Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by Ali Albarrati.
Dapagliflozin is a selective SGLT-2 inhibitor that reduces renal glucose absorption by inhibiting the SGLT-2 receptors present in the S1 region of the proximal kidney tubules.
- dapagliflozin
- heart failure
- type 2 diabetes mellitus
1. Introduction
Heart failure (HF) is a leading cause of mortality and morbidity worldwide [1]. HF is associated with several risk factors, the most prominent of which is T2DM, which has a high risk of mortality and short survival span. In addition, individuals with T2DM have a greater risk of HF independent of coronary heart disease, and some studies support HF prevention therapies [2,3,4][2][3][4]. These two abnormalities may be addressed effectively on their own, but establishing a treatment for HF associated with T2DM remains a challenge.
Sodium–glucose cotransporter-2 (SGLT-2) inhibitors are a class of hypoglycemic drug that was recently discovered to have potential cardiovascular-protective effects in adults [5,6][5][6]. SGLT-2 inhibitors modulate the sodium-glucose cotransporter in the proximal convoluted tubule of the kidney, preventing glucose reabsorption and lowering blood glucose by increasing urine glucose excretion [5]. SGLT-2 inhibitors have also been reported in recent studies to lower blood pressure and body weight, which may have cardiovascular implications [6,7,8,9,10,11][6][7][8][9][10][11]. One of the SGLT-2 inhibitors is dapagliflozin, which is commonly used in HF clinics. Several well-designed randomized controlled trials (RCTs) of dapagliflozin have shown remarkable cardiovascular benefits [7,8][7][8].
Exercise capacity is defined as the ability of the cardiovascular system to supply sufficient oxygen to the musculoskeletal system during exercise and extract the supplied oxygen from the blood by the exercising muscles [9]. Exercise capacity is usually reduced in patients with HF and is characterized by an inability to sustain physical effort [9]. Dapagliflozin has demonstrated cardiovascular safety and efficacy in reducing cardiovascular events and related hospitalizations associated with HF regardless of the incidence of T2DM [10,11,12][10][11][12]. However, there is a controversial effect on exercise capacity. Therefore, the objective of this narrative revisewarch is to highlight the effect of dapagliflozin on exercise capacity and cardiovascular risk in individuals with HF reported in clinical studies.
2. Dapagliflozin as a Drug
Dapagliflozin is a selective SGLT-2 inhibitor that reduces renal glucose absorption by inhibiting the SGLT-2 receptors present in the S1 region of the proximal kidney tubules [12]. Dapagliflozin was recently approved in the United States for lowering the risk of HF hospitalizations in patients with T2DM and cardiovascular disease risk markers based on these promising results [10]. Dapagliflozin works by altering visceral fat and blood glucose levels, which are both elements of metabolic syndrome and linked to the severity of cardiovascular disease. The physiological mechanisms by which dapagliflozin produces its cardioprotective benefits are uncertain, and more research is anticipated. Dapagliflozin is used as a monotherapy in