2. Linear Imidazole-Modified Peptides and Polymers
Using block copolymers with a poly-L-histidine (PLH) domain, the Bae group conducted a series of seminal studies on the release of hydrophobic drugs from micelles in the acidic extracellular environment of tumors
[20][21][22][23][10,39,40,41]. After comparing different molecular weights of the PLH segments (prepared by ring-opening polymerization), the molecular weight of 5000 was selected for the PLH component of the copolymer because of the lower critical micelle concentration (CMC) (2.3 μg/mL). Interestingly, the addition of polyethylene glycol (PEG) to the PLH domain (PEG-PLH) increased the pKa from 6.5 to 7.0. As with most of the histidine-containing micelles discussed
in this review, the CMC of the PEG-PLH micelles was inversely correlated with the pH. The pH dependence of the CMC and the transmittance of PEG-PLH micelles were consistent with the protonation of the imidazole groups and their disruption at mildly acidic pH levels. Although many pH-buffering micelles increased in size at acidic pH levels, these micelles became smaller as the pH was lowered
[20][10]. The PEG-PLH micelles released about 42%, 75%, and 85% of the Dox at pH 7.4, 6.8, and 5.0, respectively, over twenty-four hours at 37 °C
[21][39]. The enhanced release of Dox from micelles at lower pH levels was further corroborated by the increased inhibition of MCF-7 cancer cells in more acidic media
[21][22][39,40]. The group also determined that Dox-loaded PEG-PLH micelles had improved pharmacokinetics, enhanced tumor accumulation, and reduced tumor size compared to free Dox
[22][40].
To improve the stability at physiological pH and the release of Dox at the mildly acidic pH levels found in the extracellular tumor environment, a blend of PEG-PLH and PEG- poly-L-lactide (PEG-PLA) micelles was investigated
[21][39]. Compared to other PEG-PLH/PEG-PLA mixed micelles, the blend of PEG-PLH (75%) and PEG-PLA (25%) micelles showed improved release profiles for Dox at mildly acidic pH levels, reflective of the extracellular pH of tumors. While about 30% of the Dox was released from the optimal mixed micelle preparation at pH 7.4, nearly 75% was released at pH 6.8 over twenty-four hours. Concomitant with the release kinetics, the 75:25 mixed micelles showed enhanced cytotoxic activity toward MCF-7 cells incubated in media at pH 6.8
[21][39]. Furthermore, when Dox-loaded PEG-PLA/PEG-PLH micelles were decorated with the folate ligand, their inhibition of MCF-7 and drug-resistant MCF-7-Dox
R cells was significantly greater than that of the untargeted micelles. Notably, free Dox had little effect on the MCF-7-Dox
R xenografts. In contrast, the Dox-loaded pH-sensitive micelles, particularly the folate-targeted micelles, had a marked effect on the growth of the xenografts
[23][41]. In a later report, the authors indicated that long-term stability was an issue for these micelles
[24][42].
The improved kinetics of Dox release from mixed micelles with the hydrophobic poly-L-lactide (PLA) copolymer has stimulated interesting designs with triblock PLH-containing copolymers
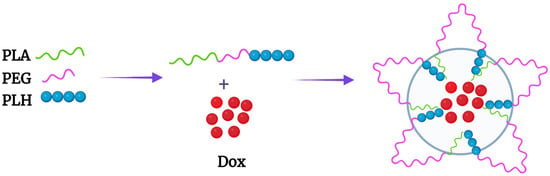
[25][26][43,44]. The Bae group designed an interesting PLA-PEG-PLH triblock copolymer that self-assembled into flower-like micelles (
Figure 1 and
Figure 2A). While the sandwich hydrophilic PEG segment was on the surface, the PLA and PLH segments on the ends made up the inner core. These micelles were approximately 80 nm in size at pH 7.4 and swelled to 580 nm at pH 6.6
[25][43]. Related to these size changes, the cumulative release of Dox from the micelles was 35% higher at pH 6.8 than the release at pH 7.4. The amounts of Dox released at various pH levels and the in vitro antitumor efficacy of these triblock micelles were similar to those of the mixed micelles
[21][39].
Figure 1.
Flower-like micelle formed by the PLA-b-PEG-b-PLH triblock copolymer. Dox was incorporated within the PLA and PLH hydrophobic core.
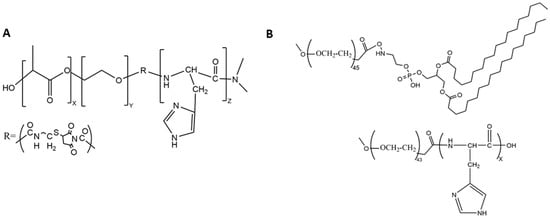
Figure 2. Chemical structures of the (A) triblock copolymer and (B) the block copolymers mPEG-PLH and PEG-DSPE, which formed micelles. The numbers of monomeric units in the block copolymer are represented by X, Y, and Z.
