The over-reliance on herbicides to reduce weed infestation in crops has led to the rapid evolution of herbicide-resistant (HR) weeds. Weed resistance to herbicides should be minimized, as this can lead to serious limitations in the food security for people around the world. Landing page resistance can occur as a result of changes in the biochemical sites of the action of one herbicide. Inappropriate resistance occurs through mechanisms that reduce the number of herbicide molecules and reach their target site. In major field crops, synthetic herbicides are used to control weeds worldwide. Cross-resistance can occur with herbicides from the same or different herbicide families, and with the same or different sites of action. Multiple resistance refers to the evolved mechanisms of resistance to more than one herbicide (e.g., resistance to inhibitors (ALS) and (ACC), and this resistance has resulted from separate selection processes). Currently, weed re-sistance has been transferred to 161 different herbicides, covering twenty-three of the twenty-six known herbicide sites. We can protect crops that are associated with herbicide tolerant weeds through biochemical, genetic and crop control strategies. The “European Green Deal” forces producers to change their approaches to plant protection. The emphasizes the importance and advantages of enhancing the natural resistance of plants to pests, with particular emphasis on the importance of oxylipins in plant protection. The summarize the latest research on the reaction of plants to pesticides, including herbicides, in order to assess the possibility of using jasmonates and brassinosteroids to stimulate the natural, induced systemic immunity of plants, as well as investigate the possibility of the interaction of oxylipins with ethylene, salicylates and other compounds.
- brassinosteroidy
- techniki zarządzania uprawą
- stres w wysokiej temperaturze
- działanie fitohormonów
- jasmonaty
- mechanizm odpowiedzi na stres
1. Introduction
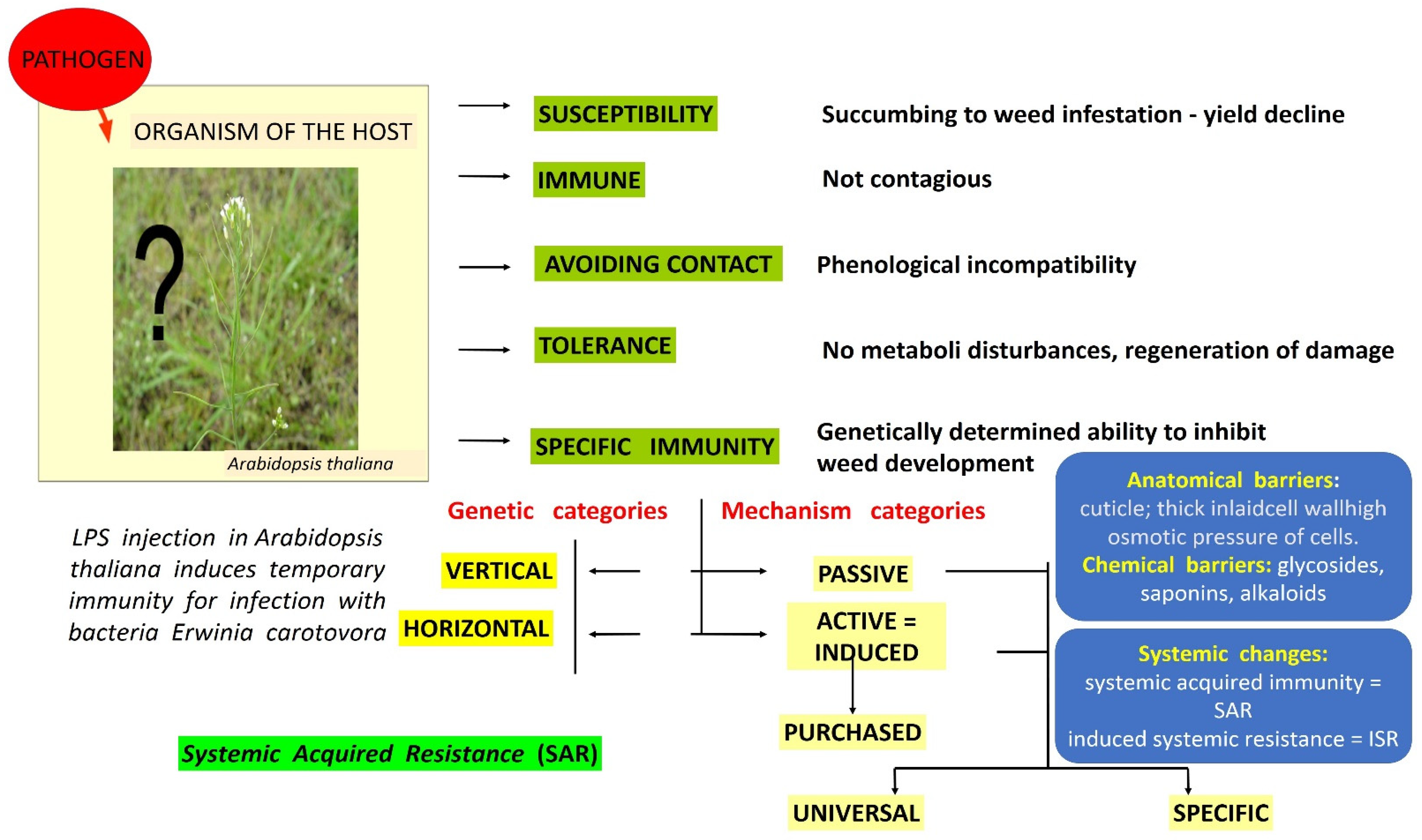
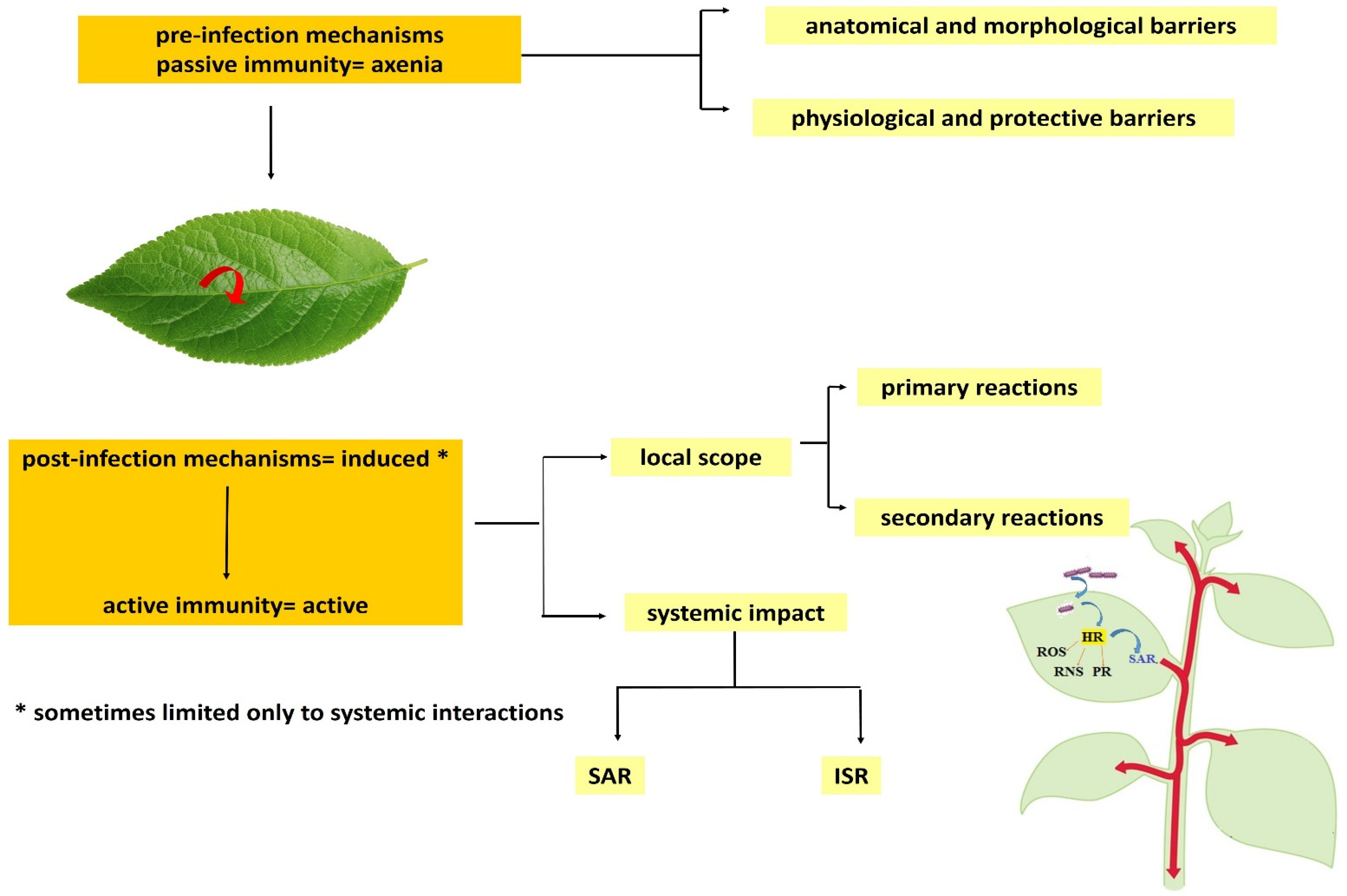
2. Resistance of Weeds to Herbicides
3. Resistance of Plants to Pesticides
- Ahmad, P.; Rasool, S.; Gul, A.; Sheikh, S.A.; Akram, N.A.; Ashraf, M.; Kazi, A.M.; Gucel, S. Jasmonates: Multifunctional Roles in Stress Tolerance. Front. Plant Sci. 2016, 7, 813. https://doi.org/10.3389/fpls.2016.00813.
- Beckie, H.J. Herbicide Resistance in Plants. Plants 2020, 9, 435. https://doi.org/10.3390/plants9040435.
- Ali, B. Practical applications of brassinosteroids in horticulture—Some field perspectives. Hortic. 2017, 225, 15–21. https://doi.org/10.1016/j.scienta.2017.06.051.
- Vigor, C.; Bertrand-Michel, J.; Pinot, E.; Oger, C.; Vercauteren, J.; Le Faouder, P.; Galano, J.-M.; Lee, J.C.-Y.; Durand, T. Non-enzymatic lipid oxidation products in biological systems: Assessment of the metabolites from polyunsaturated fatty acids. Chromatogr. B 2014, 964, 65–78. https://doi.org/10.1016/j.jchromb.2014.04.042.
