Cracking is one of the main ways that concrete ages, allowing pollutants to seep within and potentially lowering the physical and mechanical strength and endurance of concrete structures. One of the healing procedures that merits research is the use of bacterially generated calcium carbonate precipitation in concrete mixtures to mend concrete cracks.
- MICP
- self-healing concrete
- bacterial concrete
- cracking
- Sealingability
1. Introduction
2. Factor Affecting the MICP
2.1. Nucleation Site
Bacteria’s importance as crystal nucleation sites [16] has long been recognized [17]. Because of their large surface-to-volume ratio, bacteria are said to behave as crystal nucleating agents, according to geological data [18][19]. The first stage in crystal formation is the molar ratio contact of metallic ions with chemically reactive compounds created by microorganisms, which are found largely in the murein [20]. These locations nucleate the accumulation of additional mineral as chemical expedite after complexation. Only tiny-grain precipitates can be produced in this location due to the narrow intervals between wall polymers, whereas the outside surface sites have no such restriction. As a result, large-grain precipitates can form if enough metal ions are available [21]. In order to promote mineral precipitation, the hydrophilic power between the bacterium and the mineral must be lower than the interaction tension between the mixture and the mineral [22]. With his staining technique, Christian Gram established the essential systematic distinction between gram-positive and gram-negative bacterial kinds. Unaware of it, he had also distinguished between the various eu-bacterial wall types and their chemical and structural composition [23][24].2.2. Bacterial Type
Based on the many metabolic pathways used by microorganisms, the MICP process can be divided into four categories: ureaolysis, denitrification, sulphate reduction, and CO2 hydrolysis mechanism [25]. Due to its effectiveness in the deposition reaction of calcium carbonate by urease bacteria, ureaolysis has been studied in considerable detail in MICP. To increase CaCO3 deposition efficiency, different urease bacteria, like Bacillus sphaericus, have their enzyme activity and production studied [26][27], including Bacillus subtilis [28][29], Bacillus megaterium [30][31], Bacillus aerius [32], Sporosarcina pasteurii [33][34], Bacillus cereus [35], and Bacillus cohnii [36][37]. Because of its efficient enzymatic activity and biosynthesis, Sporosarcina pasteurii has emerged as one of the most popular urease bacteria research respondents among them. For calcium carbonate precipitation, several genera of bacteria discussed above can be chosen based on abiotic conditions. Urease-positive bacteria, for example, are needed to produce urease, which stimulates urea decomposition in the ureaolysis biochemical process [38]. The urease enzyme, found in bacteria like Bacillus, initiates the conversion of urea to ammonia to CO2 [39]. Urease-positive bacteria can exist in a variety of places, including the human body and soil. Besides determining whether the bacteria is suitable for the metabolic route, its feasibility and efficacy in various scenarios must also be examined. The viability of microorganisms plays a crucial role in the process efficacy when MICP is used. Discovered under anoxic conditions, the activity of Sporosarcina pasteurii (NCIMB 8841) was suppressed [40]. Bacillus species, however, exhibit increased activity in a variety of environments [41]. The results show that B. sphaericus is the bacterium most frequently used in MICP operations that use a urea-fortified medium. B. mega-terium is one of the other species in this genus [42] and B. lentus [43], have also been identified as MICP-causing microorganisms.2.3. Bacterial Concentrations and Ureolytic Activity
The efficiency of the MICP process and the crystals produced are influenced by bacterial concentration and ureolytic activity. The hydrolysis of urea is a very slow process (3 × 10−10 s−1), despite the fact that the urease enzyme can greatly speed up the process (3 × 10−4 s−1) [44]. As a result, the precursor with enhanced ureolytic activity should be chosen for increased calcium carbonate generation. Aside from that, the amount of bacteria present can influence calcium carbonate formation which seal the crack result in the strength recovery provided. The number of bacterial cells participating in the fermentation process affects how quickly urea decomposes; a greater numeral of bacterial cells in the zymolysis activity leads to increased urease enzyme synthesis, which adds to MICP’s efficiency [45]. Because urease-positive bacteria are predominantly aerobic, oxygen-limiting circumstances hinder bacterial growth and calcium carbonate production [46]. Denitrifying bacteria, however, can start MICP anaerobically by utilizing nitrate as an electrophile to oxidize organic materials to produce calcium carbonate [47]. Denitrifying strains have a reduced ability to cause calcium carbonate precipitation than ureolytic bacteria, which may influence the functionality of MICP activities [46].2.4. PH
Despite the fact that microbes affect microbial decomposition by changing almost any deposition factor [22], The ability of bacteria to create an alkaline environment through a variety of biological activities has been credited as the primary function in the formation of calcium carbonate [48]. Calcium carbonate precipitation and dissolution are mediated by microbial activity in response to environmental circumstances [49]. Generally, precipitation is influenced by the saturation index (SI) [50]; Using the formula in Equation (8), which is based on the interactions of temperature, calcium hardness, total alkalinity, and pH, which is connected to pPH medium, one may calculate the saturation index, which is a number.2.5. Nutrients
