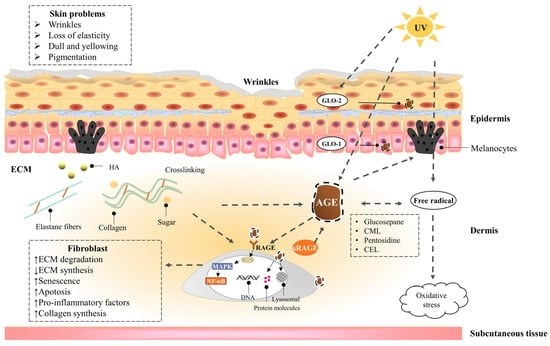
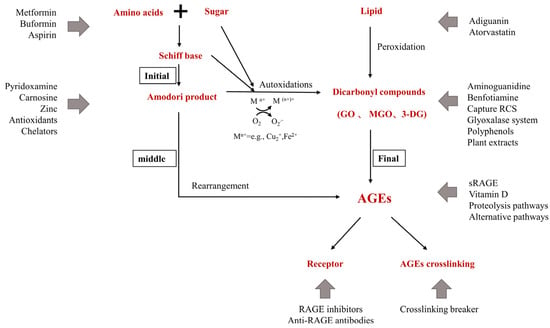
Action sites that inhibit the formation of AGEs in vivo. M
refers to transition metals. Endogenous AGEs are formed through the Maillard reaction in three main stages: early, middle, and final. The glyoxalase system include Glyoxalase I (GLO-1) and II (GLO-2) system; the proteolysis pathways include UPS and ALPS; alternative pathways include DJ-1/Park7 pathway, OPH, aldehyde dehydrogenases (ALDHs), aldo-keto reductases (AKRs), and acetoacetate degradation.
2.1. Pre-Amadori Inhibitors
Aminoguanidine (AG) is an inhibitor of late glycation reactions in vitro found in clinical trials and is an excellent dicarbonyls scavenger that captures reactive carbonyl precursors, such as MGO, GO, and 3-DG. Amadori compounds are important intermediates for AGEs formation in vivo, and CML must be formed primarily by oxidative cleavage of Amadori’s Enediol intermediate between C
2-C
3 of the ligated sugar. AG was found to have no significant effect on the CML produced during the incubation of Amadori proteins. Therefore, AG is an important pre-Amadori inhibitor. AG is toxic at higher concentrations and has been forbidden in human clinical trials
[57][100]. AG inhibits the development of diabetic complications in animal models of diabetes but does not inhibit the formation of late glycation end products of skin collagen in diabetic rats
[58][101]. Benfotiamine, a synthetic thiamine precursor, activates the enzyme transketolase to accelerate the precursors of AGEs toward the pentose phosphate pathway, thereby reducing the production of AGEs
[59][102].
2.2. Post-Amadori Inhibitors
Pyridoxamine (PM), one of the natural forms of vitamin B
6, uniquely targets the post-Amadori pathway through metal-ion chelation and blocking oxidative degradation of Amadori intermediates
[60][103]. Good post-Amadori inhibitor compounds should form stable metal-ion complexes with a higher equilibrium constant than the Amadori compound
[61][104]. PM also has the ability to scavenge toxic carbonyl products from sugar and lipid degradation, inhibit reactive oxygen species
[62][63][105,106], and increase the activation of the detoxifying enzyme GLO-1
[64][107]. The ilex paraguariensis (IP) extract is also a post-Amadori inhibitor due to its inhibition of the second stage of glycation reaction and conversion of free-radical-mediated Amadori products to AGEs
[65][108].
2.3. Crosslinking Breaker
Thiazole salts are AGEs crosslinking breakers, such as OPB-9195 and ALT-711 (alagebrium). OPB-9195 inhibits AGE formation (particularly pentosidine and CML) through the chelation of metal ions and carbonyl trapping
[66][109]. ALT-711 is the first compound in the thiazole class, which has been reported to break down established AGE-related cross-links. Another prototypic AGE cross-link breaker is N-phenacylthiazolium bromide (PTB) which break down protein cross-links by cleaving α-diketone structure. Similar effects have been observed with rosmarinic acid, tannins, and flavonoids
[67][110]. There are also other potent AGEs destroyers, such as curcumin and ALT-946
[68][111].
