Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Catherine Yang and Version 1 by Ana Figueiras.
Exosomes are defined as a type of extracellular vesicle released when multivesicular bodies of the endocytic pathway fuse with the plasma membrane. They are characterized by their role in extracellular communication, partly due to their composition, and present the ability to recognize and interact with cells from the immune system, enabling an immune response. Their targeting capability and nanosized dimensions make them great candidates for cancer therapy. As chemotherapy is associated with cytotoxicity and multiple drug resistance, the use of exosomes targeting capabilities, able to deliver anticancer drugs specifically to cancer cells, is a great approach to overcome these disadvantages.
- exosomes
- ESCRT pathway
- nanovesicles
- cancer therapy
1. Paclitaxel
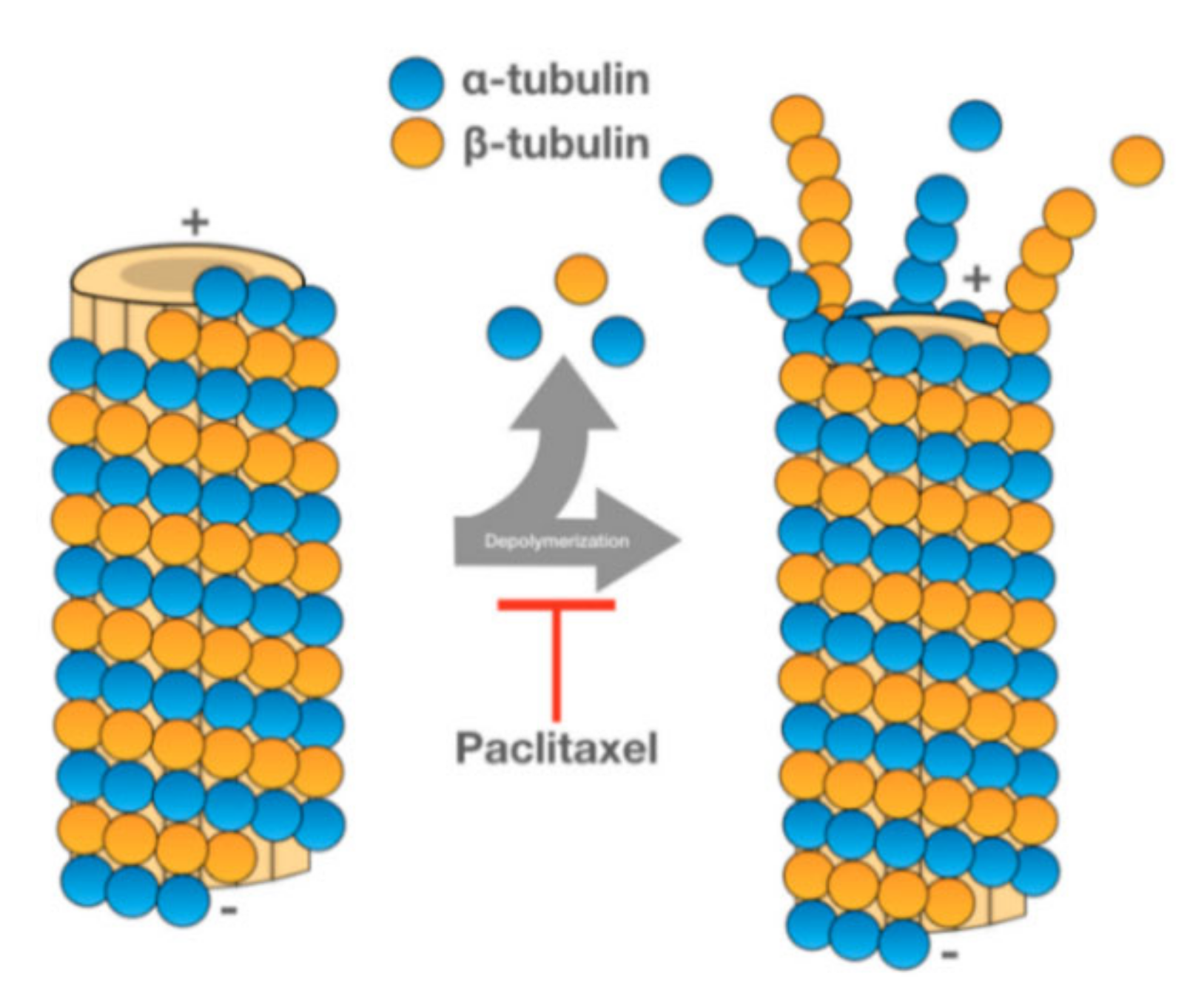
PTX is one of the most used anticancer drugs. It is naturally found in the bark of Taxus brevifolia, although new extraction methods have been developed to obtain this drug [86][1]. PTX promotes the assembly of tubulin into microtubules, stabilizing the latter and preventing their dissociation. Since the microtubules do not dissociate, cell cycle progression is blocked, and the growth of cancerous cells is hampered (see Figure 61) [87][2].
