817–823.
- Stevens, M.P.; Jenkins, A.D. Crosslinking of polystyrene via pendant maleimide groups. J. Polym. Sci. Pol. Chem. 1979, 17, 3675–3685.
- Chen, X.X.; Dam, M.A.; Ono, K.; Mal, A.; Shen, H.B.; Nutt, S.R.; Sheran, K.; Wudl, F. A thermally re-mendable cross-linked polymeric material. Science 2002, 295, 1698–1702.
- Mutlu, H.; Schmitt, C.W.; Wedler-Jasinski, N.; Woehlk, H.; Fairfull-Smith, K.E.; Blinco, J.P.; Barner-Kowollik, C. Spin fluorescence silencing enables an efficient thermally driven self-reporting polymer release system. Polym. Chem. 2017, 8, 6199–6203.
- Ahner, J.; Micheel, M.; Koetteritzsch, J.; Dietzek, B.; Hager, M.D. Thermally triggered optical ouning of π-conjugated graft copolymers based on reversible Diels-Alder reaction. RSC Adv. 2016, 6, 98221–98227.
- Ahner, J.; Dahlke, J.; Pretzel, D.; Schubert, U.S.; Dietzek, B.; Hager, M.D. Thermally switchable fluorescence resonance energy transfer via reversible Diels-Alder Reaction of π-conjugated oligo-(phenylene ethynylene)s. Macromol. Rapid Commun. 2018, 39, 1700789.
- Li, J.; Shiraki, T.; Hu, B.; Wright, R.A.E.; Zhao, B.; Moore, J.S. Mechanophore activation at heterointerfaces. J. Am. Chem. Soc. 2014, 136, 15925–15928.
- Li, H.; Zhang, Y.; Liu, Y.; Sijbesma, R.P.; Heuts, J.P.A.; Zhang, Q. Preparation of mechanoresponsive hairy particles using polymeric surfactants in emulsion polymerization. Polym. Chem. 2017, 8, 3971–3976.
- Kabb, C.P.; O’Bryan, C.S.; Morley, C.D.; Angelini, T.E.; Sumerlin, B.S. Anthracene-based mechanophores for compression-activated fluorescence in polymeric networks. Chem. Sci. 2019, 10, 7702–7708.
- Baumann, C.; Stratigaki, M.; Centeno, S.P.; Gostl, R. Multicolor mechanofluorophores for the quantitative detection of covalent bond scission in polymers. Angew. Chem. Int. Ed. 2021, 60, 13287–13293.
- Göstl, R.; Sijbesma, R.P. π-extended anthracenes as sensitive probes for mechanical stress. Chem. Sci. 2016, 7, 370–375.
- Yildiz, D.; Baumann, C.; Mikosch, A.; Kuehne, A.J.C.; Herrmann, A.; Goestl, R. Anti-stokes stress sensing: Mechanochemical activation of triplet-triplet annihilation photon upconversion. Angew. Chem. Int. Ed. 2019, 58, 12919–12923.
- Wang, X.; Cao, Y.; Peng, Y.; Wang, L.; Hou, W.; Zhou, Y.; Shi, Y.; Huang, H.; Chen, Y.; Li, Y. Concurrent and mechanochemical activation of two distinct and latent fluorophores via retro-Diels-Alder reaction of an anthracene-aminomaleimide adduct. ACS Macro Lett. 2022, 11, 310–316.
- Gomberg, M. An instance of Trivalent carbon: Triphenylmethyl. J. Am. Chem. Soc. 1900, 22, 757–771.
- Kobashi, T.; Sakamaki, D.; Seki, S. N-substituted dicyanomethylphenyl radicals: Dynamic covalent properties and formation of stimuli-responsive cyclophanes by self-Assembly. Angew. Chem. Int. Ed. 2016, 55, 8634–8638.
- Sakamaki, D.; Ghosh, S.; Seki, S. Dynamic covalent bonds: Approaches from stable radical species. Mat. Chem. Front. 2019, 3, 2270–2282.
- Imato, K.; Otsuka, H. Reorganizable and stimuli-responsive polymers based on dynamic carbonecarbon linkages in diarylbibenzofuranones. Polymer 2018, 137, 395–413.
- Sumi, T.; Goseki, R.; Otsuka, H. Tetraarylsuccinonitriles as mechanochromophores to generate highly stable luminescent carbon-centered radicals. Chem. Commun. 2017, 53, 11885–11888.
- Sakai, H.; Sumi, T.; Aoki, D.; Goseki, R.; Otsuka, H. Thermally stable radical-type mechanochromic polymers based on difluorenylsuccinonitrile. ACS Macro Lett. 2018, 7, 1359–1363.
- Kawasaki, K.; Aoki, D.; Otsuka, H. Diarylbiindolinones as substituent-tunable mechanochromophores and their application in mechanochromic polymers. Macromol. Rapid Commun. 2020, 41, 1900460.
- Ishizuki, K.; Oka, H.; Aoki, D.; Goseki, R.; Otsuka, H. Mechanochromic polymers that turn green upon the dissociation of diarylbibenzothiophenonyl: The missing piece toward rainbow mechanochromism. Chem. Eur. J. 2018, 24, 3170–3173.
More