Since nutrients provide the energy needed for bacterial production and biochemical activity, their availability has a significant impact on calcium carbonate bio mineralization [46][51]. Bacteria have a wide range of sources and amounts of critical components that are required for their functioning [52]. Calcium carbonate bio mineralization is dependent on the presence of free Ca2+ in the nearby habitat, in addition to the carbon source [53]. The disintegration of 1 mole of urea develops the creation of 1 mole of calcite, as demonstrated in Equations (1)–(3) and(7) [54]. It is obvious that a higher urea content enhances mineral precipitation. In addition, it has been discovered that inclusion a large amount of calcium salt reduces enzyme activity and, as a result, causes calcium carbonate precipitation [55][56][57][58][59][60]. Employing Bacillus genera in the existence of urea, [2] studied the influence of varying Ca2+ causes on the deposition of calcium carbonate. According to the authors, CaCl2 is the best calcium supply for biogenesis of calcium carbonate. The presence of soluble Ca2+ may cause a calcite production and urease functioning, which could be explained by adding ions species to the suspension media [55]. It’s also important to remember that, in order to obtain the maximum amount of calcium carbonate, reagent concentrations must be kept within acceptable ranges to prevent microbial growth inhibition [50].2.6. Temperature
The temperature of the incubation chamber is one of the most important (the incubation chamber temperature is the laboratory static and shaking incubator temperature for the bacteria growth that is kept 37 °C for effective growth of bacteria; Higher temperature may affect the growth of bacteria) operating parameters that might influence bacterial growth and, as a result, the bio-mineralization process [2]. The catalysis of urea hydrolysis, like other enzymatic reactions, is a temperature-dependent process [61]. The ideal temperature, however, lies between 20 and 37 °C, depending on the surrounding conditions and the concentrations of chemical reagents in the fermentative media [62] [63][64]. The rate of urea disintegration is increased by 5 and 10 times, respectively, when the reaction temperature is raised from 10 to 20 °C and between 10-15 °C, according to previous research [65]. In a solution medium with 0.02 gram/Liter of catalyst, it discovered that increasing the temperature from 20 to 50 °C enhances calcium carbonate production [66]. However, a relatively high temperature had a negative impact on bacterial metabolism and enzyme function [44]. Despite a consistent urease activity at comfortable temperature (35 °C), it discovered that at a high temperature (55 °C), the enzyme activity significantly declines [67].3. Tests for Assessing the Bio-Mineralized Calcium Carbonate Based SELF-Healing Concrete
Several methods for evaluating the crack self-healing effect were put forth in order to evaluate the potential of microbial self-healing concrete (MSHC) to close cracks. For instance, X-ray diffraction, scanning electron microscopy, and energy dispersive spectroscopy (XRD) [68][69] were employed to examine the shape of crystals that precipitated at the break. The ability of the bio-mineralized self-healing precipitate was further evaluated using water permeability, porosity, and chloride ion permeation resistance [70][71] and nondestructive detection methods including ultrasonic and acoustic emission were used to assess the effectiveness of bio-self-healing. concrete [72][73][74][75]. TEM analysis was used to assess the sealing ability or deposition of MICP by [76] on archeological gypsum plasters employed M. xanthus bacteria. However, characterization techniques like SEM, XRD, and water permeability are only suitable for testing in the lab and not real engineering since they cannot concurrently satisfy the demands of operability and nondestructive testing. Acoustic emission and ultrasonic technology are expected to be heavily used in practical engineering due to the advantages of simple detecting equipment, practical operation, and economical detection. The majority of MSHC research is based on previously published experiments.
However, several scientists have chosen to use measurements of the deposited minerals to confirm the encapsulation of bacteria in self-healing concrete [44][77][78][79][80]. In the realm of encapsulating materials in self-healing concrete, techniques of testing based on the restoration of mechanical characteristics by the transmission of ultrasonic waves are still unexamined. Quantification of precipitated crystals in cementitious and biochar materials has been investigated using TGA for concrete or mortar samples.
Microstructure tests are carried out using tools including scanning electron microscopes (SEMs), field-emission scanning electron microscopes (FESEMs), and X-ray diffraction to identify and describe embedded materials after self-healing (XRD) [81] used bio-cement with a variable urea-CaCl2 and bacterial cell density concentration. Based on its 40% greater compressive strength than conventional concrete, improved material finish, aesthetic qualities, and environmental effect, the authors came to the conclusion that bio-cement has potential as a sustainable design material.
ATRIR [82][83] revealed the chemical composition of the deposit, which consisted of a calcite and aragonite combination in addition to two CaCO3 polymorphs. For more confirmation of the characterization results, the XRD is frequently employed, along with SEM-EDX by various researchers. This method was utilized to search for verified healing agents in the precipitates based on macrostructural and SEM inspection. The most prevalent microstructural test used by investigators to trace deposition products in crack specimens was SEM analysis [84]. In addition, for qualitative and quantitative elemental analysis, several researchers have combined EDS with SEM [85][86][87][88]. X-ray tomography, Raman spectroscopy, and Nuclear Magnet Resonance (NMR) can be utilized to monitor and study crack healing qualitatively and quantitatively besides the existing microstructure level research [89][90]. The nanotechnology level study of self-restoration effectiveness by bacteria encased materials has yet to be completed. These tests are performed to ensure that the results of the microstructural testing are as reliable as possible. Nanoscale tests should be performed to determine the bonding strength within the fractures at the interface between the deposited minerals and the cement substrate [91].