PTX has been used in the treatment of several types of cancer, namely colorectal, ovarian, breast or lung cancer [86,87][1][2]. The efficiency of PTX and other chemotherapeutic drugs is limited by the emergence of multiple drug resistance (MDR) [89][4]. The overexpression of ATP-binding cassette (ABC) transporters, specifically the drug efflux P-glycoprotein (Pgp) transporter, is one of the mechanisms mediating MDR in cancer cells [12][5]. To overcome MDR, Kim et al. evaluated oncological treatment efficiency of PTX-loaded exosomes.
The exosomes used in this study were extracted from a murine macrophage cell line, using a precipitating polymer (ExoQuick-TC™ kit). As previously mentioned, three loading methods were tested in order to evaluate the loading capacity of each technique. The exosomes loaded with PTX were purified using SEC and analysed by HPLC. The exosome-PTX complex obtained by sonication was used for further studies since it showed better values of loaded PTX [12][5].
To evaluate the antineoplastic effect of exosome-PTX complex, a Lewis Lung Carcinoma mouse model was used. Using a lentiviral vector, 3LL-M27 cells (a carcinoma cell line overexpressing Pgp) were transfected in order to encode fluorescent proteins. As such, tumoral growth can be assessed via fluorescence imaging techniques [12][5].
The mice were injected intravenously with the modified 3LL-M27 cells. The carcinoma cells were allowed to establish for 48 h. To begin the treatment, the mice were split through different groups, where saline, free PTX and with the exosome-PTX system were administered, respectively. Tumour progression was accompanied by monitoring and quantifying the chemiluminescent signal emitted by the modified carcinoma cells. In vivo images show the progression of the metastasis in the different treatment groups (Figure 72a). 22 days after the administration of the 3LL-M27 cells, the mice were sacrificed. Lung sections were observed via confocal microscopy (Figure 72b). Mice administered with saline present the biggest tumour growth. Both free PTX and exosome-PTX complex inhibit tumour progression. When comparing PTX based treatments, the exosome-PTX complex proved to be more effective at stopping metastasis progression when compared with free PTX [12][5].
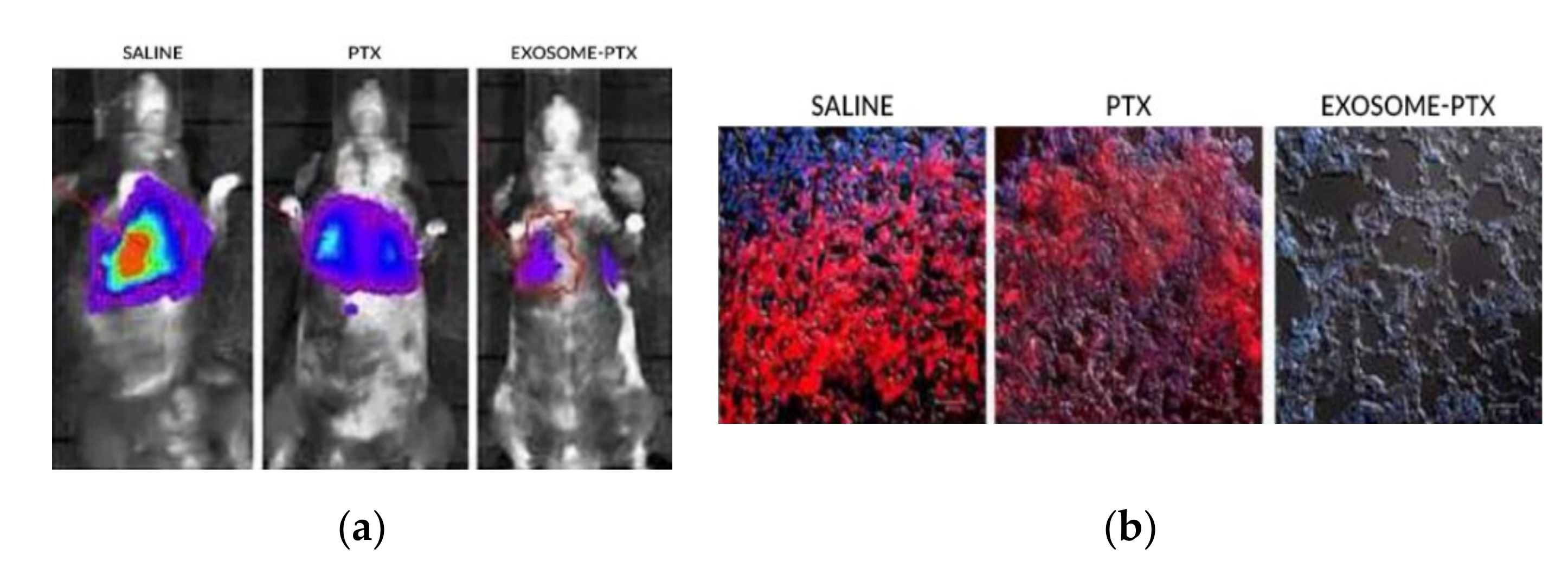
