Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Wenlu Yu and Version 2 by Catherine Yang.
Metabolic disease is a kind of multi-system abnormal disease which is manifested by diseases or disorders that disrupt normal metabolism, including hyperglycemia, dyslipidemia, hypertension, obesity, and insulin resistance, and leads to a dramatic increase in the occurrence of cardiovascular diseases such as acute myocardial infarction and stroke. T cells are involved in the inflammatory response, which can also regulate the development of metabolic diseases, CD4+ T cells and CD8+ T cells are mainly responsible for the role.
- metabolic diseases
- CD4+ T cells
- CD8+ T cells
- inflammation
1. CD4+ T Cells
CD4+ T cells are important in metabolic diseases. Functionally, CD4+ T cells are also known as Th cells. Under the stimulation of different antigens and cytokines, CD4+ T cells can differentiate into four subsets, including Th1, Th2, Th17, and inducible regulatory T (iTreg) cells [1][2][52,53]. Th1 cells, which secret IFN-γ, exert the function of helper cellular immunity against intracellular pathogens and viruses, and contribute to the progression of chronic inflammatory diseases [3][54]. Th2 cells secrete cytokines such as IL-4, IL-5, and IL-13, which promote the proliferation and differentiation of B cells, assist humoral immunity, eliminate extracellular pathogens, and play a role in allergic inflammation [4][55]. Th17 cells can eliminate extracellular pathogens. Similar to Th1 cells, Th17 cells promote progression of inflammation [5][56]. Treg cells including iTreg and nTreg inhibit inflammatory response by secreting cytokines interculin-10 (IL-10) and transforming growth factor-β (TGF-β) [4][55]. Generally, Th1 and Th17 cells play a critical role in promoting pro-inflammatory responses, whereas Th2 and Treg cells are highly correlated with anti-inflammatory responses [6][7][57,58] (Figure 13).
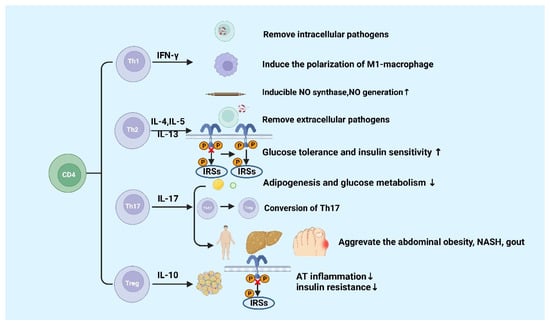
Figure 13.
Functions of CD4+ T cells in metabolic diseases.
IFN-γ is produced by Th1 cells. Studies showed that the level of IFN-γ is higher in high-fat diabetes (HFD)-induced obese mice and obese populations [8][59]. Furthermore, IFN-γ originating from Th1 promotes the conversion of macrophages to M1 macrophages, which will exacerbate AT inflammation, promote the production of chemokines and inflammatory cytokines such as TNF-α in AT [8][9][10][59,60,61]. In gouty inflammation, IFN-γ could synergistically act with monosodium urate (MSU) crystals to increase the expression of inducible NO synthase and NO production in macrophages, thereby causing pro-inflammatory immune responses [11][62]. Ricardo-Gonzalez et al. found that in mice that suffered from allergic inflammation with Th2 bias, the glucose tolerance and insulin sensitivity greatly improved [12][63]. Although the function of Th-related cytokines in inflammation response in metabolic diseases has been clearly defined, the mechanisms of Th1 and Th2 cells in metabolic diseases are not well understood [13][64].
IL-17 is mainly secreted by Th17 cells, which suppresses glucose metabolism and adipogenesis and postpones obesity progression in mice and humans [14][65]. A study of Ghannam reported that IL-17 deficiency would enhance diet-induced obesity (DIO) and change glucose homeostasis. Interestingly, it was previously reported that Th17 cells can adhere to MSCs during inflammation, which contributes to their transfer to a Treg-like phenotype [15][66]. However, further studies found that the number of Th17 cell in abdominal subcutaneous AT in insulin-resistant individuals with obesity are higher than those insulin-sensitive individuals with obesity [16][67]. It was reported that inflammatory Th17 cells increased during non-alcoholic steatohepatitis (NASH) in human and mouse disease models [17][18][19][68,69,70]. Additionally, IL-17 expression increases cytokine production in hepatocytes [20][21][12,71]. CD4+ T cells from DIO mice could secrete high levels of IL-17. The increased levels of IL-17 induce the production of IL-8, which can regulate various immune cell infiltration into AT in response to HFD [22][72]. Clinical studies have discovered that level of circulating IL-17 increases in patients with gout [23][24][73,74]. These findings suggested that Th17 cells and IL-17 might be the core factor leading to metabolic diseases, except for obesity [20][12].
Tregs exhibit an anti-inflammatory effect via IL-10 [20][12], which has been confirmed in many studies. The amount of visceral adipose tissue (VAT) Treg cells significantly reduces in HFD-fed obese mice [25][75], showing that HFD can destroy the immune system and trigger inflammation in mice. The dramatic reduction of Treg cells in VAT has been reported to be significant in adipose inflammation and insulin resistance [25][26][75,76]. Studies have found that the increase in adipose Treg cells plays a role in attenuating adipose inflammation and promoting insulin sensitivity in HFD-fed obese mice [27][28][29][77,78,79]. The transfer of Treg cells reduced the expression of TNF-α and the degree of HFD-induced hepatic inflammation [30][80]. However, a recent study showed Tregs deficiency inhibited the progression of NASH to hepatic cell carcinoma (HCC) in a mouse model of choline deficiency, HFD feeding, and diethylnitrosamine injection [31][81]. Increased pro-inflammatory T cell subgroups are recorded in the natural exhaustion of Tregs in both insulin-resistant mice and T2D patients [25][32][33][75,82,83]. In addition, Markus Feuerer and Yaron Ilan1 have also found that inflammation and insulin resistance in mice are protected by Tregs ex vivo or in vivo [25][28][75,78]. These results indicated that Tregs suppress T2D and promote metabolic health. These results indicated that Tregs inhibit inflammation and promote insulin resistance and may participate in the progression of metabolic diseases such as obesity, NASH, and T2D further. Finally, Hyperuricemia could improve spleen Th17 proportion as well as decrease Tregs proportion in mice, thus disrupting the Th17/Tregs functional balance is crucial in gout [34][84]. The specific molecular mechanisms of metabolic diseases about the different roles of Th17 and Treg cells are still poorly known and require continued investigation.