2.4. Indirect Advanced Glycation End Products Inhibitors
2.4. Indirect AGEs Inhibitors
A small number of AGEs inhibitors play a role in the early stages of glycation by disturbing the initial binding between sugars and amino groups and indirectly reducing the formation of AGEs and ALEs. Since AGEs are mostly produced by non-enzymatic glycation of sugars and lipids, hypoglycemic and lipid-lowering drugs can inhibit the production of AGEs in vivo. For example, Atorvastatin (a lipid-lowering drug) inhibits the further formation of Schiff bases and AGEs by interfering with the initial binding between reducing sugars and amino groups
[69][112]; Metformin is used to treat type II diabetes mellitus by inhibiting the production of reactive oxygen species by reducing the expression of the AGEs receptor (RAGE)
[70][113] and capturing MG and other dicarbonyls produced during glycation. Buformin inhibits the formation of AGEs by trapping the carbonyl groups of ammonia and MGO and is a more effective inhibitor of AGEs formation than metformin
[71][114]; Aspirin, or acetylsalicylic acid, inhibits the glycation process by acetylating the proteins’ free amino groups, thereby blocking the attachment of reducing sugars.
2.5. Natural Advanced Glycation End Products Inhibitors
2.5. Natural AGEs Inhibitors
Synthetic AGE inhibitors have safety concerns and side effects, so natural products with lower toxicity are the most promising alternatives for developing natural medicines with anti-glycation activity. It has been reported that tea, herbal tea, vegetables, fruits
[72][115], yogurt, and other foods have an inhibitory effect on the saccharification reaction. A large number of experiments in vitro and in vivo have shown that natural compounds have the potential to combat the formation and accumulation of AGEs, including phenols, oligosaccharides and polysaccharides, carotenoids (e.g., β-carotene), saponins
[73][116], and unsaturated fatty acids.
Plants have long been used in traditional medicine techniques to treat various diseases and are also a source of new natural medicines discovery. Plant extracts have great anti-aging potential and are rich in a variety of active ingredients, which can inhibit the formation of AGEs by scavenging free radicals, capturing dicarbonyl carbon, etc.
[74][117]. For example, C. ternatea flower extract (CTE) prevents protein glycation by trapping carbonyl groups and scavenging free radicals
[75][118]. The polyphenolic components of peanut peel include gallocatechin, phenolic acids, and resveratrol, which reduce toxicity caused by AGEs and reduce the levels of reactive oxygen species and pro-inflammatory cytokines
[76][119]. Citrus fruit extract significantly reduces the level of protein carbonyl compounds
[77][120]. Akebia quinata fruit extracts (AQFE) can act as an anti-skin aging agent by preventing oxidative stress and other complications associated with AGEs formation
[78][121]. Phenolic components of milk thistle flowers have anti-glycation activity in vitro and on human explants. Polyphenol-rich clove extract, due to its antioxidant properties, is able to inhibit the formation of AGEs and protein glycation
[79][122]. The polyphenol compounds of hazelnut bark extract can reduce the formation of AGEs in vitro
[80][123]. The hydrophobic extract of dunaliella salina, rich in colorless carotene phytoene and phytofluene, has anti-glycation and anti-inflammatory activity and helps reduce the signs of aging (wrinkles)
[81][124]. Cinnamon is a traditional spice, which contains some phenolic components in its aqueous extracts, such as catchin, epicatechin, and procyanidin B2, which inhibit the formation of AGEs through antioxidants and direct capture of active carbonyl substances
[82][125]. Black galangal extract inhibits the formation of fluorescent AGEs, pentosidine, CML, and intermediates 3-DG, GO, and MGO, and it acts on the decomposition of AGEs, thereby reducing the accumulation of AGEs in vivo
[67][110]. Salvia officinalis L. methanol extract, including rosmarinic acid, resveratrol, quercetin, rutin, and luteolin-7-O-glucoside, exerts anti-glycation effects through antioxidation and inhibition of fluorescent substances and carbonyl groups
[83][126]. Pomegranate fruit extract (PE), its phenolic constituents (punicalagin, ellagic acid, and gallic acid), and products of the degradation of ellagitannin (urolithin A and urolithin B)
[84][127] all have effective anti-glycation activities
[85][128].