4. Sealing Ability and Recovery of Mechanical and Durability Properties
The crack width healed, the precipitate’s bonding with the mixture and structure, and the precipitate’s strength would all influence the restoration of original concrete properties. For efficient self-healing, properties that are as close to those of the original concrete as possible are desirable. The capability of bio-based self-healing concrete to heal is dependent on a variety of parameters, such as the curing condition, concentrations of doable spores and nutrients, the age of the concrete, and the amount of time it takes for the concrete to heal. Healing can occur in two different formats: calcium carbonate precipitation to close the fractures, and carbon dioxide produced by bacteria metabolism reacting with unreacted portlandite at the crack region to make more deposits. The use of a bio-based restoring chemical was found to cure a wide variety of fracture widths. Although it is dependent on a variety of conditions, healing efficiency is optimal when crack width is kept between 100 and 200 µm.4.1. Recovery of Mechanical Properties
Wang [92] used glass tubes to encapsulate bacteria cells in PU and silica gel. The crack width used to measure mechanical strength recovery was around 0.35 mm. In the case of silica gel, there was more calcium carbonate precipitation, but only around 5% strength recovery. PU-containing specimens recovered between 50 and 80 percent of their potency. Conversely, the bacterial role in regaining mechanical strength was questioned because the strength recovery for live and dead bacteria cells was not significantly different. The precipitate quantity was larger in silica gel than in PU, although PU had a higher strength recovery. It’s reasonable to assume that PU, as an excellent sealing agent, played a major role in the mechanical strength recovery.
4.2. Recovery of Durability Properties
In concrete, effective self-healing means that the durability and mechanical strength are totally or almost restored to the original specimen. Water permeability and water absorption tests are frequently used to assess durability. Healing cracks also entails sealing any voids or linked pores through which foreign chemicals from the air or water could enter. As a result, water permeability and absorption are reduced. Pore blockage by calcium carbonate, which has a relatively low solubility, causes absolute permeability reduction through bacterial action [93][94][95]. Wang [96] used hydrogel as an encapsulation for bacteria spores and bio-reagents and reported a significant reduction in permeability of about 68 percent. Even for 0.3–0.4 mm cracks, the maximum crack size of 0.5 mm was repaired, albeit there was a wide range of healing ratios (40–90%). However, when compared to when only hydrogel was used, there is an improvement. Due to the proportionate dispersion of spores and bio reagents when encased along in hydrogel, better healing may be expected. Because of this encapsulation approach, bacteria would have fast reach to nutrients and precursor compounds in the case of cracking. Furthermore, in addition to bacterial precipitation, some autogenous healing helped by internal hydrogel curing may improve permeability reduction.5. Field Application of Bio-Mineralized Self-Healing Concrete
To test the capacity of Sporosarcina pasteurii cells to self-heal, broken rock was repaired with the cells [97]. In 17 h of handling, a sizable amount of calcite precipitated (around 750 gramme), and a water permeability test of a single fracture over a large area revealed a significant reduction. These results suggested that MICP can be utilised to reduce the porosity of cracked rock in practise, suggesting that a MICP-based strategy would be a suitable choice for reducing unwelcome groundwater flow through fractured channels. In another investigation, investigators used bacteria to treat the faces of limestone at various temperatures in attempt to discover the optimal microorganism