The skin represents 16% of the total body mass (in average for a person) and fulfils a protective barrier function towards the environment
[1]. On the other hand, transdermal drug delivery presents some advantages compared to the oral or parenteral routes, thereby being chosen as the uptake route for common skin treatments and long-term treatments of some chronic inflammatory skin pathologies involving atopic dermatitis (AD)
[2][3][2,3], psoriasis
[4], skin hyperpigmentation disorders
[5][6][5,6], or cancer
[7][8][7,8]. Active pharmaceutical ingredients (APIs) can be topically applied by solutions, creams, or gels for local use, improving the skin’s function and treating dermatological diseases and superficial skin tumors
[8]. However, the penetration of APIs to the target sites (such as the tumor site) is hindered due to the barrier of the stratum corneum (SC), which results in limited bioavailability and poor pharmacokinetics
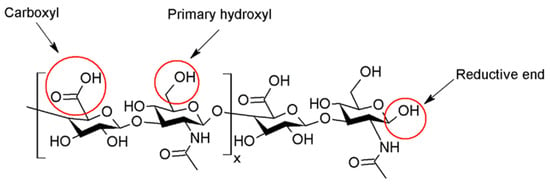
[9]. Moreover, the hindrance to the physicochemical properties of the drug is both low solubility and low permeability. Several polymers can be used for encapsulating APIs, among them hyaluronic acid. Hyaluronic acid (hyaluronan, HA) is a biopolymer ubiquitously present in the human body and bioactive. HA consists of
D-glucuronic acid (GlcA) and
N-acetyl-
D-glucosamine (GlcNAc) units and is linked by alternating
β-(1→4)- and
β-(1→3)-glycosidic bonds (see
Figure 1)
[10].
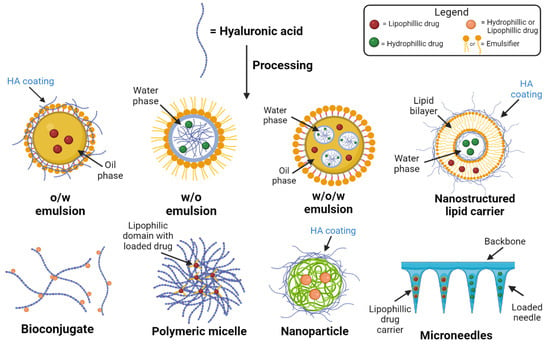
Figure 2.
Drug delivery formulations using HA and its derivatives.
The advantages of using HA for transdermal delivery of molecules are many, including the delivery of high molecular weight (Mw) proteins such as bovine serum albumin without degradation of their tertiary structure
[21][22][22,23]. Moreover, HA is soluble in biological media, is ubiquitously present in the human body, and is biodegradable and biocompatible
[10]. Furthermore, the CD44 expression on psoriatic cells could serve as a target for nanocarriers
[14]; at the same time, it stabilizes the drug delivery system
[15]. Epidermal HA is involved in forming an efficient epidermal barrier made of cornified keratinocytes. The organization of HA in the extracellular matrix and around cells is a critical component of the skin. HA is structured as compact pericellular coats maintained by CD44 on keratinocytes. Both HA and CD44 play an important role in normal epidermal physiological and keratinocyte functions (e.g., differentiation). The CD44 deficiency is always accompanied by a reduction in HA, and a marked alteration in keratinocyte barrier function, proliferation, differentiation, and lipid synthesis, resulting in altered skin barrier function
[23][24]. In the case of atopic dermatitis (AD), the epidermal barrier is characterized by several alterations; HA is produced more significantly by keratinocytes than in normal skin. Epidermal HA inside AD lesioned skin is in enlarged intercellular spaces, likely due to disease-related modifications of HA metabolism. The overexpression of HAS3 causes a massive accumulation of HA in the intercellular spaces between keratinocytes, while HAS2 remains scarcely expressed in the epidermis
[9].
