Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Malcolm Scott Duthie and Version 2 by Conner Chen.
Leishmania parasites cause a variety of discrete clinical diseases that present in regions where their specific sand fly vectors sustain transmission. Clinical and laboratory research indicate the potential of immunization to prevent leishmaniasis and a wide array of vaccine candidates have been proposed. Unfortunately, multiple factors have precluded advancement of more than a few Leishmania targeting vaccines to clinical trial.
- leishmania
- vaccine
- RNA
- clinical trials
1. Introduction
Leishmania, obligate intracellular macrophage parasites, are an important genus of parasites that can affect humans and canines for which disease manifestation is dependent upon the infecting parasite species. Each Leishmania species demonstrates a geographic range that is naturally determined by the presence of their specific sand fly vector, and thus differing forms of leishmaniasis are dispersed across endemic regions. Given migration and that the parasites can be transferred in blood, however, cases are occasionally observed in non-endemic regions. Most infections remain asymptomatic (e.g., 90% of humans infected with L. donovani do not advance to symptoms) with a key element for parasite containment being an effective antigen-specific T cell response that can prevent advancement to, or resolution from, the diseased state [1][2][1,2]. Recovery from primary infection is typically associated with long term protection against reinfection, indicating the potential for generating lasting protection through the use of durable anti-Leishmania vaccines [1][2][1,2].
In addition to their own clinical significance, experimental Leishmania infection models have served as an important tool for general immunological understanding. Seminal work in the 1980s used these models to define the Th1/2 paradigm, identifying that experimental L. major infection becomes established then typically clears as antigen-specific Th1 cells develop in resistant C57BL/6 mice whereas infection continues unabated despite the Th2 cells that predominate in the susceptible BALB/c mice [3][4][3,4]. These classic models have also been used to define numerous subsets of CD4+ T cells and reveal genetic mechanisms involved in the development of both disease and adaptive immunity [5][6][7][5,6,7]. Beyond CD4+ T cells, there is evidence that CD8+ T cells also participate in protection in both experimental and physiological situations [8][9][8,9]. Thus, Leishmania infection provides a strong basis for the evaluation of T cell-inducing vaccines and determining their durability.
2. Potential Application of RNA Technology for Leishmania Vaccines
An increase in the clinical availability and use of antileishmanial drugs has been observed in recent years, with a positive impact observed in the reduced severity of disease especially in the most lethal visceral leishmaniasis manifestation[10]. Such treatment of leishmaniasis still presents with the classical challenges of any drug treatments, including the emergence of drug resistant parasites[11], however, and prevention through immunization appears both attainable and preferred. Unfortunately, despite the plethora of preclinical evaluations multiple factors have precluded advancement of more than a few Leishmania targeting vaccines to clinical trial. The sheer volume of potential vaccine platforms and antigen targets identified, the substantial economic impact of producing these as GMP-grade, and the lack of availability of safe and effective adjuvants with which to enhance or sustain responses, has led to reticence in advancing to trial candidates that appear to have ‘room for improvement’ [12][13][14][15][12,13,14,15]. Further, given that infection is reliant on sand fly vector transmission and sand fly populations are impacted by seasonal variation and micro-geography, pre-planning for enactment in regions with sufficiently high parasite transmission at time of trial is both difficult and unassured. The risk of over-estimating infection rates and inadvertently under-powering Leishmania vaccine trials that have disease prevention as an endpoint may be mitigated by a predictive controlled human infection model (CHIM).
