You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Dean Liu and Version 1 by Ahmed Ramadan.
RNA editing is generally perceived as a repair tool to correct genomic mutation (point mutation) at the transcript level in organelles such as mitochondria and chloroplasts.
- plant chloroplast
- Transcription
- RNA editing
1. Introduction
RNA is transcribed from DNA using RNA polymerase in the nucleus, mitochondria and plastids and is further processed if needed. RNA editing represents a post-transcriptional modification (insertion, deletion or conversion) mechanism observed in many domains of life, including prokaryotes and eukaryotes [1]. The editing of RNA has already been identified in many plants, such as Arabidopsis, rice, soybean, tobacco, wheat, barley, and maize [2][3][4]. This is a powerful genetic phenomenon in organisms and was first discovered when Benne (1986) was investigating the mitochondrial cytochrome oxidase in the protozoan Trypanosoma brucei [5][6].
To begin with, RNA is transcribed normally as per the genetic code of the gene. After this, the RNA is altered in certain ways, which can incorporate uridine at certain loci and/or facilitate a change of cytidine to uridine [7]. These editing events can happen in most forms of RNA, including messenger RNA (mRNA), transfer RNA (tRNA), and ribosomal RNA (rRNA), transcribed in the cell, including organelles such as mitochondria and chloroplast [8]. The final result after such editing events might ultimately generate an RNA molecule that does not entirely reflect the complimentary genetic code in the DNA from which it was deciphered [9]. Numerous studies have shown that RNA editing events could lead to impaired organelle biogenesis, endosperm development, and response to stress conditions in plants, and thus, it becomes important to study it [10][11][12].
RNA editing can happen in multiple ways, such as the C-to-U, U-to-C, and A-to-I conversions, as well as insertions and deletions of U or G. However, in plants, RNA editing occurs mainly in the mitochondria and plastids. C-to-U-type RNA editing is primarily found [8]; however, recent studies have investigated new types of editing without suggesting the mechanisms involved in these new types, such as U to G [13]. Plant organellar RNA editing involves at least three elements: trans-acting factors that bind to cis-acting elements and thereby recruit editing enzymes to induce nucleotide conversion [14]. Plant RNA editing is mediated by a variety of pentatricopeptide repeat (PPR) proteins encoded in the nucleus [15][16]. The editosome machinery of flowering plants also requires several other non-PPR protein factors, such as RNA interacting protein/multiple organellar