patients with T2DM who are resistant to metformin or for whom it is not recommended. In terms of lowering the levels of hemoglobin A1c (HbA1c), dapagliflozin is comparable to metformin as monotherapy [13]. Furthermore, in patients with suboptimal glycemic control, dapagliflozin could be used to supplement existing antihyperglycemic drugs. Dapagliflozin has a half-life of 12.9 h and is excreted mostly in the urine as its 3-O-glucuronide metabolite [10]. There is greater systemic circulation of dapagliflozin in patients with renal and hepatic dysfunction [14]. Dapagliflozin protects the heart and circulatory system through a complex mechanism. Improvements in the circumstances of ventricular preload, cardiac metabolism and bioenergetics, Na+/H+ exchange, sugar and lipid metabolism, circulatory load, cardiovascular system, and other factors could all be part of the mechanistic strategy (Figure 1) [15]. However, the underlying mechanisms of the prevention of HF are thought to be a relief of ventricular loading conditions and reduction of preload through diuretic and natriuretic actions. Dapagliflozin has an effect on the functionality of cardiac cells and a putative effect on modulating the cardiac physiology in HF [16,17][16][17]. A number of cardioprotective sequelae of dapagliflozin contribute to altering HF pathophysiology, including diuresis/natriuresis, blood pressure reduction, erythropoiesis, enhanced cardiac energy metabolism, inflammatory minimization, suppression of the sympathetic nervous system, and prevention of adverse cardiac remodeling [18]. In vitro, dapagliflozin causes adenosine 5′-monophosphate activated protein kinase (AMPK) phosphorylation, resulting in down-regulation of protein kinase (PKC) phosphorylation in cardiac myoblast H9c2 cells after hypoxia/reoxygenation (H/R) treatment. The AMPK/PKC nicotinamide adenine dinucleotide phosphate hydrogen (NADPH) oxidase pathway has shown that dapagliflozin therapy reduces H/R-induced oxidative stress. Through AMPK/PKC/NADPH oxidase signaling, dapagliflozin also corrects H/R-induced abnormalities in PGC-1 expression, mitochondrial membrane potential, and mitochondrial DNA copy number. Dapagliflozin appears to have the ability to reduce ischemia/reperfusion (I/R) induced oxidative stress and, subsequently, cardiac apoptosis by modulating AMPK, minimizing the cardiac dysfunction mediated by I/R injury [19]. In Sprague Dawley rats, dapagliflozin has been shown to alleviate angiotensin II-induced cardiac remodeling by modulating transforming growth factor (TGF)-1/Smad signaling in a non-glucose-lowering-dependent mechanism [11]. Another study suggested that dapagliflozin significantly reduces the high glucose-induced endothelial–mesenchymal transition (EndMT) in human umbilical vein endothelial cells (HUVECs) and fibroblast collagen secretion. Similarly, dapagliflozin abolishes upregulation of TGF/Smad signaling and inhibition of AMPK activity. The anti-EndMT actions of dapagliflozin were then reversed in HUVECs using AMPK siRNA and compound C. In addition, dapagliflozin can protect against dilated cardiomyopathy and myocardial fibrosis by decreasing fibroblast activation and EndMT through suppression of AMPK-mediated TGF/Smad signaling [20]. In a mouse model with transverse aortic constriction, dapagliflozin treatment improved cardiac systolic performance and prevented myocardial fibrosis and cardiomyocyte death, suggesting that it could be used as a novel therapeutic to combat pathological cardiac remodeling in non-diabetics [21].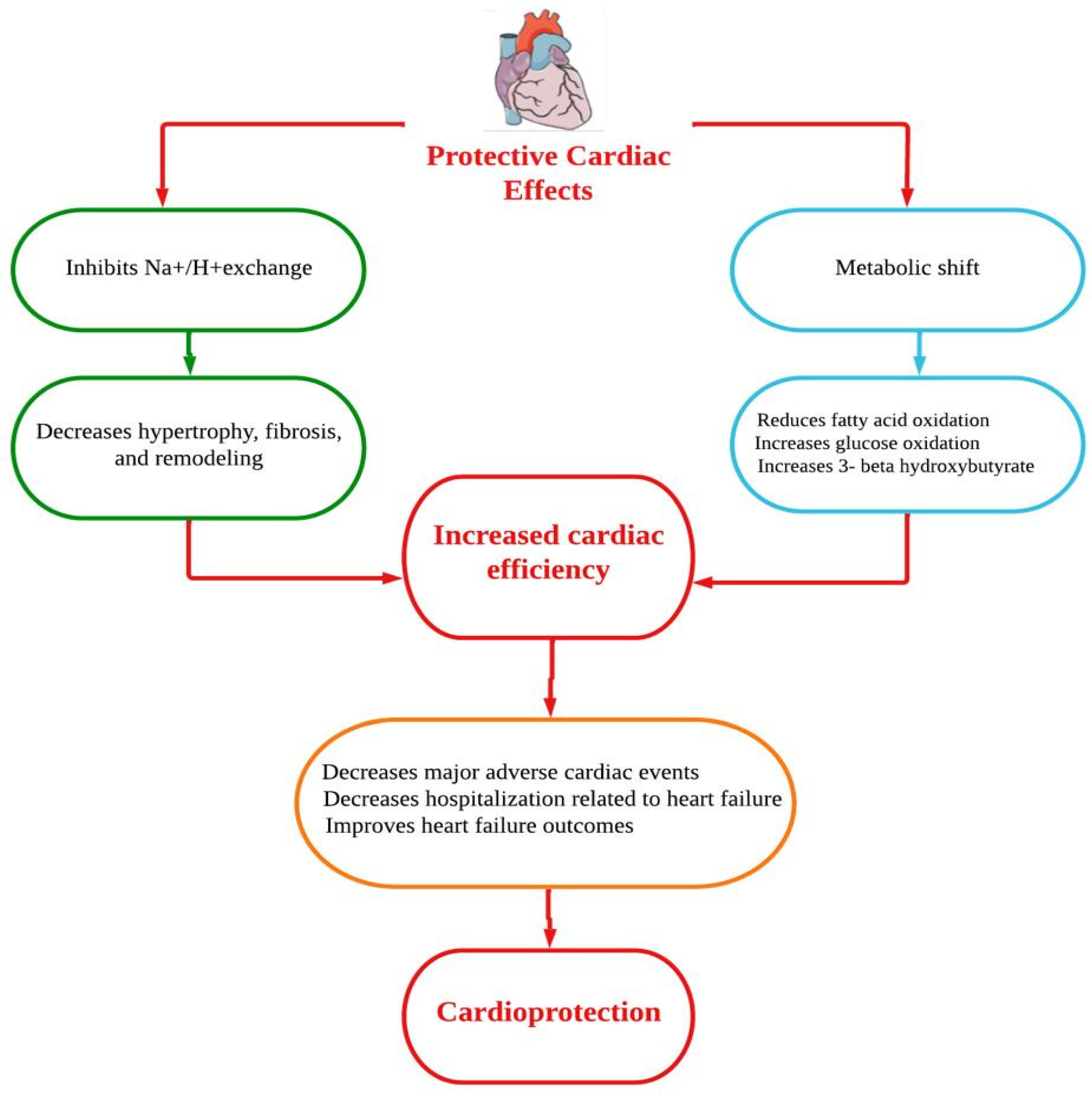