RNA-based vaccines rapidly emerged in response to the COVID-19 pandemic largely because (a) the SARS-CoV-2 Spike protein was rationally selected as the target antigen given previous work on SARS and MERS [16], and (b) RNA vaccines could be made and released at GMP grade far more rapidly than subunit vaccines involving recombinant proteins. The inserted RNA sequence can be modified with relative ease and as the COVID-19 pandemic has continued with the evolution to SARS-CoV-2 variants-of-concern, vaccines have accordingly been quickly updated to allow evaluation of revised Spike antigen sequences [17][18][19][17,18,19]. Applying the same logic to Leishmania, where multiple antigens are known to afford at least some protection in animal models, it may be possible to evaluate leads, then quickly revise them, in response to emerging clinical data. Relative ease in the design and manufacture of nucleic acid-based vaccines also suggests the potential for inexpensive and somewhat generic production. One considerable logistical advantage of RNA-based vaccines over the majority of other platforms is that the RNA can be produced in a cell-free environment by in vitro transcription, removing the need for cultured cells in the manufacturing process and avoiding the quality and safety issues associated with their use. In this way, it is possible to perform simple downstream purification to provide both faster and more cost-effective manufacturing, and robust manufacturing processes have now been established for both mRNA and self-amplifying replicon RNA (repRNA) constructs. Thus, RNA vaccines possess an inherent nimbleness that recombinant proteins of defined subunit vaccines do not. Further, the composition of T cell responses elicited by different vaccine platforms are qualitatively distinct: the intracellular localization of RNA vaccines allows for MHC I presentation and generation of associated CD8+ T cell responses that is not typically observed in response to immunization with subunit vaccines (Figure 1). The utility of this is debated for COVID-19 in which a neutralizing antibody response is the most desired initial outcome, recent data indicates that generation of longer term T cell responses provides an extended benefit [20][21][20,21]. For chronic infections such as Leishmaniasis the generation of both CD4+ and CD8+ T cell responses may be the optimal profile for affording protection[22].
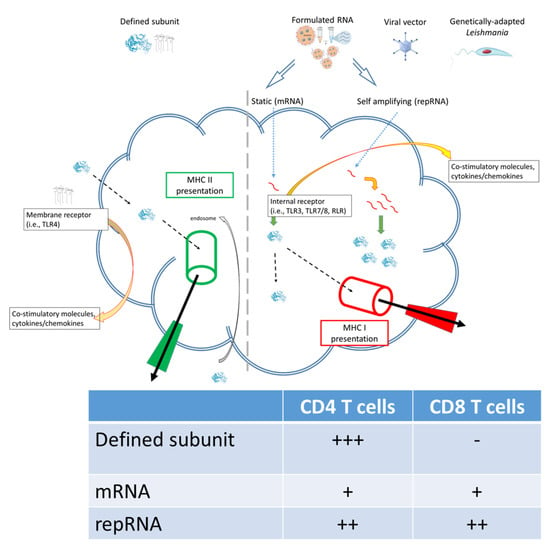
Figure 1.
Subunit and RNA replicon vaccines can generate different quality of immune response.
3. Current Challenges for Leishmania Vaccines
3.1. Development
Numerous diverse technological platforms have been explored as Leishmania vaccine candidates, including live-attenuated or whole-killed parasites (first generation), recombinant proteins (second generation) and DNA vaccines (third generation). Theavailability of genetic information has significantly aided the development process. Several live attenuated Leishmania species have been rendered by genetic modification of critical parasite virulence or survival genes, perhaps most notably the Centrin gene-deleted series that includes L. braziliensis, L. donovani, L. major and L. mexicana parasites [23]. Significant effort has also been focused on defining antigens associated with a protective immune response against Leishmania. Selected targets have been delivered as formulated protein, or as DNA sequences either alone or within vectors such as adenoviruses or even within Leishmania themselves, and measurements have included cellular immune responses and protection [23][24][25][26][27][28][29][30][31][23,24,25,26,27,28,29,30,31]. These studies clearly demonstrate that delivery of defined antigens (or antigenic sequences) in a manner that induces appropriate T cell responses affords protection in animals. Although single antigens may prove to be effective vaccines it is possible, especially when attempting to induce protection against multiple parasite species, that a multi-antigen approach would be desirable. Several defined subunit vaccines consisting of recombinant fusion proteins formulated with adjuvant that elicit protective Th1 responses have been developed [32][33][34][32,33,34]. Although this reduces manufacturing costs, further attempts should be made to address the practical aspects of vaccine production during early development. Case in point is the M72 tuberculosis vaccine candidate that was produced with a scientifically sound approach but which failed to advance beyond phase 2 clinical trials due to costs of production and limited availability of adjuvant components.