2.6. Polyphenolic Compounds
As major and ubiquitous phytochemicals, including flavonoids, phenolic acids, alfalfa, and lignans, polyphenols exert AGEs inhibition through ROS inhibition, dicarbonyls capture (MGO and GO), and disruption of protein crosslinking
[81][86][124,129]. Glycation and oxidative stress are closely related, with all steps of sugar oxidation producing oxygen radicals and ultimately resulting in the formation of AGEs. In addition, glycated proteins activate membrane receptors such as RAGE through AGEs and induce intracellular oxidative stress and pro-inflammatory states
[87][130]. Therefore, compounds that scavenge free radicals can effectively inhibit glycation. For example, resveratrol (3,4′,5-Trihydroxystilbene) is a plant polyphenol that reduces oxidative stress
[88][131] and inhibits AGEs-induced proliferation, collagen synthesis, and RAGE receptors
[89][132]. Asiatic acid (AA), a pentacyclic triterpenoid, occurs naturally in many vegetables and fruits. AA pretreatment effectively protects HaCaT cells from subsequent AGE-BSA-induced oxidative and inflammatory stresses, exerting an anti-glycation effect
[90][133]. The natural antioxidant “ellagic acid” (EA) exerts its inhibitory effect on AGEs in diabetic rats by inhibiting glycated ntermediates (including dicarbonyls) and interrupting the auto-oxidation pathway
[91][134].
Flavonoids (flavones, flavanones, isoflavones, and flavonols) are the most common class of polyphenol compounds and have shown significant inhibitory effects on protein glycation and AGEs formation. These mechanisms may involve capturing reactive amino groups, so that they cannot react with glucose or scavenging carbonyl compounds, chelating with trace metal ions that catalyze glycation, scavenging hydroxyl radicals, and inhibiting oxidative degradation of various intermediates
[92][135]. For example, garlic can inhibit protein glycation and dicarbonyls in vitro; quercetin is a phenolic compound found in garlic
[93][136], which has a more effective anti-glycation effect than aminoguanidine
[94][137]. Dietary antioxidants such as quercetin can prevent free radical toxicity
[95][138]. Rutin (flavonoids) is found in fruits and vegetables, making it unable to react with glucose through mechanisms such as capturing reactive amino groups. All five metabolites formed after ingestion effectively inhibit the formation of CML
[92][135]. Anthocyanins are the main flavonoids in blackcurrants that effectively prevent the formation of AGEs by capturing methylglyoxal
[96][139].
Phenolic acids are secondary metabolites that are widely present in plants, including a large distribution of hydroxycinnamic acid (coumalic acid, caffeic acid, ferulic acid, coumarin) and hydroxybenzoic acid (Protocatechuic acid, gallic acid, hydrobenzoic acid, and ellagitannin). These metabolites are also found to have anti-aging potential. For example, ferulic acid inhibits the formation of fluorescent AGEs and CML and reduces fructosamine levels. This leads to the prevention of protein oxidation through the reduction in protein carbonyl formation and protein thiol modification
[97][140]. Isoferulic acid (IFA) is a powerful antioxidant and has an effective inhibitory effect on protein glycation and sugar oxidation
[98][141]. Cinnamic acid and its derivatives reduce the levels of fructosamines, the formation of CML, and the level of amyloid cross-β structures
[99][142].
2.7. Other Advanced Glycation End Products Inhibitors
2.7. Other AGEs Inhibitors
Carnosine is a naturally occurring dipeptide (beta-alanyl-l-histidine), which hinders the formation of protein carbonyl groups and has the ability to chelate transition metal ions, prevent MG-induced glycation, and reduce sugar-induced crosslinking
[100][143], leading to a significantly lower AGEs levels in the epidermis and reticular dermis of human skin explants
[101][144]. Piperazine-2,5-dione reduces the number of late glycation end products accumulated in human dermal fibroblasts with age. Vitamin D therapy may help lower AGEs levels, significantly reduce NF-κB activation, and increase sRAGE levels
[102][145]. Zinc has antioxidant, anti-inflammatory, and anti-apoptotic potential. Zinc deficiency may stimulate the formation of AGEs, while zinc supplementation may inhibit the formation of AGEs and protein carbonyl groups through a variety of signaling pathways and improve AGEs-induced apoptosis and oxidative stress
[103][146].