for practical use [98]. The limestone was reinforced to depths of 30 mm after a surface modification with bacteria twice in 12 h. The treatment cost was reduced to within the reach of common consolidates by optimizing urease dosages and carbonate precursor solutions. Subsurface drilled well fluid leaking in typical oil and gas exploitation or carbon capture technologies can be stopped by using the MICP a novel way by sealing gaps and porosity and lowering the system’s liquid penetration. So because activation solutions used in MICP-based sealants are water-soluble and have lower viscosities than those used in cement-based sealants, they are simpler to move into the consolidation deposit and are therefore more appropriate for usage with cracked structures. The study used conventional fluid conveyance methods to treat sandstone strata fractures 340.8 m below ground level with a S. pasteurii culture and a urea-calcium mixture (packer and bailer). Leading to decreased in insertion rate of flow from 1.9 to 0.47 L/min and a reduction in well pressure gradient from more than 30 percent to 7 percent. Moreover, following MICP therapy, the crack extension stress while re-fracturing improved in comparison to before MICP administration. The applications for which bio-concrete might be especially advantageous are shown in Figure 1. These applications’ main goals were to cut maintenance costs and prevent water infiltration. Additional uses included those for hard-to-reach locations, the nuclear sector, water-retaining structures, and airports [99].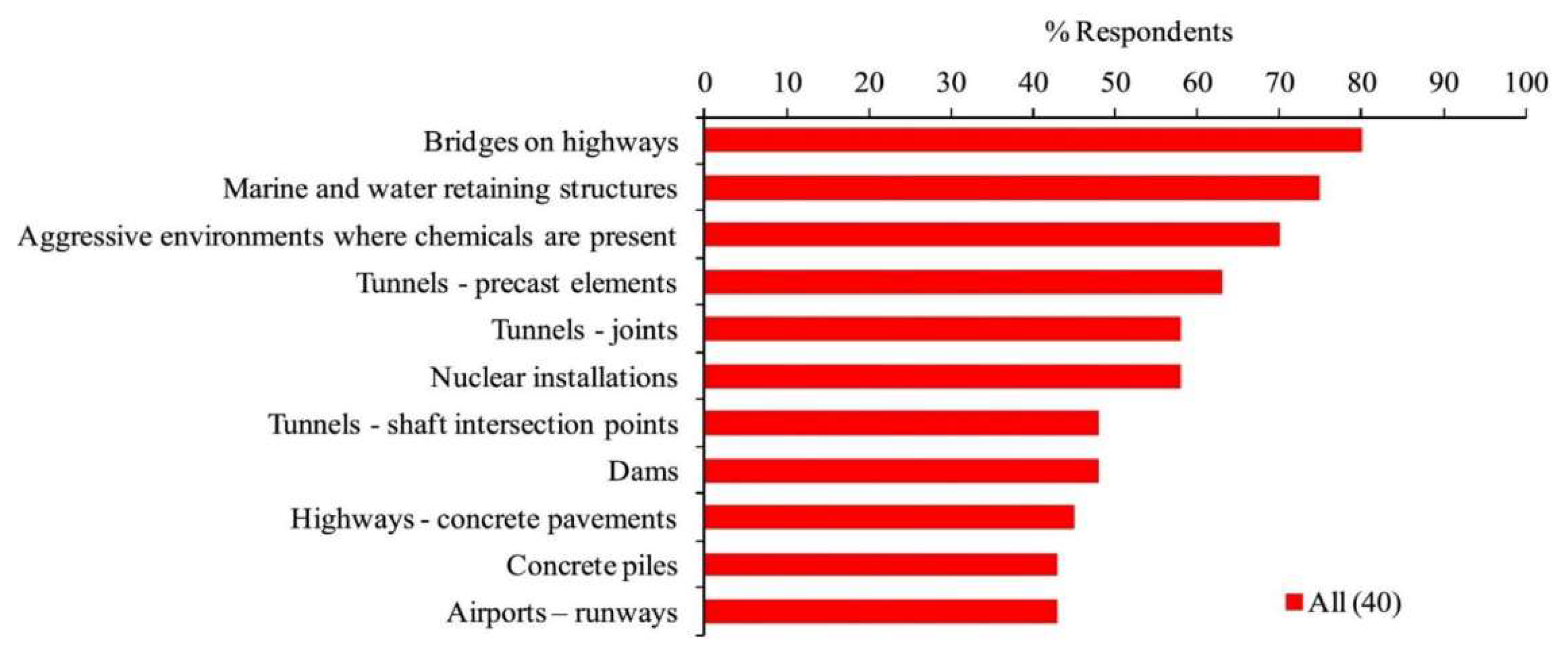
References
- Horszczaruk, E.; Sikora, P.; Cendrowski, K.; Mijowska, E.J.C.; Materials, B. The effect of elevated temperature on the properties of cement mortars containing nanosilica and heavyweight aggregates. Constr. Build. Mater. 2017, 137, 420–431.
- Seifan, M.; Samani, A.K.; Berenjian, A. Induced calcium carbonate precipitation using Bacillus species. Appl. Microbiol. Biotechnol. 2016, 100, 9895–9906.
- Luhar, S.; Luhar, I.; Shaikh, F.U.A. A Review on the Performance Evaluation of Autonomous Self-Healing Bacterial Concrete: Mechanisms, Strength, Durability, and Microstructural Properties. J. Compos. Sci. 2022, 6, 23.
- Chapman, J.; Regan, F.; Sullivan, T. Nanoparticles in Anti-Microbial Materials: Use and Characterisation; Royal Society of Chemistry: London, UK, 2012; Volume 23.
- Biondini, F.; Camnasio, E.; Palermo, A. Lifetime seismic performance of concrete bridges exposed to corrosion. Struct. Infrastruct. Eng. 2014, 10, 880–900.
- Virmani, Y.P.; Clemena, G.G. Corrosion Protection: Concrete Bridges; Federal Highway Administration: Washington, DC, USA, 1998.
- Müller, H.S.; Haist, M.; Vogel, M. Assessment of the sustainability potential of concrete and concrete structures considering their environmental impact, performance and lifetime. Constr. Build. Mater. 2014, 67, 321–337.
- Roels, E.; Terryn, S.; Iida, F.; Bosman, A.W.; Norvez, S.; Clemens, F.; Van Assche, G.; Vanderborght, B.; Brancart, J. Processing of Self-Healing Polymers for Soft Robotics. Adv. Mater. 2022, 34, 2104798.
- Palmer, F. Using Emergent Technologies to Develop Sustainable Architectural Composites. Ph.D. Thesis, Auckland University of Technology, Auckland, New Zealand, 2009.
- Ismail, A.; Adan, N.H. Effect of oxygen concentration on corrosion rate of carbon steel in seawater. Am. J. Eng. Res. 2014, 3, 64–67.
- Andrade, C.; Alonso, C.; Sarria, J. Influence of relative humidity and temperature on-site corrosion rates. Mater. Constr. 1998, 48, 5–17.
- Shevtsov, D.; Cao, N.L.; Nguyen, V.C.; Nong, Q.Q.; Le, H.Q.; Nguyen, D.A.; Zartsyn, I.; Kozaderov, O.J.S. Progress in Sensors for Monitoring Reinforcement Corrosion in Reinforced Concrete Structures—A Review. Sensors 2022, 22, 3421.
- Osterminski, K. Zur Voll-Probabilistischen Modellierung der Korrosion von Stahl in Beton: Ein Beitrag zur Dauerhaftigkeitsbemessung von Stahlbetonbauteilen. Ph.D. Thesis, Technische Universität München, München, Germany, 2013.