Figure 72. (a) In vivo imaging of chemiluminescent signal monitoring in each treatment group [12][5]. (b) Confocal microscopy of lung sections of the sacrificed mice. It is possible to observe no detection of fluorescence in the Exosome-PTX treated cells, when compared to the non-treated control group (saline) [12][5].
2. siRNA
siRNA belongs to a class of small, double stranded, non-coding RNAs, composed of 20–30 nucleotides. Through a mechanism of RNA interference, these molecules can target complementary mRNA. This way, it causes mRNA degradation and subsequent gene silencing [90][6].
Due to their mechanism and the possibility of exosome loading, exosome-siRNA complexes are emerging as a therapeutic agent in oncologic conditions. Due to an uncontrolled tumour growth and metastasis, head and neck cancer (HNC) still has a poor prognosis [13][7]. One of the reasons that make HNC so malignant is the epithelial-mesenchymal transition (EMT). EMT is a transforming process that some epithelial cells go through, leading to the formation of mesenchymal cells. EMT is usually associated with tumour growth and cancer progression. Cancer cells become more invasive and form metastases easier [91][8]. The transient receptor potential polycystic 2 (TRPP2), an ion channel, is one of the regulating mechanisms of EMT in HCN. Targeting TRPP2 could be a way to inhibit tumour progression in HNC, which was the goal of a developed study by Wang et al. [13][7].
Wang et al. investigated if TRPP2 siRNA would have any effect in TRPP2 gene knockdown and further influence HNC growth and metastasis. To achieve this, a TRPP2 siRNA-exosome complex was prepared and used. Exosomes were obtained from HEK 293 cells using PEG as the precipitating agent (a centrifugation was previously performed to remove cells and cell debris). Exosomes were incubated with the TRPP2 siRNA, with the latter being loaded and encapsulated. siRNA is susceptible to the action of many enzymes, such as nucleases, so it’s fundamental that exosomes provide some sort of protection to the cargo they carry. To assess this, an agarose gel electrophoresis was performed to evaluate the stability of free TRPP2 siRNA and the TRPP2 siRNA-exosome complex against RNA nucleases (see Figure 83). Free TRPP2 siRNA was degraded after 5 min, whereas exosome-encapsulated TRPP2 siRNA maintained stability, proving that exosomes shield siRNA from enzyme degradation [13][7].