Liu et al. reported somewhat different findings for micelles prepared with the same components but in a different order, a triblock mPEG-b-PLH-PLA copolymer
[26][44]. In contrast to the PEG being sandwiched between two hydrophobic polymers, PEG was external to these domains. In a twenty-four hour period, about 35% and 80% of the Dox was released from the micelles at pH 7.4 and pH 5.0, respectively. Because there was no quick release of Dox from these micelles at pH 5, the authors speculated that Dox was primarily located in the hydrophobic PLA core at this acidic pH. The greater degree of polymerization of PLA compared to other block polymers
[25][27][43,45] may have played a role in the entrapment of Dox at the lower pH. Significant amounts of Dox were unlikely to have been released from these micelles at pH values between 6.3 and 7.0 since no burst release was observed at pH 5.0. In any event, no release data were reported at a pH of 6.3, even though the micelles reached their maximum size at this pH prior to their becoming smaller. In contrast, there was a burst release of Dox at pH 6.5 and 5.0 in which PLH formed the inner core of the micelle (mPEG-PLA-PLH)
[28][46]. Therefore, the order of the triblock polymer and perhaps the length of the polymeric block components may be important in determining whether micelles release Dox at mildly acidic pH levels between 6.5 and 7.0.
Compared to PLA, the copolymer poly(lactide-co-glycolide) (PLGA) is generally preferred because its biocompatibility, biodegradability, and mechanical strength can be controlled by varying the ratios of its monomers. Li et al. synthesized block copolymers of PLGA and tocopheryl polyethylene glycol succinate (TPGS-PLGA) with or without poly-L-histidine (TPGS-PLGA-PLH)
[27][45]. Among these components, the PEG-containing TPGS segment formed the outer shell, improving the stability of the nanoparticles and inhibiting the multidrug resistance (MDR) transporter, while both the hydrophobic PLGA middle and PLH inner shell components entrapped Dox efficiently. Moreover, the Dox-loaded particles with PLH showed enhanced release of Dox at acidic pH and more significant cytotoxicity toward Dox-sensitive and -resistant breast cancer cells compared to particles without histidine. Although these results demonstrated the importance of PLH in the release of Dox
[27][45], the Dox readily leaked from the cores of the two nanoparticles at pH 7.4 (TPGS-PLGA-PLH, TPGS-PLGA, ~55% in twelve hours)
.
To reduce the release of Dox from nanoparticles, Johnson et al. synthesized a diblock copolymer composed of poly(2-hydroxyethyl methacrylate (pHEMA) and PLH domains (p(HEMA)-b-PLH)
[29][47]. The number of monomeric histidines in the PLH domain markedly affected the biophysical characteristics of the micelle and the release profile of the Dox. The pHEMA component formed a hydrophilic shield, whereas the polyhistidine formed a hydrophobic core incorporating the Dox. Upon varying the number of histidines (15, 25, 35, and 45) in the PLH domain, the size of the Dox-loaded micelle was affected, ranging in size from about 124 to 194 nm. The more histidines in the diblock copolymer, the larger the Dox-loaded micelle and the greater the release rate of Dox at physiological pH and acidic pH. Despite the differences in size and release rates of Dox at different pH levels, the micelles with varying histidine content showed similar cytotoxicity for cancer cells yet reduced cytotoxicity compared to free Dox. Notably, cytotoxicity was progressively increased when the Dox-loaded micelles were incubated with the cells at lower pH levels. In a later study from the same group, a similar pH-dependent enhanced release of Dox was observed from micelles formed from the triblock copolymer (PEG- p(Lys)
25-p(His)
100)
[30][31][48,49]. Although nucleic acids could presumably have been loaded into these micelles, only Dox was. It is possible that the nearly 50% release of Dox from the micelles at pH 7.4 was due to the self-repelling poly-L-lysine layer, and, with the addition of siRNA to neutralize the poly-L-lysine component, the release of the drug might have been reduced, as reported by others
[31][49].
With their high numbers of hydroxyl groups, polysaccharides such as dextran likely behave similarly to PEG in reducing nonspecific interactions of serum proteins with nanoparticles. Dextran is a neutral, biodegradable polysaccharide made up of glucose molecules of 1,6-glycosidic linkages with varying degrees of length and branching. The lower the degree of branching of dextran, the fewer the allergic side effects
[32][50]. Moreover, dextran may have advantages over PEG, since severe allergic side effects may occur less frequently
[33][34][51,52].