Figure 1.
Putative cardioprotective mechanisms of dapagliflozin.
3. Discussion
HF is an extremely incapacitating affliction affecting millions of people worldwide. There is still a significant unmet need for HF prevention through early detection and treatment of people who are symptomatic or at high risk of developing adverse events related to HF. The American College of Cardiology, the European Society of Cardiology, and the UK National Institute for Health and Care Excellence have recommended dapagliflozin in the treatment of patients with HFrEF [36,37,38][22][23][24]. The recommended dose of dapagliflozin is 10 mg once a day with or without food for patients with HF who have no severe liver dysfunction or renal dialysis.
Dapagliflozin has been associated with a lower risk of MACEs in patients with HF compared to placebo, regardless of the presence or absence of T2DM. The DEFINE-HF, DAPA-HF, and DECLARE–TIMI 58 trials demonstrated the cardiovascular benefits of dapagliflozin in patients with HFrEF, including reduced hospitalizations and death related to HF [8,29,33][8][25][26]. A recent meta-analysis looked into the effect of dapagliflozin on cardiovascular events as reported in 21 clinical studies [35][27]. This meta-analysis included a total of 9339 patients: 5936 patients received dapagliflozin (2.5–10 mg) and 3403 received a placebo. Remarkably, dapagliflozin administration led to a significantly decreased rate of hospitalization related to HF in patients with HFrEF compared to the control group.
Similarly, the DELIVER trial demonstrated the beneficial effects of dapagliflozin in patients with HFpEF, including reduced worsening HF and cardiovascular death [34][28]. The beneficial effect of dapagliflozin on exercise capacity in patients with HF remains controversial. However, patients with HF have reported functional outcome measures that demonstrate a positive effect. The controversy between the trials is due to the heterogeneity of the sample recruited and the outcomes used to measure exercise capacity in these clinical trials. The DAPA-VO2 trial was designed primarily to evaluate the effect of dapagliflozin on exercise capacity and showed a positive effect on peak VO2, which is the gold standard for measuring cardiopulmonary exercise capacity [25][29]. This improvement may not be solely related to dapagliflozin, and other contributing factors, including weight loss associated with dapagliflozin may have overestimated the effect on peak VO2. Although there are several data available to show improvement in exercise capacity using subjective exercise testing measures, there are not enough data to show improvement in objective measures, and no conclusion has yet been reached. Furthermore, well-controlled studies using objective exercise testing are needed on patients with HF without comorbidities that affect exercise capacity.