- Xian, X.; Zhang, D.; Lin, H.; Shao, Y. Ambient pressure carbonation curing of reinforced concrete for CO2 utilization and corrosion resistance. J. CO2 Util. 2022, 56, 101861.
- Tuutti, K. Corrosion of Steel in Concrete; Cement-Och Betonginst: Stockholm, Schwedisch, 1982.
- Zhang, J.; Shi, X.; Chen, X.; Huo, X.; Yu, Z. Microbial-Induced Carbonate Precipitation: A Review on Influencing Factors and Applications. Adv. Civ. Eng. 2021, 2021, 9974027.
- Jain, S.; Fang, C.; Achal, V. A critical review on microbial carbonate precipitation via denitrification process in building materials. Bioengineered 2021, 12, 7529–7551.
- Ferris, G.R.; Frink, D.D.; Galang, M.C.; Zhou, J.; Kacmar, K.M.; Howard, J.L. Perceptions of organizational politics: Prediction, stress-related implications, and outcomes. Hum. Relat. 1996, 49, 233–266.
- Warren, L.A.; Haack, E.A. Biogeochemical controls on metal behaviour in freshwater environments. Earth-Sci. Rev. 2001, 54, 261–320.
- Chuo, S.C.; Mohamed, S.F.; Mohd Setapar, S.H.; Ahmad, A.; Jawaid, M.; Wani, W.A.; Yaqoob, A.A.; Mohamad Ibrahim, M.N. Insights into the current trends in the utilization of bacteria for microbially induced calcium carbonate precipitation. Materials 2020, 13, 4993.
- Walker, S.; Flemming, C.; Ferris, F.; Beveridge, T.; Bailey, G. Physicochemical interaction of Escherichia coli cell envelopes and Bacillus subtilis cell walls with two clays and ability of the composite to immobilize heavy metals from solution. Appl. Environ. Microbiol. 1989, 55, 2976–2984.
- Hammes, F.; Verstraete, W. Key roles of pH and calcium metabolism in microbial carbonate precipitation. Rev. Environ. Sci. Biotechnol. 2002, 1, 3–7.
- Beveridge, T.; Davies, J.A. Cellular responses of Bacillus subtilis and Escherichia coli to the Gram stain. J. Bacteriol. 1983, 156, 846–858.
- Salton, M.R.J. The relationship between the nature of the cell wall and the Gram stain. Microbiology 1963, 30, 223–235.
- Zhu, J.; Shen, D.; Jin, B.; Wu, S. Theoretical investigation on the formation mechanism of carbonate ion in microbial self-healing concrete: Combined QC calculation and MD simulation. Constr. Build. Mater. 2022, 342, 128000.
- Wang, J.; Snoeck, D.; Van Vlierberghe, S.; Verstraete, W.; De Belie, N. Application of hydrogel encapsulated carbonate precipitating bacteria for approaching a realistic self-healing in concrete. Constr. Build. Mater. 2014, 68, 110–119.
- Wang, J.; Jonkers, H.M.; Boon, N.; De Belie, N. Bacillus sphaericus LMG 22257 is physiologically suitable for self-healing concrete. Appl. Microbiol. Biotechnol. 2017, 101, 5101–5114.
- Shahid, S.; Aslam, M.A.; Ali, S.; Zameer, M.; Faisal, M. Self-healing of cracks in concrete using Bacillus strains encapsulated in sodium alginate beads. ChemistrySelect 2020, 5, 312–323.
- Khaliq, W.; Ehsan, M.B. Crack healing in concrete using various bio influenced self-healing techniques. Constr. Build. Mater. 2016, 102, 349–357.
- Andalib, R.; Abd Majid, M.Z.; Hussin, M.W.; Ponraj, M.; Keyvanfar, A.; Mirza, J.; Lee, H.-S. Optimum concentration of Bacillus megaterium for strengthening structural concrete. Constr. Build. Mater. 2016, 118, 180–193.
- Dhami, N.K.; Reddy, M.S.; Mukherjee, A. Improvement in strength properties of ash bricks by bacterial calcite. Ecol. Eng. 2012, 39, 31–35.
- Siddique, R.; Singh, K.; Singh, M.; Corinaldesi, V.; Rajor, A. Properties of bacterial rice husk ash concrete. Constr. Build. Mater. 2016, 121, 112–119.
- Chahal, N.; Siddique, R.; Rajor, A. Influence of bacteria on the compressive strength, water absorption and rapid chloride permeability of concrete incorporating silica fume. Constr. Build. Mater. 2012, 37, 645–651.
- Raut, S.H.; Sarode, D.; Lele, S.S. Biocalcification using B. pasteurii for strengthening brick masonry civil engineering structures. World J. Microbiol. Biotechnol. 2014, 30, 191–200.
- Castanier, S.; Le Métayer-Levrel, G.; Perthuisot, J.-P. Ca-carbonates precipitation and limestone genesis—The microbiogeologist point of view. Sediment. Geol. 1999, 126, 9–23.
- Jonkers, H.M.; Thijssen, A.; Muyzer, G.; Copuroglu, O.; Schlangen, E. Application of bacteria as self-healing agent for the development of sustainable concrete. Ecol. Eng. 2010, 36, 230–235.