RNA technology has the potential to provide an effective and practical solution to vaccine development for a multitude of diseases, including many neglected tropical diseases [14]. RNA vaccine development requires only the target gene sequence be known and removes the need for pathogen culture or scaled recombinant protein production. Due to activation of various pattern-recognition receptors, RNA vaccines can be very immunogenic and have demonstrated a capacity for rapid induction of antibody responses to several emerging pathogens [16][35][36][37][38][39][16,35,36,37,38,39]. From their initial conception, by mimicking immunization with a live vaccine, nucleic acid vaccines, delivered virally, such as with viral replicon particles or similar systems have also held promise as an effective way to induce T cell immunity. While mRNA vaccines are translated directly from the incoming RNA molecules, introduction of repRNA into cells initiates ongoing biosynthesis of antigen-encoding RNA that results in dramatically increased transcription and hence greater protein yields for each RNA molecule delivered [40][41][42][40,41,42]. In addition, repRNA vaccines mimic an alphavirus infection in that viral-sensing stress factors are triggered and innate pathways are activated through Toll-like receptors (TLR) and retinoic acid inducible gene (RIG)-I to produce interferons, pro-inflammatory factors and chemotaxis of APCs, as well as promoting antigen cross-priming. As a consequence, repRNA typically elicit stronger immune responses than similar quantities of mRNA, or equivalent responses when provided at substantially lower doses [42].
3.2. Clinical Evaluation
Addressing the on-the-ground reality of inconsistent pockets of local Leishmania transmission within much larger overall endemic regions, establishing controlled human infection models (CHIM) provides an opportunity to evaluate vaccines in the context of assured infection rates, with a marked impact in terms of reducing study complexity and cost. For example, estimates suggest that use of a CHIM for sand fly transmitted Leishmania major may require as few as 30 subjects per arm to detect vaccine efficacy of 60%. This compares favorably to the need for many hundreds if not thousands of subjects in conventional natural exposure trials. CHIM studies, by virtue of the known time of exposure, also provide an excellent opportunity to identify immunological correlates of protection and to further understand disease pathogenesis. Such findings from CHIM studies can then be evaluated and used to interpret data within larger field trials. CHIM studies and prospective human infection studies exist for a diverse array of pathogens, including for SARS-CoV-2 [43][44][45][46][47][48][49][64,65,66,67,68,69,70]. Importantly, CHIM can have surprising outcomes, such as a CHIM for malaria that did not fulfill the hypothesis that strong cellular immune responses impacted parasite growth rates but rather directed focus to achieving sufficient antibody titers [50][71].
It is important to note that experimental infection of humans with Leishmania spp. is extremely well established for both needle challenge and sand fly initiated infection [51][52][72,73]. Many factors weigh on the decision to develop a CHIM, however, including (a) poor disease control and impact on morbidity and mortality; (b) lack of successful vaccines, despite a number of candidate vaccines/antigens in the development pipeline; (c) absence of effective treatments and/or evidence of drug resistance; and perhaps most important, (d) a defined and treatable pathogen strain or species relevant to clinical disease. Leishmaniasis appears to satisfy each of these criteria: despite vector control efforts, highly endemic regions persist and cause suffering; several Leishmania species have been characterized genetically and multiple antigens have been proposed as vaccine candidates; despite improving drugs, drug resistance has and continues to emerge; and finally, establishing well characterized, and preferably GMP compliant, parasite banks for clinical use is being addressed [53][74]. Beyond establishing the rational and required tools, several practical and safety concerns with any proposed Leishmania CHIM then present themselves. A study involving non-infected sand fly biting was used to establish parameters for challenge and importantly to gauge and incorporate public perceptions of this type of study into a challenge protocol ([54][55][75,76] and clinicaltrials.gov: NCT03999970). A clinical study to evaluate the reproducibility of a CHIM for sand fly transmitted cutaneous leishmaniasis has similarly gained ethical and institutional approval and is ongoing (clinicaltrials.gov: NCT04512742).