References
- Ponikowski, P.; Anker, S.D.; AlHabib, K.F.; Cowie, M.R.; Force, T.L.; Hu, S.; Filippatos, G. Heart failure: Preventing disease and death worldwide. ESC Heart Fail. 2014, 1, 4–25.
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E., Jr.; Colvin, M.M.; Drazner, M.H.; Filippatos, G.S.; Fonarow, G.C.; Givertz, M.M.; et al. ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J. Card. Fail. 2017, 23, 628–651.
- Kenny, H.C.; Abel, E.D. Heart Failure in Type 2 Diabetes Mellitus. Circ. Res. 2019, 124, 121–141.
- Martín-Timón, I.; Sevillano-Collantes, C.; Segura-Galindo, A.; Del Cañizo-Gómez, F.J. Type 2 diabetes and cardiovascular disease: Have all risk factors the same strength? World J. Diabetes 2014, 5, 444–470.
- Syed, S.; Gosavi, S.; Shami, W.; Bustamante, M.; Farah, Z.; Teleb, M.; Abbas, A.; Said, S.; Mukherjee, D. A Review of Sodium Glucose Co-transporter 2 Inhibitors Canagliflozin, Dapagliflozin and Empagliflozin. Cardiovasc. Hematol. Agents Med. Chem. 2015, 13, 105–112.
- Voelker, R. Update on SGLT2 Inhibitor Warning. JAMA 2016, 315, 243.
- McMurray, J.J.V.; DeMets, D.L.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Langkilde, A.M.; Martinez, F.A.; Bengtsson, O.; Ponikowski, P.; Sabatine, M.S.; et al. A trial to evaluate the effect of the sodium–glucose co-transporter 2 inhibitor dapagliflozin on morbidity and mortality in patients with heart failure and reduced left ventricular ejection fraction (DAPA-HF). Eur. J. Heart Fail. 2019, 21, 665–675.
- Nassif, M.E.; Windsor, S.L.; Tang, F.; Khariton, Y.; Husain, M.; Inzucchi, S.E.; Kosiborod, M. Dapagliflozin Effects on Biomarkers, Symptoms, and Functional Status in Patients with Heart Failure with Reduced Ejection Fraction: The DEFINE-HF Trial. Circulation 2019, 140, 1463–1476.
- Tavazzi, L.; Giannuzzi, P.; Dubach, P.; Opasich, C.; Myers, J.; Perk, J. Recommendations for exercise testing in chronic heart failure patients Working Group on Cardiac Rehabilitation & Excercise Physiology and Working Group on Heart Failure of the European Society of Cardiology. Eur. Heart J. 2001, 22, 37.
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2019, 380, 347–357.
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008.
- Zhang, Y.; Lin, X.; Chu, Y.; Chen, X.; Du, H.; Zhang, H.; Chai, D. Dapagliflozin: A sodium-glucose cotransporter 2 inhibitor, attenuates angiotensin II-induced cardiac fibrotic remodeling by regulating TGFbeta1/Smad signaling. Cardiovasc. Diabetol. 2021, 20, 121.
- Kasichayanula, S.; Liu, X.; LaCreta, F.; Griffen, S.C.; Boulton, D.W. Clinical Pharmacokinetics and Pharmacodynamics of Dapagliflozin, a Selective Inhibitor of Sodium-Glucose Co-transporter Type 2. Clin. Pharmacokinet. 2013, 53, 17–27.
- Saleem, F. Dapagliflozin: Cardiovascular Safety and Benefits in Type 2 Diabetes Mellitus. Cureus 2017, 9, e1751.
- Zhai, M.; Du, X.; Liu, C.; Xu, H. The Effects of Dapagliflozin in Patients with Heart Failure Complicated With Type 2 Diabetes: A Meta-Analysis of Placebo-Controlled Randomized Trials. Front. Clin. Diabetes Heal. 2021, 2, 703937.