RNA editing factor (RIP/MORF family), organelle RNA recognition motif (ORRM family), and organelle zinc finger (OZ family) [16]. Advancements in gene sequencing, computational methods, and data analysis pipelines have led to high-resolution access to RNA editing events and predictions towards it [17]. Many online and offline resources are available to predict and assist researchers in searching for RNA editing events in their study organism and comparing them with other plants with an evolutionary background. Phylogenetic analysis, statistical correlations and regression models, machine learning approaches and artificial intelligence based on available datasets further empower the research into RNA editing and understanding it in a larger context [18].
2. Transcription and RNA Editing in Plant Chloroplasts
The expression of chloroplast DNA (cpDNA) genes depends on post-transcriptional changes such as polycistronic mRNA processing, intron splicing, and RNA editing [19]. Angiosperm chloroplast genes are transcribed by bacterial-like multi-subunit RNA polymerase (PEP) and phage-like RNA polymerase (NEP) [20]. The PEP enzyme is made from subunits derived from nuclear and cpDNA genes. The subunits α, β, β’ and β’’ are encoded by cp genes. However, sigma (SIG) factors and polymerase-associated proteins are encoded by nuclear genes [21]. These types of RNA polymerases are recognized at bacterial-like promoters in chloroplast genome-like psaS genes [22]. NEP is a single subunit enzyme that has similarity to bacteriophage-type RNA polymerase, which is responsible for promoting the transcription of cp PEP subunits [21]. There are three types of NEP polymerases coded by RPOT genes: RPOTp, RPOTm and RPOTmp [20][22] (Figure 1). The NEP polymerases are divided into three class (Ia, Ib and II) depending on the features of recognized promoters [22].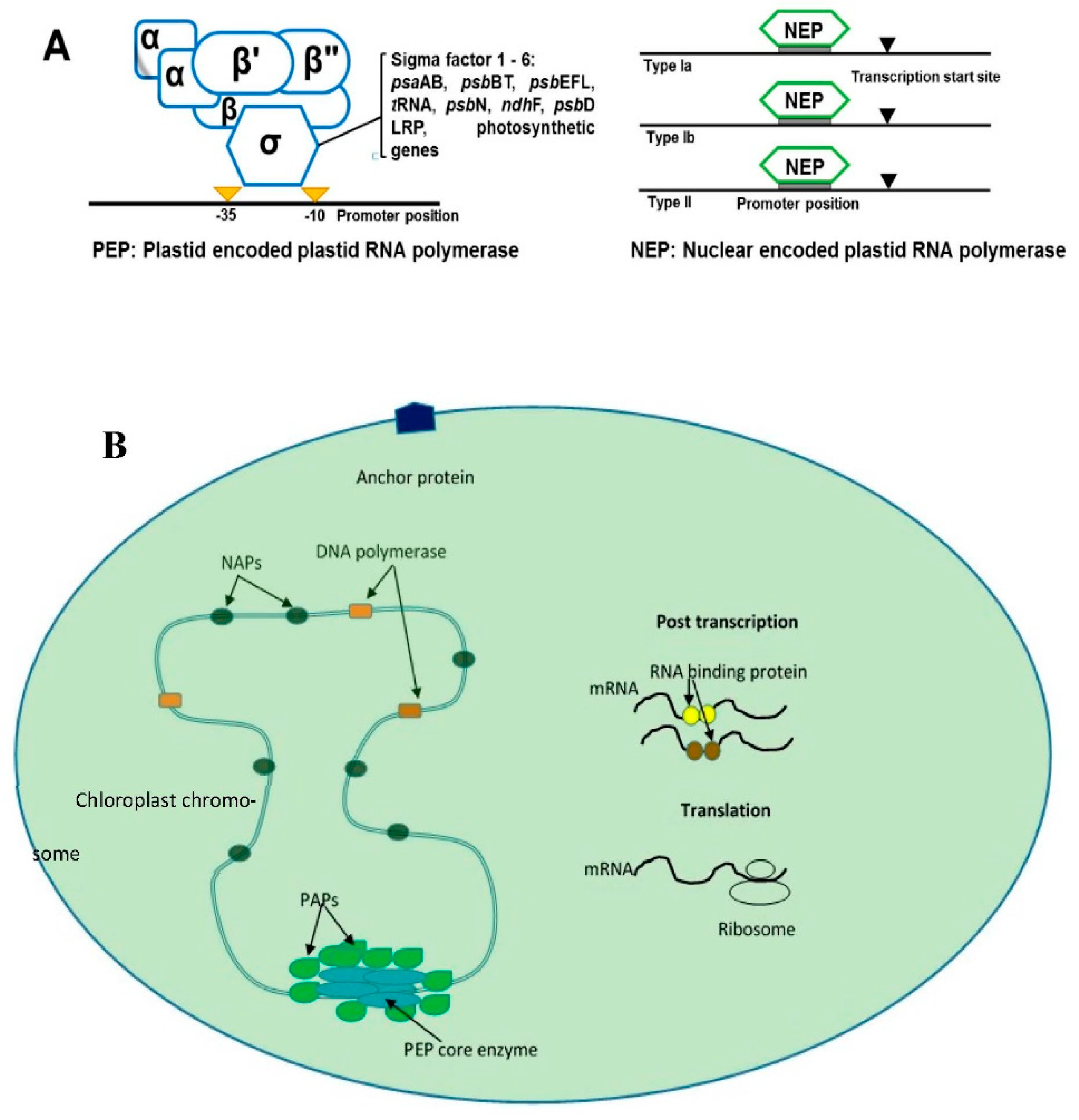
Figure 1. The figures show the general scheme of chloroplast transcription. (A) shows the two types of chloroplast RNA polymerase transcription that occurs in plants: plastid-encoded plastid RNA polymerase (PEP, which recognizes bacterial-like promoters such as PSA gene promoters) and nucleus-encoded plastid RNA polymerase (NEP), which recognizes housekeeping genes in chloroplast-like rpo genes. NEB is divided into subclasses Ia, Ib and II depending on the structure of recognized promoters. (B) shows the general scheme of plants’ transcription that involves relevant proteins in nucleoid. PEP: plastid RNA polymerase, white blue color; PAPs: RNA polymerase-associated proteins, green color; NAPs: plastid nucleoid-associated proteins; mRNA binding proteins: such as PPR and mTERF, yellow and brown color.