A dextran-b-poly(L-histidine) (Dex-b-PLH) block copolymer was synthesized by Hwang and his colleagues
[35][53]. Poly-L-histidine of two molecular weights (~5800 and ~12,600) was conjugated to the reductive end of the low-molecular-weight dextran (~6000). There were modest differences in the drug loading capacity, pH-dependent size, and release of Dox between these two Dex-b-PLH particles. The release of Dox at both neutral and acidic pH levels from particles containing the higher-molecular-weight (MW) PLH segment was reduced compared to those with the lower-MW PLH segment. This was in contrast to the micelles formed from the diblock polymer (p(HEMA)-b-PLH), in which the higher-MW PLH domain enhanced the release of Dox
[29][47]. Whether this was due to the different block copolymers conjugated to the PLH domain in the two studies is not known. Still, with Dex-b-PLH micelles at pH 7.4, about 40% of Dox was released from the particles with the high-MW PLH in twenty-four hours. Additionally, the cellular uptake rate of Dox-loaded Dex-PLH was higher than that of free Dox, particularly at the lower pH. Consistent with these uptake studies, the cytotoxicity of Dox-loaded particles toward cholangiocarcinoma cells (HuCC-T1) was pH-dependent and greater than that of free Dox
[35][53].
Similarly to dextran, the auricularia auricula polymer (AAP) is a water-soluble polysaccharide that is biodegradable and has potential as a drug carrier. In contrast to dextran, AAP has not been as well characterized as dextran and may be immunogenic
[36][54]. Wang and colleagues formed a nanoparticle named AAP-His by conjugating histidines to a high-molecular-weight AAP. Unlike most polymers described
in this section which contain PLH, the hydroxyl groups on the monomeric unit of AAP were modified by a single histidine. The poorly water-soluble drug paclitaxel (PTX) was incorporated within the hydrophobic unprotonated histidine segment of the micelles. The size and the in vitro cytotoxicity of the PTX-loaded AAP-His micelles were pH-responsive. With longer incubation times (seventy-two hours) and at lower drug dosages (0.01 μg/mL), the PTX micelles inhibited the viability of MCF-7 cells to a greater degree than the free drug (MTT assay). The cumulative release rates over a twelve-hour period at pH 7.4 and 5.0 at 37 °C were about 65% and 85%, respectively. If more than a single histidine was conjugated to the monomeric unit of AAP, improved retention at pH 7.4 and greater pH-dependent release of PTX may occur. Notably, in tumor-bearing (sarcoma-180) mouse models, these PTX-loaded micelles significantly inhibited the tumor weight by about 60% more than free PTX (
p < 0.01)
[37][55]. Although aspects of the design of these AAP-His nanoparticles may be helpful for future drug delivery systems, the use of AAP may be limited because of the induction of cytokines
[36][54]. The CMC was not given in this or the prior dextran study.
It has been suggested that negatively charged nanoparticles have fewer undesirable effects than positively charged nanoparticles. In an interesting report, it was noted that negatively charged micelles at physiological pH that progressively become positive in a slightly acidic environment may target tumors
[38][56]. Specifically, if the charge-reversed micelles become positive (or at least more positive) between 6.5 and 7.0, cellular uptake by tumor cells could be increased significantly. Kim et al. developed one of the two charge-reversed micelles discussed
in this review. After the diblock PEG-PAsp (polyaspartic) copolymer was synthesized, 60% of the PAsp groups were modified with imidazole groups (PEG-PAsp-(im). These PEG-PAsp-(im) micelles, formed via the thin-film rehydration method, had zeta potentials ranging from −16 at pH 7.4 to +1 at pH 4.0. Furthermore, the CMC changed dramatically between pH 7.0 and 6.5, going from about 5 to 65 μg/mL. Consistent with the CMC changes, the size increased from 110 to 275 nm between pH 7.4 and 6.5, respectively. As a result, these micelles have the potential to release hydrophobic drugs at pH levels consistent with the extracellular pH levels of tumors. Although no uptake studies were done with these micelles,
researcherswe think that the less negative zeta potential of the micelles at pH 6.5 would increase their uptake.
One significant problem has been the poor retention of hydrophobic drugs within histidine-containing nanoparticles or micelles at pH 7.4. This was partially addressed in a study by Kim et al. in which a copolymer of histidine and phenylalanine was conjugated to the hydrophilic PEG (PEG-PLH/F)
[39][57]. Notably, the pKa of the polymer varied based on the percentage of phenylalanine and the presence or absence of PEG. As the percentage of phenylalanine increased, the pKa of the diblock PEG-PLH/F decreased. The micelles in which the peptide segment of the block copolymer had a higher molecular weight (M
w of peptide: 5600) released about 5% of pyrene after two days at physiological pH. In contrast, the micelles released about 45% and 60% of the pyrene at pH 6.4 and 6.0, respectively. Unfortunately, these investigations do not seem to have explored this particle further. As a result, it is not known whether the release of pyrene from these micelles correlates with the release of Dox, PTX, or other hydrophobic drugs. More studies are needed to discover whether these histidine-containing micelles stably incorporate hydrophobic drugs at physiological pH.