- bbs, M.; Leng, S.; Devassy, J.G.; Monirujjaman, M.; Aukema, H.M. Advances in Our Understanding of Oxylipins Derived from Dietary PUFAs. Nutr. 2015, 6, 513–540. https://doi.org/10.3945/an.114.007732.
- Bao, A.B.; Won, O.J.; Sin, H.T.; Lee, J.J.; Park, K.W. Mechanisms of herbicide resistance in weeds. Korean J. Agric. Sci. 2017, 44, 1–15.
- Dekker, J.; Duke, S.O. Herbicide-resistant field crops. Agron. 1995, 54, 69–116. https://core.ac.uk/download/pdf/38933693.pdf (accessed: 24.10.2022)
- Porta, H.; Rocha-Sosa, M. Plant Lipoxygenases. Physiological and Molecular Features. Plant Physiol. 2002, 130, 15–21. https://doi.org/10.1104/pp.010787.
- Cristensen, S.A.; Kolomiets, M.V. The lipid language of plant–fungal interactions. Fungal Genet. Biol. 2011, 48, 4–14. DOI: 10.1016/j.fgb.2010.05.005
- Noor, J.; Ullah, A.; Saleem, M.H.; Tariq, A.; Ullah, S.; Waheed, A.; Okla, M.K.; Al-Hashimi, A.; Chen, Y.; Ahmed, Z.; et al. Effect of Jasmonic Acid Foliar Spray on the Morpho-Physiological Mechanism of Salt Stress Tolerance in Two Soybean Varieties (Glycine max). Plants 2022, 11, 651. https://doi.org/10.3390/plants11050651.
- Howe, G.A.; Schilmiller, A.L. Oxylipin metabolism in response to stress. Opin. Plant Biol. 2002, 5, 230–236. https://doi.org/10.1016/s1369-5266(02)00250-9.
- Heap, I.M. The International Survey of Herbicide Resistant Weeds. Available online: http://weedscience.org/ (accessed on 12 September 2022).