References
- Gerber, A.; O’Connell, M.A.; Keller, W. Two forms of human double-stranded RNA-specific editase 1 (hRED1) generated by the insertion of an Alu cassette. RNA 1997, 3, 453–463.
- Ramadan, A.M. Light/heat effects on RNA editing in chloroplast NADH-plastoquinone oxidoreductase subunit 2 (ndhB) gene of Calotropis (Calotropis procera). J. Genet. Eng. Biotechnol. 2020, 18, 49.
- Kim, S.; Yang, J.; Moon, S.; Ryu, C.; An, K.; Kim, K.; Yim, J.; An, G. Rice OGR1 encodes a pentatricopeptide repeat–DYW protein and is essential for RNA editing in mitochondria. Plant J. 2009, 59, 738–749.
- Zheng, P.; Wang, D.; Huang, Y.; Chen, H.; Du, H.; Tu, J. Detection and analysis of C-to-U RNA editing in rice mitochondria-encoded ORFs. Plants 2020, 9, 1277.
- Pusnik, M.; Small, I.; Read, L.K.; Fabbro, T.; Schneider, A. Pentatricopeptide repeat proteins in Trypanosoma brucei function in mitochondrial ribosomes. Mol. Cell. Biol. 2007, 27, 6876–6888.
- Göringer, H.U. ‘Gestalt’, composition and function of the trypanosoma brucei editosome. Annu. Rev. Microbiol. 2012, 66, 65–82.
- Wedekind, J.E.; Dance, G.S.; Sowden, M.P.; Smith, H.C. Messenger RNA editing in mammals: New members of the APOBEC family seeking roles in the family business. Trends Genet. 2003, 19, 207–216.
- Hao, W.; Liu, G.; Wang, W.; Shen, W.; Zhao, Y.; Sun, J.; Yang, Q.; Zhang, Y.; Fan, W.; Pei, S.; et al. RNA editing and its roles in plant organelles. Front. Genet. 2021, 12, 1747.
- Herzel, H.; Weiss, O.; Trifonov, E.N. 10-11 bp periodicities in complete genomes reflect protein structure and DNA folding. Bioinformatics 1999, 15, 187–193.
- Ciuzan, O.; Hancock, J.; Pamfil, D.; Wilson, I.; Ladomery, M. The evolutionarily conserved multifunctional glycine-rich RNA-binding proteins play key roles in development and stress adaptation. Physiol. Plantarum 2015, 153, 1–11.
- Yan, J.; Zhang, Q.; Yin, P. RNA editing machinery in plant organelles. Sci. China Life Sci. 2018, 61, 162–169.
- Ramadan, A.M.; Alnufaei, A.A.; Khan, T.K.; Ali, H.M.; Eissa, H.F.; Hassan, S.M. The first report of RNA U to C or G editing in the mitochondrial NADH dehydrogenase subunit 5 (Nad5) transcript of wild barley. Mol. Biol. Rep. 2021, 48, 6057–6064.
- Ramadan, A.M.; Said, O.A.M.; Abushady, A.M. Salinity stress reveals three types of RNA editing sites in mitochondrial Nad7 gene of wild barley both in silico and in qRT-PCR experiments. Theor. Exp. Plant Physiol. 2022, 34, 13–22.
- Takenaka, M.; Zehrmann, A.; Verbitskiy, D.; Kugelmann, M.; Härtel, B.; Brennicke, A. Multiple organellar RNA editing factor (MORF) family proteins are required for RNA editing in mitochondria and plastids of plants. Proc. Natl. Acad. Sci. USA 2012, 109, 5104–5109.
- Lo Giudice, C.; Pesole, G.; Picardi, E. REDIdb 3.0: A comprehensive collection of RNA editing events in plant organellar genomes. Front. Plant Sci. 2018, 9, 482.
- Sun, T.; Bentolila, S.; Hanson, M.R. The Unexpected Diversity of Plant Organelle RNA Editosomes. Trends Plant Sci. 2016, 21, 962–973.