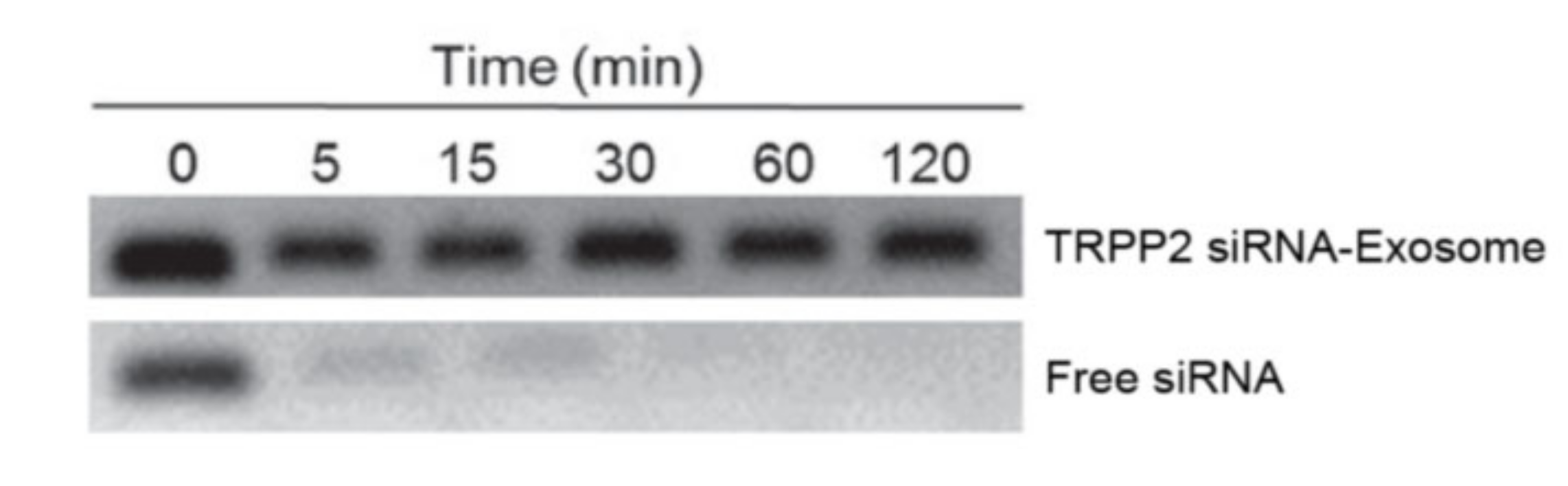
Figure 83. Results of agarose gel electrophoresis. Each sample was incubated with RNA nucleases, free siRNA was mostly degraded after 5 min [13].
Results of agarose gel electrophoresis. Each sample was incubated with RNA nucleases, free siRNA was mostly degraded after 5 min [7].
FaDu cells, a human squamous carcinoma cell line, were used to evaluate TRPP2 siRNA-exosome complex efficiency. The cells were incubated with free TRPP2 siRNA (used as the control) and with the exosomes. Western blot analysis was performed to confirm if TRPP2 suppression occurred. The results indicated that there was a significant reduction of TRPP2 expression in FaDu cells treated with the TRPP2 siRNA-exosomes (see Figure 94). Since TRPP2 is associated with EMT, the authors of the study also confirmed the influence that the treatment had in this process. Common biomarkers of EMT are E-cadherin (at low levels), vimentin and N-cadherin (both with increased levels). For these biomarkers, western blot analysis was also performed, all of them with promising results (Figure 94). There was an increase in the expression of E-cadherin and a reduction of both vimentin and N-cadherin, showing the potential of TRPP2 siRNA-exosomes as a treatment option for HNC [13][7].

Figure 94. Results of western blot analysis for TRPP2 expression and EMT biomarkers. Free siRNA was used as control [13].
Results of western blot analysis for TRPP2 expression and EMT biomarkers. Free siRNA was used as control [7].
3. Doxorubicin-Loaded Nanoparticles
DOX belongs to anthracyclines, a class of drugs routinely used in chemotherapy. Its use is recommended in the treatment of several cancers (ovarian, breast, lung, Hodgkin’s lymphoma). The way DOX fights cancer cells has been described by two mechanisms. One occurs in the nucleus of the cell, where DOX intercalates itself with DNA, impairing the activity of topoisomerase-II and blocking nucleic acid transcription. The other way involves oxidation of DOX into semiquinone, with posterior transformation into DOX again. This process generates reactive oxygen species that cause oxidative stress and cell membrane damage, eventually causing cell death [92][9]. Besides MDR caused by ABC transporters (like the previously mentioned Pgp), the use of DOX is limited due to the cardiotoxic effect it causes.