- Panozzo, S.; Collavo, A.; Sattin, M. Sensitivity Analysis of Italian Lolium spp. to Glyphosate in Agricultural Environments. Plants 2020, 9, 165. https://doi.org/10.3390/plants9020165.
- Göbel, C.; Feussner, I.; Hamberg, M.; Rosahl, S. Oxylipin profiling in pathogen-infected potato leaves. i Biochim Biophys Acta 2002, 1584, 55–64. https://doi.org/10.1016/s1388-1981(02)00268-8.
- Hossain, R.; Quispe, C.; Saikat, A.S.M.; Jain, D.; Habib, A.; Janmeda, D.; Islam, M.T.; Radha, J.; Daştan, S.D.; Kumar, M.; et al. Biosynthesis of secondary metabolites based on co-operation with microRNA. BioMed Res. Int. 2022, 2022, https://doi.org/10,1155/2022/9349897.
- Shearer, G.C.; Walker, R. An overview of the biologic effects of omega-6 oxylipins in humans. Prostaglandins Leukot. Essent. Fat. Acids 2018, 137, 26–38. https://doi.org/10.1016/j.plefa.2018.06.005.
- Liakh, I.; Pakiet, A.; Sledzinski, T.; Mika, A. Methods of the Analysis of Oxylipins in Biological Samples. Molecules 2020, 25, 349. https://doi.org/10.3390/molecules25020349.
- Sugimoto, K.; Allmann, S.; Kolomiets, M.V. Editorial: Oxylipins: The Front Line of Plant Interactions. Plant Sci. 2022, 13, 878765. https://doi.org/10.3389/fpls.2022.878765.
- Sawicka, B. Loss of Agricultural Products After Harvest. Zero Hunger; ilho, W.L., Azul, A.M., Brandli, L., Özuyar, P.G., Wall, T., Eds.; Springer: Cham, Switzerland, 2020. https://doi.org/10.1007/978-3-319-69626-3.
- FAO/WHO. World Health Statistics 2020: Monitoring Health for the SDGs, Sustainable Development Goals? WHO: Geneva, Switzerland, 2020; ISBN 9789240005112 ).
- Egbuna, C.; Sawicka, B.; Tijjani, H. iopesticides, Safety Issues and Market Trends. In: Natural Remedies for Pest, Disease and Weed Control; Egbuna, C., Sawicka, B, Eds.; Chapter:;, Academic Press: Cambridge, MA, USA, 2020; pp. 43–54, ISBN: 9780128193044, .
- Jang, S.; Marjanovic, J.; Gornicki, P. Resistance to herbicides caused by single amino acid mutations in acetyl-CoA carboxylase in resistant populations of grassy weeds. New Phytol. 2013, 197, 1110–1116.
- Sawicka, B.; Barbaś, P.; Pszczółkowski, P.; Skiba, D.; Yeganehpoor, F.; Krochmal-Marczak, B. Climate Changes in Southeastern Poland and Food Security. Climate 2022, 10, 57. https://doi.org/10.3390/cli10040057.
- Synowiec, A.; Jop, B.; Domaradzki, K.; Podsiadło, C.; Gawęda, D.; Wacławowicz, R.; Wenda-Piesik, A.; Nowakowski, M.; Bocianowski, J.; Marcinkowska, K.; et al. Environmental Factors Effects on Winter Wheat Competition with Herbicide-Resistant or Susceptible Silky Bentgrass (Apera spica-venti) in Poland. Agronomy 2021, 11, 871. https://doi.org/10.3390/agronomy11050871.
- Stankiewicz-Kosyl, M.; Haliniarz, M.; Wrochna, M.; Synowiec, A.; Wenda-Piesik, A.; Tendziagolska, E.; Sobolewska, M.; Domaradzki, K.; Skrzypczak, G.; et al. Herbicide Resistance of Centaurea cyanus in Poland in the Context of Its Management. Agronomy 2021, 11, 1954. https://doi.org/10.3390/agronomy11101954.
- Wrzesińska, B.; Praczyk, T. Genetic Variability of Acetolactate Synthase (ALS) Sequence in Centaurea cyanus Plants Resistant and Susceptible to Tribenuron-Methyl. Agronomy 2021, 11, 2311. https://doi.org/10.3390/agronomy11112311.