- Zhang, J.; Liu, Y.; Feng, T.; Zhou, M.; Zhao, L.; Zhou, A.; Li, Z. Immobilizing bacteria in expanded perlite for the crack self-healing in concrete. Constr. Build. Mater. 2017, 148, 610–617.
- Seifan, M.; Sarabadani, Z.; Berenjian, A. Development of an innovative urease-aided self-healing dental composite. Catalysts 2020, 10, 84.
- Stocks-Fischer, S.; Galinat, J.K.; Bang, S.S. Microbiological precipitation of CaCO3. Soil Biol. Biochem. 1999, 31, 1563–1571.
- Tomalia, D.A.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. A new class of polymers: Starburst-dendritic macromolecules. Polym. J. 1985, 17, 117–132.
- Seifan, M.; Samani, A.K.; Berenjian, A. New insights into the role of pH and aeration in the bacterial production of calcium carbonate (CaCO3). Appl. Microbiol. Biotechnol. 2017, 101, 3131–3142.
- Achal, V.; Pan, X.; Zhang, D. Remediation of copper-contaminated soil by Kocuria flava CR1, based on microbially induced calcite precipitation. Ecol. Eng. 2011, 37, 1601–1605.
- Ning, F.; Cong, W.; Qiu, J.; Wei, J.; Wang, S. Additive manufacturing of carbon fiber reinforced thermoplastic composites using fused deposition modeling. Compos. Part B Eng. 2015, 80, 369–378.
- De Belie, N.; Gruyaert, E.; Al-Tabbaa, A.; Antonaci, P.; Baera, C.; Bajare, D.; Darquennes, A.; Davies, R.; Ferrara, L.; Jefferson, T. A review of self-healing concrete for damage management of structures. Adv. Mater. Interfaces 2018, 5, 1800074.
- Krajewska, B. Urease-aided calcium carbonate mineralization for engineering applications: A review. J. Adv. Res. 2018, 13, 59–67.
- Seifan, M.; Berenjian, A. Application of microbially induced calcium carbonate precipitation in designing bio self-healing concrete. World J. Microbiol. Biotechnol. 2018, 34, 168.
- Algaifi, H.A.; Bakar, S.A.; Sam, A.R.M.; Ismail, M.; Abidin, A.R.Z.; Shahir, S.; Altowayti, W.A.H. Insight into the role of microbial calcium carbonate and the factors involved in self-healing concrete. Constr. Build. Mater. 2020, 254, 119258.
- De Muynck, W.; De Belie, N.; Verstraete, W. Microbial carbonate precipitation in construction materials: A review. Ecol. Eng. 2010, 36, 118–136.
- Kempe, S.; Kazmierczak, J. The role of alkalinity in the evolution of ocean chemistry, organization of living systems, and biocalcification processes. Bull. Inst. Océanograph. 1994, 13, 61–117.
- Gowthaman, S.; Iki, T.; Nakashima, K.; Ebina, K.; Kawasaki, S. Feasibility study for slope soil stabilization by microbial induced carbonate precipitation (MICP) using indigenous bacteria isolated from cold subarctic region. SN Appl. Sci. 2019, 1, 1480.
- Seifan, M.; Berenjian, A. Microbially induced calcium carbonate precipitation: A widespread phenomenon in the biological world. Appl. Microbiol. Biotechnol. 2019, 103, 4693–4708.
- Nannipieri, P.; Ascher, J.; Ceccherini, M.; Landi, L.; Pietramellara, G.; Renella, G. Microbial diversity and soil functions. Eur. J. Soil Sci. 2003, 54, 655–670.
- Rusznyák, A.; Akob, D.M.; Nietzsche, S.; Eusterhues, K.; Totsche, K.U.; Neu, T.R.; Frosch, T.; Popp, J.; Keiner, R.; Geletneky, J. Calcite biomineralization by bacterial isolates from the recently discovered pristine karstic Herrenberg cave. Appl. Environ. Microbiol. 2012, 78, 1157–1167.
- Li, Y.H.; Chen, Y.Y.M.; Burne, R.A. Regulation of urease gene expression by Streptococcus salivarius growing in biofilms. Environ. Microbiol. 2000, 2, 169–177.
- Gorospe, C.M.; Han, S.-H.; Kim, S.-G.; Park, J.-Y.; Kang, C.-H.; Jeong, J.-H.; So, J.-S. Effects of different calcium salts on calcium carbonate crystal formation by Sporosarcina pasteurii KCTC 3558. Biotechnol. Bioprocess Eng. 2013, 18, 903–908.
- Nemati, M.; Greene, E.; Voordouw, G. Permeability profile modification using bacterially formed calcium carbonate: Comparison with enzymic option. Process Biochem. 2005, 40, 925–933.
- Rivadeneyra, M.A.; Delgado, G.; Soriano, M.; Ramos-Cormenzana, A.; Delgado, R. Precipitation of carbonates by Nesterenkonia halobia in liquid media. Chemosphere 2000, 41, 617–624.
- Sadeghzadeh, M.; Maddah, H.; Ahmadi, M.H.; Khadang, A.; Ghazvini, M.; Mosavi, A.; Nabipour, N. Prediction of thermo-physical properties of TiO2-Al2O3/water nanoparticles by using artificial neural network. Nanomaterials 2020, 10, 697.