Of the co-block polymers, only the PTX-loaded micelles developed by Wu et al. were tested for long-term stability
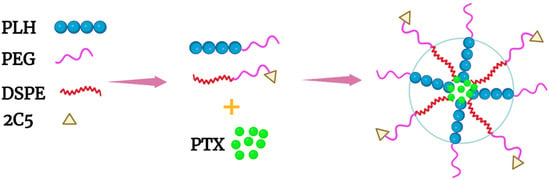
[40][58]. These mixed micelles, made of mPEG-PLH and PEG-1,2-distearoylphostidylethanolamine (PEG-DSPE) copolymers in a ~1:1 weight ratio, delivered PTX effectively (
Figure 2B and
Figure 3). Notably, these micelles were stable and released about 10% of PTX at pH 7.4, whereas the micelles released nearly 50% and 65% of PTX at pH 6.0 and 5.0, respectively, over the same time period (twenty-four hours). The release of PTX at pH 5.0 was dramatic, with a burst release of 60% (twelve hours). Consistent with the PTX release data, 4T1 cells incubated in media at pH 5.8 were very sensitive to the cytotoxic effects of the PTX-loaded micelles compared to cells incubated in media at the same pH with free PTX. Since the pH 5.8 medium had no cytotoxic effect on 4T1 cells, the toxicity was attributed to the PTX or the PTX-loaded mixed micelles. Moreover, the inclusion of an antinucleosomal antibody (2C5-PEG-DSPE) on the surface of the micelles further enhanced their cytotoxicity. Notably, with PLH and DSPE forming the hydrophobic core, the PTX-containing micelles were stable for several months at 4 °C. Since these micelles have several attractive properties, this formulation deserves further study. However, because of the size of the 2C5 monoclonal antibody and its possible lack of tumor specificity
[41][59], single-chain antibodies, as well as other small tumor-specific ligands, should be investigated with this mixed micelle preparation.
Figure 3. Schematic of a mixed micelle formed with the copolymers PEG-PLH, PEG-DSPE, and 2C5-PEG-DSPE. The hydrophobic drug PTX was incorporated within the inner core, which comprised PLH and DSPE.
Another promising approach for delivering Dox was reported by Liang et al., who entrapped a histidine–arginine co-peptide within their NPs
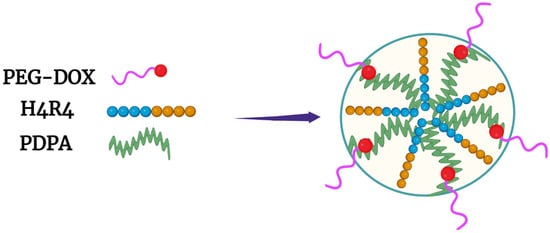
[42][8]. The PEG-Dox conjugate, together with the hydrophobic PDPA polymer (also named PDPAEMA), was mixed with various ratios of the H4R4 co-peptide (HHHHRRRR) (
Figure 4). The H4R4 was incorporated into the NPs to enhance endosomal escape/lysis of the PEG-Dox conjugate. Importantly, the H4R4 did not affect the release of the PEG-Dox conjugate. The PDPA, with a pKa of 6.4, was the primary factor in disrupting the NPs and releasing the H4R4 co-peptide and the PEG-Dox conjugate. At a weight percentage of 14%, the incorporated H4R4 increased the cytotoxicity 30-fold compared to the Dox-loaded NPs. This underscores how vital endosomal lysis was in enhancing the efficacy of Dox. Notably, the Dox-loaded NPs were quite stable and released about 10% of the Peg-Dox at pH 7.4, while the NPs released about 90% of the PEG-Dox at pH 5.5 over the same time (thirty-six hours). It is likely that most of the Dox was bioavailable from the PEG-Dox conjugate, since the Dox-loaded NPs showed markedly more cytotoxicity than the free Dox toward HeLa cells (IC
50: 0.063 vs. 1 μM). An interesting comparison would perhaps be to examine the non-pH-dependent amide bond between PEG and Dox
in this study using a pH-dependent linkage
[43][60]. Nonetheless, the amide bond seemed to be readily cleaved in HeLa cells.
Figure 4. Micelle formed with PEG-Dox conjugate and the hydrophobic PDPA polymer. The H4R4 peptide enhanced endosomal lysis, increasing Dox release into the cytosol.
Several questions arise
byfrom reserchersthis study. Would incorporating the H4R4 peptide into histidine-rich or other pH-dependent micelles enhance the cytosolic delivery of the drug? Since the R4 peptide was likely on the surface, would a longer histidine segment add greater stability to the micelle? Why did the pH-sensitive PDPA not effectively lyse endosomes? It is of note that most block or grafted PLH polymers discussed
in this review were not tested for their endosomal lysis potential.