- Wrzesińska, B.; Kościelniak, K.; Frąckowiak, P.; Praczyk, T.; Obrępalska-Stęplowska, A. The analysis of reference genes expression stability in susceptible and resistant Apera spica-venti populations under herbicide treatment. Rep. 2021, 11, 22145. https://doi.org/10.1038/s41598-021-01615-6.
- Banasiak, J. System odpornościowy roślin—Model zygzakowy. Postępy Biochemii / Biochem. 2022, 68, 123–128. (In Polish). https://doi.org/10.18388/pb.2021_427.
- Sharma, A.; Kumar, V.; Kumar, R.; Shahzad, B.; Thukral, A.K.; Bhardwaj, R.; Tejada Moral, M. Brassinoster-oid-mediated pesticide detoxification in plants: A mini-review. Cogent Food Agric. 2018, 4, 1436212. https://doi.org/10.1080/23311932.2018.1436212.
- Bajguz, A.; Tretyn, A. The chemical characteristic and distribution of brassinosteroids in plants. Phytochemistry 2003, 62, 1027–1046.
- Bajguz, A.; Hayat, S. Effects of brassinosteroids on the plant responses to environmental stresses. Plant Physiol. Biochem. 2009, 47, 1–8. https://doi.org/10.1016/j.plaphy.2008.10.002.
- Bajguz, A. Brassinosteroids—Occurrence and Chemical Structures in Plants; Brassino-Steroids: A Class of Plant Hormone; Hayat, S., Ahmad, A., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 1–29.
- Bajguz, A.; Pietrasz, M.; Piotrowska-Niczyporuk, A. Rola Brassinosteroidów w Odpowiedzi Roślin na Niekorzystne Czynniki Środowiska. In Różnorodność biologiczna. Rośliny i grzyby w Zmieniających się Warunkach Środowiska; Ciereszko, I., Bajguz, A., Eds.; Polskie Towarzystwo Botaniczne: Białystok, Poland, 2013; pp. 53–67. ISBN: 9788362069378.
- Ali, S.; Baek, K.-H. Jasmonic Acid Signaling Pathway in Response to Abiotic Stresses in Plants. J. Mol. Sci. 2020, 21, 621. https://doi.org/10.3390/ijms21020621.
- Gudesblat, G.E.; Russinova, E. Plants grow on brassinosteroids. Opin. Plant Biol. 2011, 14, 530–537.
- Starck, Z. Plant physiology: Yesterday, today and what will bring tomorrow? Probl. Biol. Sci. 2014, 63, 569–589. (In Polish).
- A Professionally Selected Database of Abstracts and Citations. Available online: https://www.elsevier.com/pl-pl/solutions/scopus (accessed on 7 March 2022).
- VOS Viewer 2021. Available online: https://www.vosviewer.com/VOSviewer (accessed on 7 March 2022).
- Jones, J.D.G.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. https://doi.org/10.1038/nature05286.
- Owen, M.D.K.; Zelaya, I.A. Herbicide-resistant crops and weed resistance to herbicides. Pest Manag. Sci. 2005, 61, 301–311. https://doi.org/10.1002/ps.1015.
- Tharayil-Santhakumar, N. Mechanism of Herbicide Resistance in Weeds; Plant and Soil Science University of Massachusetts: Amherst, MA, USA, 2003; pp. 20. Available online: https://www.weedresearch.com/paper/Mechanism (ccessed on10.2022).
- Nandula, V. Herbicide resistance in weeds: Survey, characterization and mechanisms. Indian J. Weed Sci. 2016, 48, 128–131. https://doi.org/10.5958/0974-8164.2016.00033.2.
- Adamczewski, K.; Matysiak, K.; Kierzak, R.; Kaczmarek, S. Significant increase of weed resistance to herbicides in Poland. Plant Prot. Res. 2019, 59, 139–150. https://doi.org/10.24425/jppr.2019.129293.
- Adamczewski, K.; Kierzek, R.; Matysiak, K. Multiple resistance to acetolactate synthase (ALS)- and acetyl-coenzyme A carboxylase (ACCase)-inhibiting herbicides in black-grass (Alopecurus myosuroides) populations from Poland. J. Plant Prot. Res. 2016, 56, 402–410. https://doi.org/10.1515/jppr-2016-0059.