- Shabani, S.; Samadianfard, S.; Sattari, M.T.; Mosavi, A.; Shamshirband, S.; Kmet, T.; Várkonyi-Kóczy, A.R. Modeling pan evaporation using Gaussian process regression K-nearest neighbors random forest and support vector machines; comparative analysis. Atmosphere 2020, 11, 66.
- Nabipour, N.; Mosavi, A.; Hajnal, E.; Nadai, L.; Shamshirband, S.; Chau, K.-W. Modeling climate change impact on wind power resources using adaptive neuro-fuzzy inference system. Eng. Appl. Comput. Fluid Mech. 2020, 14, 491–506.
- Mitchell, A.C.; Ferris, F.G. The coprecipitation of Sr into calcite precipitates induced by bacterial ureolysis in artificial groundwater: Temperature and kinetic dependence. Geochim. Cosmochim. Acta 2005, 69, 4199–4210.
- Okwadha, G.D.; Li, J. Optimum conditions for microbial carbonate precipitation. Chemosphere 2010, 81, 1143–1148.
- Mosavi, A.; Sajedi Hosseini, F.; Choubin, B.; Goodarzi, M.; Dineva, A.A.; Rafiei Sardooi, E. Ensemble boosting and bagging based machine learning models for groundwater potential prediction. Water Resour. Manag. 2021, 35, 23–37.
- Ghalandari, M.; Shamshirband, S.; Mosavi, A.; Chau, K.-W. Flutter speed estimation using presented differential quadrature method formulation. Eng. Appl. Comput. Fluid Mech. 2019, 13, 804–810.
- Ferris, F.G.; Phoenix, V.; Fujita, Y.; Smith, R. Kinetics of calcite precipitation induced by ureolytic bacteria at 10 to 20 C in artificial groundwater. Geochim. Cosmochim. Acta 2004, 68, 1701–1710.
- Nemati, M.; Voordouw, G. Modification of porous media permeability, using calcium carbonate produced enzymatically in situ. Enzym. Microb. Technol. 2003, 33, 635–642.
- Dhami, N.K.; Reddy, M.S.; Mukherjee, A. Synergistic role of bacterial urease and carbonic anhydrase in carbonate mineralization. Appl. Biochem. Biotechnol. 2014, 172, 2552–2561.
- Zamani, M.; Nikafshar, S.; Mousa, A.; Behnia, A. Bacteria encapsulation using synthesized polyurea for self-healing of cement paste. Constr. Build. Mater. 2020, 249, 118556.
- Pang, B.; Zhou, Z.; Hou, P.; Du, P.; Zhang, L.; Xu, H. Autogenous and engineered healing mechanisms of carbonated steel slag aggregate in concrete. Constr. Build. Mater. 2016, 107, 191–202.
- Zhang, X.; Jin, Z.; Li, M.; Qian, C. Effects of carrier on the performance of bacteria-based self-healing concrete. Constr. Build. Mater. 2021, 305, 124771.
- Qian, C.; Zhang, Y.; Xie, Y. Effect of ion concentration in crack zone on healing degree of microbial self-healing concrete. Constr. Build. Mater. 2021, 286, 122969.
- Qian, C.; Zheng, T.; Zhang, X.; Su, Y. Application of microbial self-healing concrete: Case study. Constr. Build. Mater. 2021, 290, 123226.
- Aggelis, D.; Leonidou, E.; Matikas, T.E. Subsurface crack determination by one-sided ultrasonic measurements. Cem. Concr. Compos. 2012, 34, 140–146.
- Shamshirband, S.; Mosavi, A.; Rabczuk, T.; Nabipour, N.; Chau, K.-W. Prediction of significant wave height; comparison between nested grid numerical model, and machine learning models of artificial neural networks, extreme learning and support vector machines. Eng. Appl. Comput. Fluid Mech. 2020, 14, 805–817.
- Mousavi, S.M.; Ghasemi, M.; Dehghan Manshadi, M.; Mosavi, A. Deep learning for wave energy converter modeling using long short-term memory. Mathematics 2021, 9, 871.
- Jroundi, F.; Gonzalez-Muñoz, M.T.; Garcia-Bueno, A.; Rodriguez-Navarro, C. Consolidation of archaeological gypsum plaster by bacterial biomineralization of calcium carbonate. Acta Biomater. 2014, 10, 3844–3854.
- Xu, J.; Yao, W. Multiscale mechanical quantification of self-healing concrete incorporating non-ureolytic bacteria-based healing agent. Cem. Concr. Res. 2014, 64, 1–10.
- Taherei Ghazvinei, P.; Hassanpour Darvishi, H.; Mosavi, A.; Yusof, K.b.W.; Alizamir, M.; Shamshirband, S.; Chau, K.-W. Sugarcane growth prediction based on meteorological parameters using extreme learning machine and artificial neural network. Eng. Appl. Comput. Fluid Mech. 2018, 12, 738–749.
- Asadi, E.; Isazadeh, M.; Samadianfard, S.; Ramli, M.F.; Mosavi, A.; Nabipour, N.; Shamshirband, S.; Hajnal, E.; Chau, K.-W. Groundwater quality assessment for sustainable drinking and irrigation. Sustainability 2019, 12, 177.
- Kalbasi, R.; Jahangiri, M.; Mosavi, A.; Dehshiri, S.J.H.; Dehshiri, S.S.H.; Ebrahimi, S.; Etezadi, Z.A.-S.; Karimipour, A. Finding the best station in Belgium to use residential-scale solar heating, one-year dynamic simulation with considering all system losses: Economic analysis of using ETSW. Sustain. Energy Technol. Assess. 2021, 45, 101097.