As previously mentioned, the use of nano-based technologies in drug delivery seems like an almost perfect solution. However, exogenous particles have the disadvantage of being recognized and eliminated by the immune system [63][10]. To overcome this, Yong et al. developed biomimetic NPs, converging the efficiency of NPs with the endogenous benefits of exosomes [14][11].
Porous silicon NPs were loaded with DOX. The choice of these NPs was based on their biocompatibility and drug loading capacity. Afterwards, the DOX-loaded NPs were incubated with H22 cells, a mouse hepatocellular carcinoma cell line. Exosomes were obtained by centrifugation followed by differential centrifugation. Cells were able to incorporate DOX-NPs and release DOX-NP-Exosomes. The same procedure was applied to other cell lines, originating DOX-NP-Exosomes from different origins [14][11].
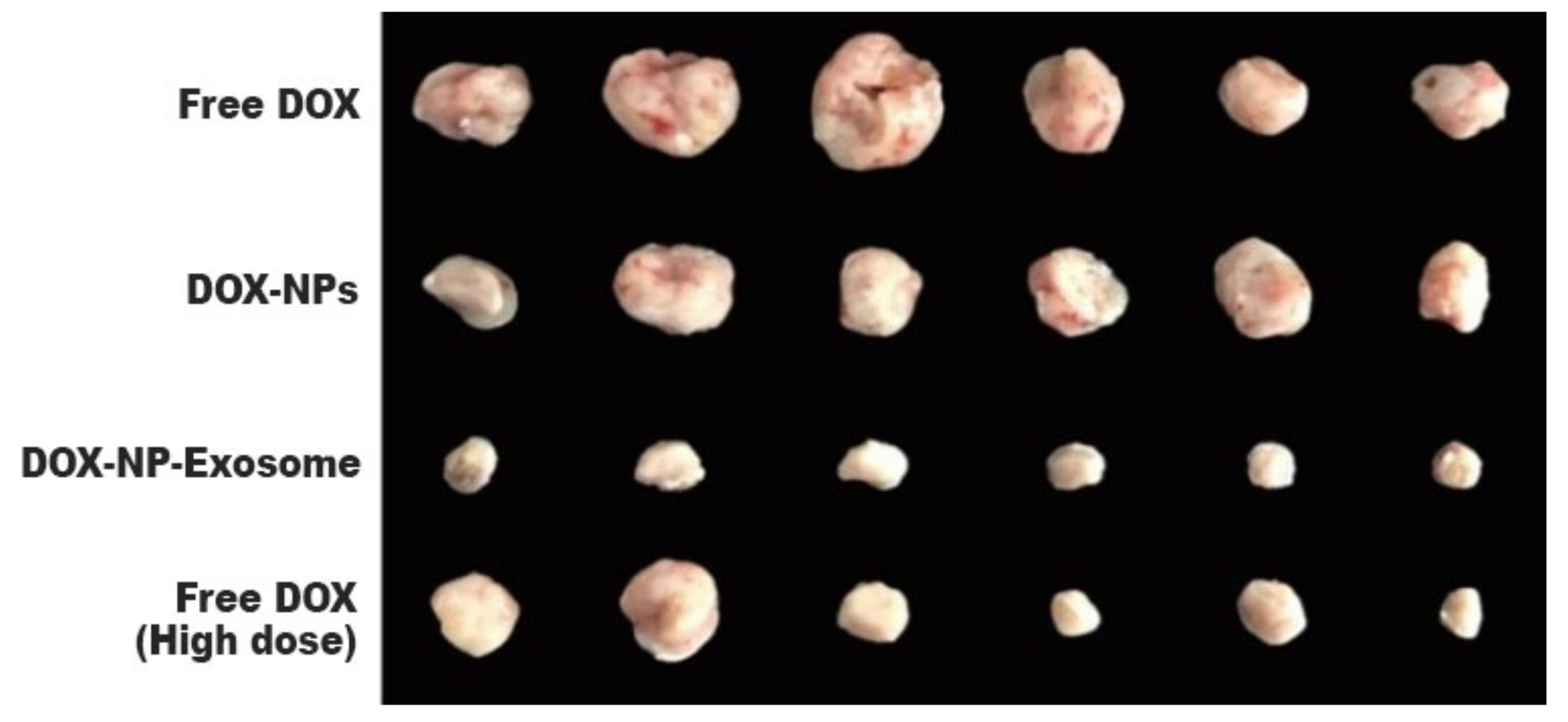
Another cause of MDR is the existence of cancer stem cells (CSC). This cell population has high expressions of ABC transporters and an elevated self-renewal rate. These properties render most treatments ineffective. In this sense, H22 CSCs were treated with DOX-NP-Exosomes to evaluate the cytotoxic effectiveness of the nano-complex. The procedure was done with different groups, containing free DOX, DOX-NPs and DOX-NP-Exosomes, respectively. The results are represented in Figure 105. In comparison with the other methods, there is an accentuated reduction of H22 CSCs in cells treated with the DOX-NP-Exosome complex [14][11].