- Janjic, V.; Milosevic, D.; Djalovic, I.; Týr, S. Weed resistance to herbicides—Mechanisms and molecular basis. Acta Agric. Ser-Bica 2007, 12, 19–40.
- Adamczewski, K.; Kierzek, R. Problem odporności chwastów na herbicydy w Polsce. Plant Prot. 2011, 51, 1665–1674. (In Polish).
- Hull, R.; Moss, S. A rapid test for ALS herbicide resistance in black grass (Alopecurus myosuroides). In Proceedings of the 14th European Weed Research Society Symposium, Hamar, Norway, 17–21 June 2017; p. 151.
- Adamczewski, K.; Matysiak, K. The mechanism of resistance to ALS inhibiting herbicides in biotypes of wind bent grass (Apera spica-venti L.) with cross and multiple resistance. J. Agron. 2012, 10, 3–8.
- Adamczewski, K.; Matysiak, K.; Kierzek, R. 2017. Występowanie biotypów miotły zbożowej (Apera spica-venti) odpornej na isoproturon. Fragm. Agron. 2017, 34, 7–13.
- Yu, Q.; Han, H.; Vila-Aiub, M.M.; Powles, B. AHAS herbicide resistance endowing mutations: Effect on AHAS functionality and plant growth. Exp. Bot. 2010, 61, 3925–3934. https://doi.org/10.1093/jxb/erq205.
- Holt, J.S.; Powles, S.B.; Holtum, J.A.M. Mechanisms and agronomic aspects of herbicide resistance. Rev. Plant Physiol. Plant Mol. Biol. 1993, 44, 203–209.
- Burnet, M.W.M.; Hildebrand, O.B.; Holtum, J.A.M.; Powles, S.B. Amitrole, Triazine, Substituted Urea, and Metribuzin Resistance in a Biotype of Rigid Ryegrass (Lolium rigidum). Weed Sci. 1992, 39, 317–323. https://doi.org/10.1017/s0043174500072994.
- Hall, L.M.; Moss, S.R.; Powles, S.B. Mechanisms of resistance to Aryloxyphenoxypropionate herbicides in two resistant biotypes of Alopecurus myosuroides Huds. (blackgrass): Herbicide metabolism as a cross-resistance mechanism. Biochem. Physiol. 1997, 57, 87–98.
- Busi, R.; Goggin, D.E.; Heap, I.M.; Horak, M.J.; Jugulam, M.; Masters, R.A.; Napier, R.M.; Riar, D.S.; Satchivi, N.M.; Torra, J.; et al. Weed resistance to synthetic auxin herbicides. Pest Manag. Sci. 2017, 74, 2265–2276. https://doi.org/10.1002/ps.4823.
- Zheng, D.; Kruger, G.R.; Singh, S.; Davis, V.M.; Tranel, P.J.; Weller, S.C.; Johnson, W.G. Cross-resistance of horseweed (Conyza canadensis) populations with three different ALS mutations. Pest Manag. Sci. 2011, 67, 1486–1492. https://doi.org/10.1002/ps.2190.
- Nowak, D.; Zborowski, D. Resiherb-system doradczy w zakresie zarządzania odpornością chwastów na herbicydy. Zagadnienia Doradz. Rol. 2021, 2, 60–69.
- Wojtasik, W.; Kuma, A. Plant response to biotic stress factors. Postępy Biol. Komórki 2016, 43, 453–476. (In Polish).
- Avdiushko, S.; Croft, K.; Brown, G.C.; Jackson, D.M.; Hamilton-Kemp, T.R.; Hildebrand, D. Effect of Volatile Methyl Jasmonate on the Oxylipin Pathway in Tobacco, Cucumber, and Arabidopsis. Plant Physiol. 1995, 109, 1227–1230. https://doi.org/10.1104/pp.109.4.1227.
- Brodhun, F.; Feussner, I. Oxylipins in fungi. FEBS J. 2011, 278, 1047–1063.
- Lewak, S.; Kopcewicz, J.; Jaworski, K. Fizjologia Roślin; Wydawnictwo Naukowe PWN: Warsaw: Poland, 2019; pp. 220. ISBN: 9788301207199. (In Polish).
References
- Banasiak, J. System odpornościowy roślin—Model zygzakowy. Postępy Biochemii / Adv. Biochem. 2022, 68, 123–128. (In Polish)
- Jones, J.D.G.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329.