- Lytvyn, Y.; Bjornstad, P.; Udell, J.A.; Lovshin, J.A.; Cherney, D.Z.I. Sodium Glucose Cotransporter-2 Inhibition in Heart Failure: Potential Mechanisms, Clinical Applications, and Summary of Clinical Trials. Circulation 2017, 136, 1643–1658.
- Seferovic, P.M.; Fragasso, G.; Petrie, M.; Mullens, W.; Ferrari, R.; Thum, T.; Rosano, G.M.C. Sodium-glucose co-transporter 2 inhibitors in heart failure: Beyond glycaemic control. A position paper of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2020, 22, 1495–1503.
- Lopaschuk, G.D.; Verma, S. Mechanisms of Cardiovascular Benefits of Sodium Glucose Co-Transporter 2 (SGLT2) Inhibitors: A State-of-the-Art Review. Basic Transl. Sci. 2020, 5, 632–644.
- Tsai, K.L.; Hsieh, P.L.; Chou, W.C.; Cheng, H.C.; Huang, Y.T.; Chan, S.H. Dapagliflozin attenuates hypoxia/reoxygenation-caused cardiac dysfunction and oxidative damage through modulation of AMPK. Cell. Biosci. 2021, 11, 44.
- Tian, J.; Zhang, M.; Suo, M.; Liu, D.; Wang, X.; Liu, M.; Pan, J.; Jin, T.; Fengshuang, A. Dapagliflozin alleviates cardiac fibrosis through suppressing EndMT and fibroblast activation via AMPKα/TGF-β/Smad signalling in type 2 diabetic rats. J. Cell. Mol. Med. 2021, 25, 7642–7659.
- Shi, L.; Zhu, D.; Wang, S.; Jiang, A.; Li, F. Dapagliflozin Attenuates Cardiac Remodeling in Mice Model of Cardiac Pressure Overload. Am. J. Hypertens. 2019, 32, 452–459.
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Kathrine Skibelund, A. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 3599–3726.
- Rosano, G.M.; Moura, B.; Metra, M.; Böhm, M.; Bauersachs, J.; Ben Gal, T.; Adamopoulos, S.; Abdelhamid, M.; Bistola, V.; Čelutkienė, J.; et al. Patient profiling in heart failure for tailoring medical therapy. A consensus document of the Heart Failure Association of the European Society of Cardiology. Eur. J. Hear. Fail. 2021, 23, 872–881.
- National Institute of Health and Care Excellence. Dapagliflozin for Treating Chronic Heart Failure with Reduced Ejection Fraction. 2021. Available online: http://www.nice.org.uk (accessed on 13 July 2022).
- Butt, J.H.; Docherty, K.F.; Petrie, M.C.; Schou, M.; Kosiborod, M.N.; O’Meara, E.; Kober, L. Efficacy and Safety of Dapagliflozin in Men and Women with Heart Failure with Reduced Ejection Fraction: A Prespecified Analysis of the Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure Trial. JAMA 2021, 6, 678–689.
- Zelniker, T.A.; Morrow, D.A.; Mosenzon, O.; Goodrich, E.L.; Jarolim, P.; Murphy, S.A.; Wiviott, S.D. Relationship between baseline cardiac biomarkers and cardiovascular death or hospitalization for heart failure with and without sodium–glucose co-transporter 2 inhibitor therapy in DECLARE-TIMI 58. Eur. J. Hear. Fail. 2021, 23, 1026–1036.
- Sonesson, C.; Johansson, P.A.; Johnsson, E.; Gause-Nilsson, I. Cardiovascular effects of dapagliflozin in patients with type 2 diabetes and different risk categories: A meta-analysis. Cardiovasc. Diabetol. 2016, 15, 37.
- Solomon, S.D.; McMurray, J.J.; Claggett, B.; de Boer, R.A.; De Mets, D.; Hernandez, A.F.; Langkilde, A.M. Dapagliflozin In Heart Failure With Mildly Reduced Or Preserved Ejection Fraction. N. Engl. J. Med. 2022, 387, 1089–1098.
- Palau, P.; Amiguet, M.; Domínguez, E.; Sastre, C.; Mollar, A.; Seller, J.; Núñez, J. Short-term effects of dapagliflozin on maximal functional capacity in heart failure with reduced ejection fraction (DAPA-VO2): A randomized clinical trial. Eur. J. Heart Fail. 2022.
More