- Chateigner-Boutin, A.L.; Small, I. A rapid high-throughput method for the detection and quantification of RNA editing based on high-resolution melting of amplicons. Nucleic Acids Res. 2007, 35, e114.
- Lo Giudice, C.; Hernandez, I.; Ceci, L.R.; Pesole, G.; Picardi, E. RNA editing in plants: A comprehensive survey of bioinformatics tools and databases. Plant Physiol. Biochem. 2019, 137, 53–61.
- Nishikura, K. Functions and regulation of RNA editing by ADAR deaminases. Annu. Rev. Biochem. 2010, 79, 321–349.
- Morley, S.A.; Ahmad, N.; Nielsen, B.L. Plant organelle genome replication. Plants 2019, 8, 358.
- Tadini, L.; Jeran, N.; Peracchio, C.; Masiero, S.; Colombo, M.; Pesaresi, P. The plastid transcription machinery and its coordination with the expression of nuclear genome: Plastid-Encoded Polymerase, Nuclear-Encoded Polymerase and the Genomes Uncoupled 1-mediated retrograde communication. Philos. Trans. R. Soc. 2020, 375, 1801.
- Yagi, Y.; Shiina, T. Recent advances in the study of chloroplast gene expression and its evolution. Front. Plant Sci. 2014, 5, 61.
- Börner, T.; Aleynikova, A.Y.; Zubo, Y.O.; Kusnetsov, V.V. Chloroplast RNA polymerases: Role in chloroplast biogenesis. Biochim. Biophys. Acta (BBA)-Bioenerg. 2015, 1847, 761–769.
- Boniecka, J.; Prusińska, J.; Dąbrowska, G.B.; Goc, A. Within and beyond the stringent response-RSH and (p) ppGpp in plants. Planta 2017, 246, 817–842.
- Zhang, Y.; Zhang, A.; Li, X.; Lu, C. The role of chloroplast gene expression in plant responses to environmental stress. Int. J. Mol. Sci. 2020, 21, 6082.
- Bang, S.; Lee, H.; Park, S.-H.; Lee, D.-K.; Seo, J.; Kim, Y.; Park, S.-C.; Kim, J.-K. OsCRP1, a ribonucleoprotein gene, regulates chloroplast mRNA stability that confers drought and cold tolerance. Int. J. Mol. Sci. 2021, 22, 1673.
- Covello, P.; Gray, M.W. On the evolution of RNA editing. Trends Genet. 1993, 9, 265–268.
- Takenaka, M.; Zehrmann, A.; Verbitskiy, D.; Härtel, B.; Brennicke, A. RNA editing in plants and its evolution. Annu. Rev. Genet. 2013, 47, 335–352.
- Verma, S.; Tomar, R.S.; Rathode, V.; Thakker, J.; Shubham, S.; Bhagwaat, N.; Raval, S.; Antala, T.; Jogia, Z.; Golakiya, B.A. Genome sequencing analysis of macrophomina phaseolina resistant and susceptible castor genotype. Biosci. Biotechnol. Res. Asia 2018, 15, 195–215.
- Oldenkott, B.; Yamaguchi, K.; Tsuji-Tsukinoki, S.; Knie, N.; Knoop, V. Chloroplast RNA editing going extreme: More than 3400 events of C-to-U editing in the chloroplast transcriptome of the lycophyte Selaginella uncinata. RNA 2014, 20, 1499–1506.
- Lin, C.; Ko, C.; Kuo, C.; Liu, M.; Schafleitner, R.; Chen, L. Transcriptional slippage and RNA editing increase the diversity of transcripts in chloroplasts: Insight from deep sequencing of vigna radiata genome and transcriptome. PLoS ONE 2015, 10, e0129396.
- Chen, T.; Liu, Y.; Wang, X.; Wu, C.; Huang, C.; Chang, C. Whole plastid transcriptomes reveal abundant RNA editing sites and differential editing status in Phalaenopsis aphrodite subsp. formosana. Bot. Stud. 2017, 58, 38.
- Zhang, A.; Jiang, X.; Zhang, F.; Wang, T.; Zhang, X. Dynamic response of RNA editing to temperature in grape by RNA deep sequencing. Funct. Integr. Genom. 2020, 20, 421–432.
More