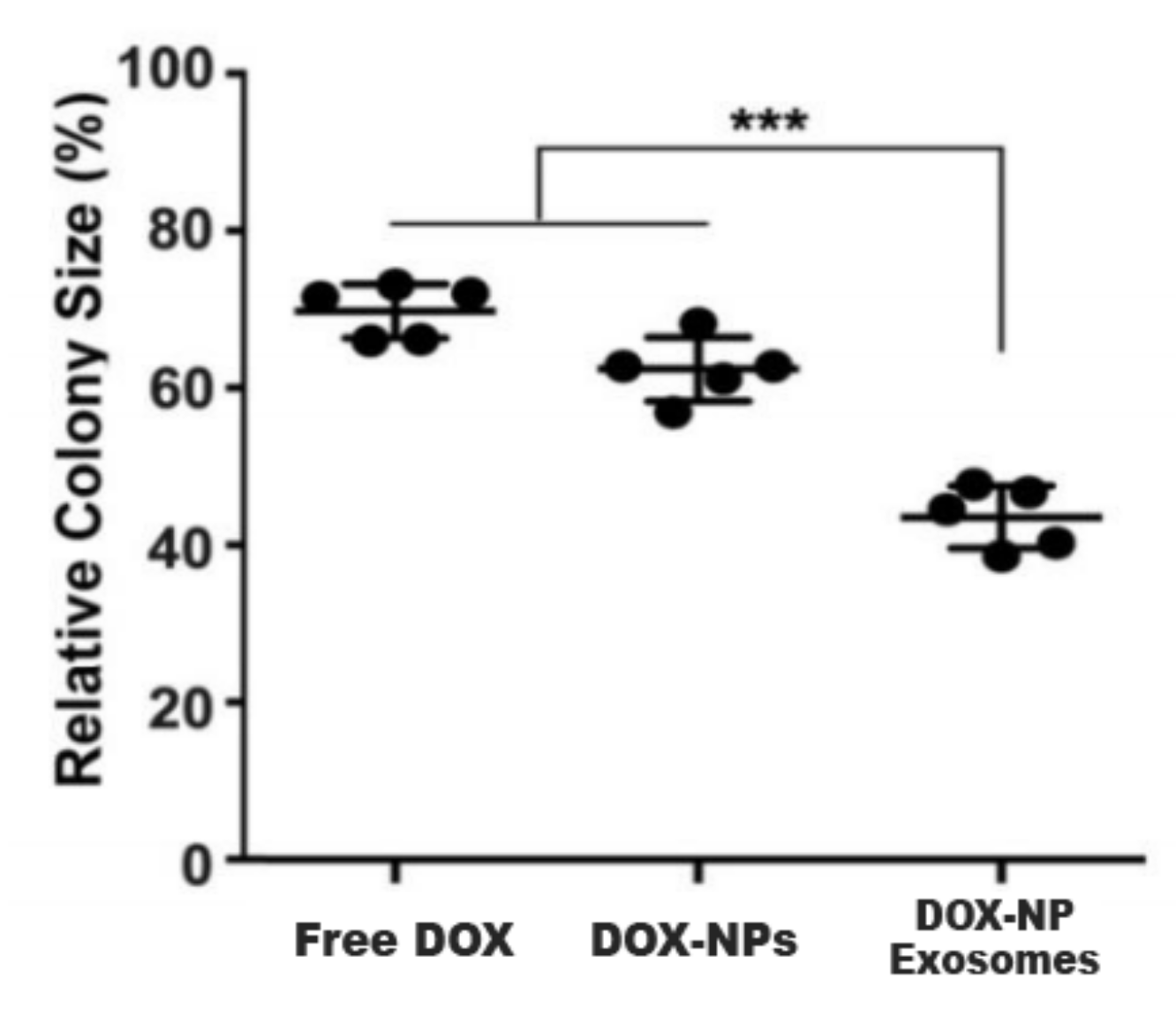
To assess in vivo results, a model was used with H22 tumour-bearing mice. The mice were administered with different DOX formulations (free DOX, DOX-NPs and DOX-NP-Exosomes), with a drug concentration of 0.5 mg/kg. A fourth group was administered with free DOX at a higher concentration, namely 4 mg/kg. Free DOX and DOX-NPs (at a dosage of 0.5 mg/kg) showed weak tumour growth inhibiting capabilities. The most effective treatment was with DOX-NP-Exosomes, which proved to be even more effective than high dose of free DOX (4 mg/kg). This formulation achieved the biggest reduction in tumour mass (see Figure 116) and also increased mice survival time. Yong et al. developed an exosomal formulation, containing NPs loaded with a therapeutic agent. The presented drug delivery system proved itself to be biocompatible, not triggering an immune response. In terms of MDR, this system exhibits a higher tumour accumulation in comparison with other formulations (like free DOX) that suffer efflux by the action of ABC transporters [14][11].
References
- Zhu, L.; Chen, L. Progress in research on paclitaxel and tumor immunotherapy. Cell. Mol. Biol. Lett. 2019, 24, 1–11.
- Weaver, B.A. How Taxol/paclitaxel kills cancer cells. Mol. Biol. Cell 2014, 25, 2677–2681.
- Fong, A.; Durkin, A.; Lee, H. The potential of combining tubulin-targeting anticancer therapeutics and immune therapy. Int. J. Mol. Sci. 2019, 20, 586.