- Lee, C.; Lee, H.; Kim, O.B. Biocement fabrication and design application for a sustainable urban area. Sustainability 2018, 10, 4079.
- Cagatay Ersan, Y.; Hernandez Sanabria, E.; Boon, N.; De Belie, N. Enhanced crack closure performance of microbial mortar through nitrate reduction. Cem. Concr. Compos. 2016, 70, 159–170.
- Wiktor, V.; Jonkers, H.M. Quantification of crack-healing in novel bacteria-based self-healing concrete. Cem. Concr. Compos. 2011, 33, 763–770.
- Brandl, E.; Heckenberger, U.; Holzinger, V.; Buchbinder, D. Additive manufactured AlSi10Mg samples using Selective Laser Melting (SLM): Microstructure, high cycle fatigue, and fracture behavior. Mater. Des. 2012, 34, 159–169.
- Wang, J.; Mignon, A.; Trenson, G.; Van Vlierberghe, S.; Boon, N.; De Belie, N. A chitosan based pH-responsive hydrogel for encapsulation of bacteria for self-sealing concrete. Cem. Concr. Compos. 2018, 93, 309–322.
- Palin, D.; Wiktor, V.; Jonkers, H.M. A bacteria-based self-healing cementitious composite for application in low-temperature marine environments. Biomimetics 2017, 2, 13.
- Mohammadzadeh, S.D.; Kazemi, S.-F.; Mosavi, A.; Nasseralshariati, E.; Tah, J.H. Prediction of compression index of fine-grained soils using a gene expression programming model. Infrastructures 2019, 4, 26.
- Ghalandari, M.; Ziamolki, A.; Mosavi, A.; Shamshirband, S.; Chau, K.-W.; Bornassi, S. Aeromechanical optimization of first row compressor test stand blades using a hybrid machine learning model of genetic algorithm, artificial neural networks and design of experiments. Eng. Appl. Comput. Fluid Mech. 2019, 13, 892–904.
- Snoeck, D.; Malm, F.; Cnudde, V.; Grosse, C.U.; Van Tittelboom, K. Validation of Self-Healing Properties of Construction Materials through Nondestructive and Minimal Invasive Testing. Adv. Mater. Interfaces 2018, 5, 1800179.
- Liu, W.; Li, Y.-Q.; Tang, L.-P.; Dong, Z.-J. XRD and 29Si MAS NMR study on carbonated cement paste under accelerated carbonation using different concentration of CO2. Mater. Today Commun. 2019, 19, 464–470.
- Muhammad, N.Z.; Shafaghat, A.; Keyvanfar, A.; Majid, M.Z.A.; Ghoshal, S.; Yasouj, S.E.M.; Ganiyu, A.A.; Kouchaksaraei, M.S.; Kamyab, H.; Taheri, M.M. Tests and methods of evaluating the self-healing efficiency of concrete: A review. Constr. Build. Mater. 2016, 112, 1123–1132.
- Wang, J.; Van Tittelboom, K.; De Belie, N.; Verstraete, W. Use of silica gel or polyurethane immobilized bacteria for self-healing concrete. Constr. Build. Mater. 2012, 26, 532–540.
- Johannesson, B.; Utgenannt, P. Microstructural changes caused by carbonation of cement mortar. Cem. Concr. Res. 2001, 31, 925–931.
- Choubin, B.; Mosavi, A.; Alamdarloo, E.H.; Hosseini, F.S.; Shamshirband, S.; Dashtekian, K.; Ghamisi, P. Earth fissure hazard prediction using machine learning models. Environ. Res. 2019, 179, 108770.
- Mahmoudi, M.R.; Heydari, M.H.; Qasem, S.N.; Mosavi, A.; Band, S.S. Principal component analysis to study the relations between the spread rates of COVID-19 in high risks countries. Alex. Eng. J. 2021, 60, 457–464.
- Imtiaz, L.; Rehman, S.K.; Ali Memon, S.; Khizar Khan, M.; Faisal Javed, M. A review of recent developments and advances in eco-friendly geopolymer concrete. Appl. Sci. 2020, 10, 7838.
- Cuthbert, M.O.; McMillan, L.A.; Handley-Sidhu, S.; Riley, M.S.; Tobler, D.J.; Phoenix, V.R. A field and modeling study of fractured rock permeability reduction using microbially induced calcite precipitation. Environ. Sci. Technol. 2013, 47, 13637–13643.
- De Muynck, W.; Verbeken, K.; De Belie, N.; Verstraete, W. Influence of temperature on the effectiveness of a biogenic carbonate surface treatment for limestone conservation. Appl. Microbiol. Biotechnol. 2013, 97, 1335–1347.
- Amran, M.; Onaizi, A.M.; Fediuk, R.; Vatin, N.I.; Muhammad Rashid, R.S.; Abdelgader, H.; Ozbakkaloglu, T. Self-Healing Concrete as a Prospective Construction Material: A Review. Materials 2022, 15, 3214.
- Gardner, D.; Lark, R.; Jefferson, T.; Davies, R. A survey on problems encountered in current concrete construction and the potential benefits of self-healing cementitious materials. Case Stud. Constr. Mater. 2018, 8, 238–247.
