Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Conner Chen and Version 1 by Ángel R. De Lera.
Within the 2,5-dioxopiperazine-containing natural products generated by “head-to-tail” cyclization of peptides, those derived from tryptophan allow further structural diversification due to the rich chemical reactivity of the indole heterocycle, which can generate tetracyclic fragments of hexahydropyrrolo[2,3-b]indole or pyrrolidinoindoline skeleton fused to the 2,5-dioxopiperazine. Even more complex are the dimeric bispyrrolidinoindoline epi(poly)thiodioxopiperazines (BPI-ETPs), since they feature transannular (poly)sulfide bridges connecting C3 and C6 of their 2,5-dioxopiperazine rings. Homo- and heterodimers composed of diastereomeric epi(poly)thiodioxopiperazines increase the complexity of the family.
- bispyrrolidinoindoline epi(poly)thiodioxopiperazine alkaloids
- isolation
- structural elucidation
1. Introduction
Dimeric bispyrrolidinoindoline epi(poly)thiodioxopiperazines (BPI-ETPs) are a family of highly complex natural products that biogenetically derive from dioxopiperazines formed by the double intramolecular condensation of dipeptides containing tryptophan and an additional amino acid, followed by a variety of structural modifications [1,2,3][1][2][3]. The monomeric units contain cyclic dipeptide (CDP) substructures fused to pyrrolidinoindoline cores and feature transannular (poly)sulfide connections between C3 and C6 (for the numbering indicated in Scheme 1, see [4]) of their 2,5-dioxopiperazine rings [1]. Relevant features of these privileged structures include the conformational constraint, which has been linked to their greater stability and conformational rigidity and, therefore, higher resistance to protease degradation than acyclic counterparts, as well as their ability to cross the intestinal barrier and the blood–brain barrier [5]. The ability to mimic preferential peptide conformations, with two hydrogen bond donor and acceptor sites, favors interactions with putative biological targets. Thus, their pharmacological potency is boosted when compared with the monomeric unit through multipoint interactions on chemical space with their biological targets [6,7][6][7].
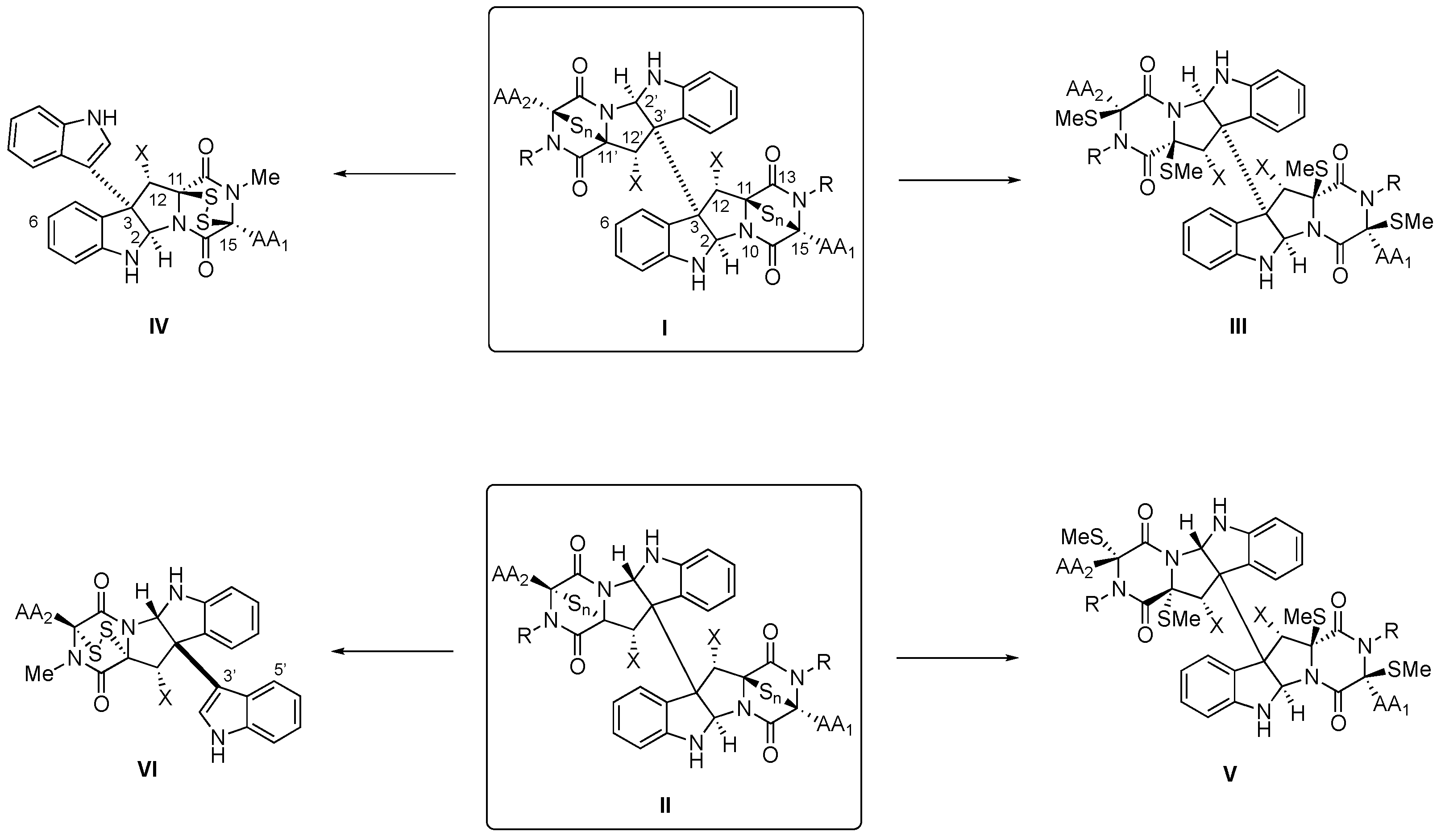
Scheme 1. General structures (and atom numbers) of tryptophan-derived BPI-ETP alkaloids (I,II), their transformation into the methylsulfanyl derivatives (III,V), and partial degradation products (IV,VI).