The biological effect of HA is molecular weight-dependent. Furthermore, HA signaling properties are unique among other biologically active molecules, and depend on fragment size
[24][25]. Indeed, high molecular weight HA (HMW-HA) with a Mw (≥10
3 kDa) regulates the distribution of cytoskeletal proteins, preserves tissues, has immunosuppressive functions
[25][26], and increases skin hydration
[26][27]. Even though HMW-HA is present in homeostatic skin, fragmentation occurs under prolonged exposure to UV light or sun exposition
[27][28]. Medium molecular weight HA (MMW-HA) is characterized by a molecular weight between 250 to1000 kDa, increases expression levels of TNF-α (tumour necrosis factor TNF), interleukin 6 and 1β, (IL-6 and IL-1 β), and chemokine ligand 2 (CCL2) in lipopolysaccharide-stimulated macrophages
[28][29]. Low molecular weight HA (LMW-HA) is characterized by Mw from 10 to 250 kDa, activates inflammatory and immune responses, e.g., interleukins (IL-12, IL-1, IL-2, IL-4), and showed significantly higher skin penetration than HMW-HA
[29][30]. Oligosaccharides of HA or fragments are characterized by Mw lower than 10 kDa. They promoted the differentiation of the epidermis and exhibited a more potent moisturizing effect than HMW-HA. Moreover, the HA disaccharide (ΔHA2) inhibited lipopolysaccharide (LPS)-induced inflammation. Therefore, the anti-inflammatory activity depended on HAs polymerization degree, acetyl group, and configuration
[22][23].
2. The Use of Native Versus Modified Hyaluronan in Products
HA injections are one of the most common aesthetic procedures performed worldwide and represent an essential part of modern aesthetic practices, as well as in osteoarthritis treatment. However, native HA has a half-life of only 24–48 h in tissue and skin due to a fast-enzymatic degradation
[30][31], making it unsuitable for internal use. The use of chemically modified HA improves stability, shelf-life, and viscoelasticity. Chemical derivatization and crosslinking impart appropriate mechanical properties and create an adequate macromolecular architecture. Chemical modification of HA involves the two functional sites: hydroxyl and carboxyl moieties. Furthermore, the modification can be performed at the terminal end (
Figure 1). Successful clinical translations of products that use modified HA are unfortunately slowed down by poor chemical characterization of bioconjugates, batch-to-batch variability, complicated multi-step manufacturing process, and inflammatory responses caused by the lack of purity of the products
[31][32]. Nonetheless, HA is a valuable cosmetic ingredient, improving skin plumpness, increasing skin hydration, and inhibiting photoaging.
Several authors demonstrated that the immunomodulatory activities of HA could be refined, enhanced, and uncoupled from its molecular size by using chemical modification. For example, Jouy et al. described that sulphated-HA (sHA, degree of sulphation, 3.4%) exhibited a strong anti-inflammatory effect on human macrophages by decreasing IL-12, MCP-1, and IL-6. The derivative promoted the activation of regulatory functions independent of its Mw. Sulphated-HA was reported to be internalized by inflammatory macrophages mediated by CD44
[32][33]. Furthermore, Gao et al. reported that in contrast to conventional LMW-HA, that can trigger inflammation and immunostimulation. A butyric HA derivative demonstrated anti-inflammatory activities by modulating the cytokine expression, indicating its capacity for preventing wounds in a chronic inflammatory state
[33][34], followed by the results of Hauck et al., who studied the role of highly-sulphated HA (sHA), HMW-HA, which served as a positive anti-inflammatory control, and LMW-HA of the same molecular size as sHA, serving as size control. All cells were stimulated with the Toll-like receptor 4 (TLR4) agonist lipopolysaccharide (LPS) to mimic a pro-inflammatory activation as it occurs in wounds. The authors detected no anti-inflammatory macrophage regulation by HMW-HA. This difference may arise from the different experimental set-ups and the different cells that were used (primary murine macrophages in this study vs. primary human macrophages or murine macrophage cell lines). The results showed that sHA improved dermal wound healing in diabetic mice by reducing the inflammatory activity of cytokines and chemokines tumor necrosis factor (TNF), IL-6 and chemokine (C-C motif) ligand 2 identified as CCL2
[34][35].
Yuan and collaborators prepared an amphiphilic HA derivative (HA-ODA) based on LMW-HA (10 kDa) and octadecylamine by amidation. The derivative was used for coating transfersomes. These HA-modified transfersomes were prepared by thin film hydration method and membrane extrusion. The HA coating resulted in a significantly lower release rate of indomethacin. Furthermore, an enhanced transdermal effect was observed for HA-based compositions. HA probably changed the microstructure of skin layers and decreased the skin barrier function, and then the vesicle was able to squeeze through the intercellular space. The in-vivo study showed an enhanced antinociceptive effect without skin irritation
[16].