- Beckie, H.J. Herbicide Resistance in Plants. Plants 2020, 9, 435.
- Synowiec, A.; Jop, B.; Domaradzki, K.; Podsiadło, C.; Gawęda, D.; Wacławowicz, R.; Wenda-Piesik, A.; Nowakowski, M.; Bocianowski, J.; Marcinkowska, K.; et al. Environmental Factors Effects on Winter Wheat Competition with Herbicide-Resistant or Susceptible Silky Bentgrass (Apera spica-venti L.) in Poland. Agronomy 2021, 11, 871.
- Bao, A.B.; Won, O.J.; Sin, H.T.; Lee, J.J.; Park, K.W. Mechanisms of herbicide resistance in weeds. Korean J. Agric. Sci. 2017, 44, 1–15.
- Dekker, J.; Duke, S.O. Herbicide-resistant field crops. Adv. Agron. 1995, 54, 69–116. Available online: https://core.ac.uk/download/pdf/38933693.pdf (accessed on 20 October 2022).
- Liakh, I.; Pakiet, A.; Sledzinski, T.; Mika, A. Methods of the Analysis of Oxylipins in Biological Samples. Molecules 2020, 25, 349.
- Owen, M.D.K.; Zelaya, I.A. Herbicide-resistant crops and weed resistance to herbicides. Pest Manag. Sci. 2005, 61, 301–311.
- Jang, S.; Marjanovic, J.; Gornicki, P. Resistance to herbicides caused by single amino acid mutations in acetyl-CoA carboxylase in resistant populations of grassy weeds. New Phytol. 2013, 197, 1110–1116.
- Noor, J.; Ullah, A.; Saleem, M.H.; Tariq, A.; Ullah, S.; Waheed, A.; Okla, M.K.; Al-Hashimi, A.; Chen, Y.; Ahmed, Z.; et al. Effect of Jasmonic Acid Foliar Spray on the Morpho-Physiological Mechanism of Salt Stress Tolerance in Two Soybean Varieties (Glycine max L.). Plants 2022, 11, 651.
- Egbuna, C.; Sawicka, B.; Tijjani, H. Biopesticides, Safety Issues and Market Trends. In Natural Remedies for Pest, Disease and Weed Control; Egbuna, C., Sawicka, B., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 43–54. ISBN 9780128193044.
- Heap, I.M. The International Survey of Herbicide Resistant Weeds. Available online: http://weedscience.org/ (accessed on 12 September 2022).
- Tharayil-Santhakumar, N. Mechanism of Herbicide Resistance in Weeds; Plant and Soil Science University of Massachusetts: Amherst, MA, USA, 2003; p. 20. Available online: https://www.weedresearch.com/paper/Mechanism (accessed on 20 October 2022).
- Nandula, V. Herbicide resistance in weeds: Survey, characterization and mechanisms. Indian J. Weed Sci. 2016, 48, 128–131.
- Adamczewski, K.; Matysiak, K.; Kierzak, R.; Kaczmarek, S. Significant increase of weed resistance to herbicides in Poland. J. Plant Prot. Res. 2019, 59, 139–150.
- Adamczewski, K.; Kierzek, R.; Matysiak, K. Multiple resistance to acetolactate synthase (ALS)- and acetyl-coenzyme A carboxylase (ACCase)-inhibiting herbicides in black-grass (Alopecurus myosuroides Huds.) populations from Poland. J. Plant Prot. Res. 2016, 56, 402–410.
- Janjic, V.; Milosevic, D.; Djalovic, I.; Týr, S. Weed resistance to herbicides—Mechanisms and molecular basis. Acta Agric. Ser-Bica 2007, 12, 19–40.
- Adamczewski, K.; Kierzek, R. Problem odporności chwastów na herbicydy w Polsce. Prog. Plant Prot. 2011, 51, 1665–1674. (In Polish)
- Hull, R.; Moss, S. A rapid test for ALS herbicide resistance in black grass (Alopecurus myosuroides). In Proceedings of the 14th European Weed Research Society Symposium, Hamar, Norway, 17–21 June 2017; p. 151.