Naïve CD4 T cells differentiate into different subsets in various circumstances, including Th1, Th2, Th17, and Treg cells. Generally, Th1 and Th17 cells secrete pro-inflammatory cytokines, and Th2 and Treg cells produce anti-inflammatory cytokines. The figure was constructed with BioRender (https://biorender.com, accessed on 7 September 2022).
2. CD8+ T Cells
CD8+ T cells differentiate into cytotoxic T lymphocyte (CTL) after being stimulated by antigens. CTL kills target cells by secreting cytotoxic substances such as perforin and granzyme, and induces apoptosis through the Fas/FasL pathway [32][82]. It was reported that CD8+ T cells play a crucial role in chronic inflammation and metabolic disorders induced by metabolic disease [35][85]. It has been revealed that the population of CD8+ T cells increases in the AT of both DIO and gene-induced obese mice [8][36][59,86]. Additionally, the proportion of CD4+ to CD8+ T cells decreases in obese AT prior to infiltration of macrophages [37][87]. Notably, the CD8+ T cells also infiltrated in human AT [35][85]. Moreover, in CD8-deficient obese mice, the M1 macrophages and CD8+ T cells in AT significantly reduce, AT inflammation, glucose intolerance, and the insulin resistance also decrease [32][82].
CD8+ T cell infiltration increase in both obese humans and mice with nonalcoholic fatty liver disease (NAFLD) [38][88]. In mouse models, the consumption of CD8+ T cells reduces the liver damage in NASH, indicating that CD8+ T cells may directly promote disease development [39][40][89,90]. It is reported that compared to regular NASH mice, CD8+T cell-deficient mice are associated with higher hepatic insulin sensitivity, lower liver damage, and lower fibrosis [38][39][41][88,89,91]. There are several probable mechanisms about activated CD8+ T cells leading to NASH development. In the early transition from steatosis to NASH, type I interferons contribute to the activation of cytotoxic CD8+ T cells, resulting in increased production of pro-inflammatory cytokines such as IFN-γ and TNF-α [41][91]. During the establishment of NASH, the amount of liver tissue-resistant CXCR6+CD8T cells subsets increased, which could stimulate the role of self-attack to hepatic cells and instigate the disease development [42][92]. Importantly, during NASH, exhausted CD8 T cells would accumulate in liver tissues, which not only damages liver tissues but impairs immunosurveillance and promotes the conversion from NASH to hepatocellular cancer [43][93]. However, CD8+ tissue-resident memory T cells are also beneficial for murine NASH subsiding through inducing activated hepatic stellate cells (HSCs) to apoptosis [44][94]. Therefore, CD8+ T cells play a potential dichotomous role in NAFLD progression and resolution.
Nishimura et al. discovered that the accumulation of CD8+ T cells results in insulin resistance and chronic inflammation in mice [32][82]. Chronic inflammation can increase the amount of exhausted CD8+ T cells. The amount of exhausted CD8+ T cells and the pro-inflammatory cytokines produced by them are positively correlated with fasting blood glucose. Notably, the increase in CD8+ T cells accompanied by a senile phenotype in the peripheral blood and AT may accelerate the hyperglycemia in humans [45][46][14,95]. Moreover, the research also proclaimed that the exhausted CD8+ T cells impaired insulin sensitivity in both humans and mice [45][46][14,95]. Studies showed that in DIO, chemokines secreted by CD8+ T cells that infiltrate AT induce the activation of macrophages and facilitate the recruitment of macrophages into AT [32][82]. Overall, these researchers prove that CD8+ T cells promote the development of metabolic diseases through pro-inflammatory activities; Figure 24 summarizes it.
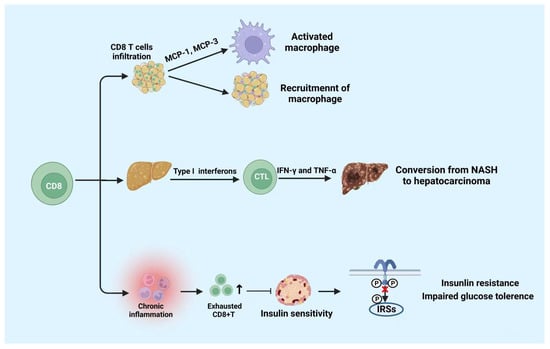
Figure 24.
Functions of CD8+ T cells in metabolic diseases.
CD8+ T cells play a role in pro-inflammatory activities. CD8+ T cells infiltration promotes the activation and recruitment of macrophages. Moreover, CD8+ T cells infiltration in liver tissue is beneficial to the conversion from NASH to hepatocarcinoma. Moreover, cumulated exhausted CD8+ T cells suppress the insulin sensitivity which leads to insulin resistance and impaired glucose tolerance. NASH: non-alcoholic steatohepatitis. The figure was constructed with BioRender (https://biorender.com, accessed on 7 September 2022).