- Liu, J.; Song, L.; Liu, S.; Zhao, S.; Jiang, Q.; Ding, B. A Tailored DNA Nanoplatform for Synergistic RNAi-/Chemotherapy of Multidrug-Resistant Tumors. Angew. Chem. Int. Ed. 2018, 57, 15486–15490.
- Kim, M.S.; Haney, M.J.; Zhao, Y.; Mahajan, V.; Deygen, I.; Klyachko, N.L.; Inskoe, E.; Piroyan, A.; Sokolsky, M.; Okolie, O.; et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 655–664.
- Dana, H.; Chalbatani, G.M.; Mahmoodzadeh, H.; Karimloo, R.; Rezaiean, O.; Moradzadeh, A.; Mehmandoost, N.; Moazzen, F.; Mazraeh, A.; Marmari, V.; et al. Molecular Mechanisms and Biological Functions of siRNA. Int. J. Biomed. Sci. 2017, 13, 48–57.
- Wang, C.; Chen, L.; Huang, Y.; Li, K.; Jinye, A.; Fan, T.; Zhao, R.; Xia, X.; Shen, B.; Du, J.; et al. Exosome-delivered TRPP2 siRNA inhibits the epithelial-mesenchymal transition of FaDu cells. Oncol. Lett. 2019, 17, 1953–1961.
- Dave, B.; Mittal, V.; Tan, N.M.; Chang, J.C. Epithelial-mesenchymal transition, cancer stem cells and treatment resistance. Breast Cancer Res. 2011, 14, 1–5.
- Thorn, C.F.; Oshiro, C.; Marsh, S.; Hernandez-Boussard, T.; McLeod, H.; Klein, T.E.; Altman, R.B. Doxorubicin pathways: Pharmacodynamics and adverse effects. Pharm. Genom. 2011, 21, 440–446.
- Luan, X.; Sansanaphongpricha, K.; Myers, I.; Chen, H.; Yuan, H.; Sun, D. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol. Sin. 2017, 38, 754–763.
- Yong, T.; Zhang, X.; Bie, N.; Zhang, H.; Zhang, X.; Li, F.; Hakeem, A.; Hu, J.; Gan, L.; Santos, H.A.; et al. Tumor exosome-based nanoparticles are efficient drug carriers for chemotherapy. Nat. Commun. 2019, 10, 1–16.
More