- Adamczewski, K.; Matysiak, K. The mechanism of resistance to ALS inhibiting herbicides in biotypes of wind bent grass (Apera spica-venti L.) with cross and multiple resistance. Pol. J. Agron. 2012, 10, 3–8.
- Adamczewski, K.; Matysiak, K.; Kierzek, R. 2017. Występowanie biotypów miotły zbożowej (Apera spica-venti L.) odpornej na isoproturon. Fragm. Agron. 2017, 34, 7–13.
- Yu, Q.; Han, H.; Vila-Aiub, M.M.; Powles, B. AHAS herbicide resistance endowing mutations: Effect on AHAS functionality and plant growth. J. Exp. Bot. 2010, 61, 3925–3934.
- Holt, J.S.; Powles, S.B.; Holtum, J.A.M. Mechanisms and agronomic aspects of herbicide resistance. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1993, 44, 203–209.
- Burnet, M.W.M.; Hildebrand, O.B.; Holtum, J.A.M.; Powles, S.B. Amitrole, Triazine, Substituted Urea, and Metribuzin Resistance in a Biotype of Rigid Ryegrass (Lolium rigidum). Weed Sci. 1992, 39, 317–323.
- Hall, L.M.; Moss, S.R.; Powles, S.B. Mechanisms of resistance to Aryloxyphenoxypropionate herbicides in two resistant biotypes of Alopecurus myosuroides Huds. (blackgrass): Herbicide metabolism as a cross-resistance mechanism. Pestic. Biochem. Physiol. 1997, 57, 87–98.
- Busi, R.; Goggin, D.E.; Heap, I.M.; Horak, M.J.; Jugulam, M.; Masters, R.A.; Napier, R.M.; Riar, D.S.; Satchivi, N.M.; Torra, J.; et al. Weed resistance to synthetic auxin herbicides. Pest Manag. Sci. 2017, 74, 2265–2276.
- Zheng, D.; Kruger, G.R.; Singh, S.; Davis, V.M.; Tranel, P.J.; Weller, S.C.; Johnson, W.G. Cross-resistance of horseweed (Conyza canadensis) populations with three different ALS mutations. Pest Manag. Sci. 2011, 67, 1486–1492.
- Nowak, D.; Zborowski, D. Resiherb-system doradczy w zakresie zarządzania odpornością chwastów na herbicydy. Zagadnienia Doradz. Rol. 2021, 2, 60–69.
- Wrzesińska, B.; Praczyk, T. Genetic Variability of Acetolactate Synthase (ALS) Sequence in Centaurea cyanus Plants Resistant and Susceptible to Tribenuron-Methyl. Agronomy 2021, 11, 2311.
- Sawicka, B.; Barbaś, P.; Pszczółkowski, P.; Skiba, D.; Yeganehpoor, F.; Krochmal-Marczak, B. Climate Changes in Southeastern Poland and Food Security. Climate 2022, 10, 57.
- Wrzesińska, B.; Kościelniak, K.; Frąckowiak, P.; Praczyk, T.; Obrępalska-Stęplowska, A. The analysis of reference genes expression stability in susceptible and resistant Apera spica-venti populations under herbicide treatment. Sci. Rep. 2021, 11, 22145.
- Gabbs, M.; Leng, S.; Devassy, J.G.; Monirujjaman, M.; Aukema, H.M. Advances in Our Understanding of Oxylipins Derived from Dietary PUFAs. Adv. Nutr. 2015, 6, 513–540.
- Panozzo, S.; Collavo, A.; Sattin, M. Sensitivity Analysis of Italian Lolium spp. to Glyphosate in Agricultural Environments. Plants 2020, 9, 165.
- Stankiewicz-Kosyl, M.; Haliniarz, M.; Wrochna, M.; Synowiec, A.; Wenda-Piesik, A.; Tendziagolska, E.; Sobolewska, M.; Domaradzki, K.; Skrzypczak, G.; Łykowski, W.; et al. Herbicide Resistance of Centaurea cyanus L. in Poland in the Context of Its Management. Agronomy 2021, 11, 1954.
- Sharma, A.; Kumar, V.; Kumar, R.; Shahzad, B.; Thukral, A.K.; Bhardwaj, R.; Tejada Moral, M. Brassinoster-oid-mediated pesticide detoxification in plants: A mini-review. Cogent Food Agric. 2018, 4, 1436212.