More than 100 alkaloids containing at least a unit of indole-derived [8] pyrrolidinoindoline epi(poly)thiodioxopiperazine (PI-ETP) [9] have already been isolated from terrestrial and marine fungi (about a third, from marine sources) [10[10][11][12][13],11,12,13], including species of the Leptosphaeria, Chaetomium, [14] Tilachlidium, Verticillium, Gliocladium, Aspergillus sp. and Penicillium genera [1[1][15][16],15,16], but they have also been obtained from bacteria (Streptomyces species) [17], plants, and even animals [5,13,18][5][13][18]. Homo- and heterodimeric BPI ETPs compose about half of the family.
(+)-Chaetocin A (1, Figure 1A) [19] [19] and (+)-verticillin A (8, Figure 2) [20][20] were first discovered in 1970, although antibacterial monomeric (−)-gliotoxin (7, see Figure 1B) had been isolated more than 30 years before [21,22][21][22]. Relative to the monomers, the homo- and heterodimeric BPI ETP family members feature a greater level of structural diversity and complexity since the characteristic dioxopiperazine scaffold is bridged by a variable number (1 to 4) of sulfur atoms. Nondimeric family members connected at C3 through the indole nitrogen of either simple indole units or epi(poly)thiodioxopiperazines will not be covered.
Biogenetic generation of CDPs in nature usually involves either nonribosomal peptide synthetases (NRPSs) in fungi [23,24][23][24] or cyclodipeptide synthases (CDPSs) in bacteria [25,26,27][25][26][27]. Whereas fungi generate DKP monomers by the activation of the free amino acids through adenylation by the action of NRPSs, bacteria use cyclodipeptide synthetases (CDPSs) and employ aminoacyl-tRNAs (aa-TRNAs) as substrates [26,27,28,29,30][26][27][28][29][30].
The diversification of the skeleton is further achieved through the biogenetically controlled action of their tailoring enzymes, which are usually found in dedicated biosynthetic gene clusters [31]. Whereas, for the bispyrrolidinoindoline dioxopiperazine, family various types of oxidoreductases, hydrolases, transferases, and ligases have been isolated and structurally characterized [32], for the BPI-ETP family, these chemical modifications are rather limited. In addition to the role of Cyt P450 oxidation enzymes [33], as well as enzymatic dimerization [34[34][35],35], only hydroxylation/dehydroxylation processes leading ultimately to the modulation of the oxidation level of the pyrrolidinoindoline and DKP scaffolds and the amino acid side chains have been noted in some members of the family of dimeric alkaloids. In contrast, enzymes responsible for structural modifications of the DKP scaffold in simpler bispyrrolidinoindoline dioxopiperazine alkaloids have been characterized in several biogenetic gene clusters of fungal and microbial secondary metabolites [1,5,29,36,37,38,39,40,41,42][1][5][29][36][37][38][39][40][41][42].
The conformationally constrained DKP scaffold of dimeric dioxopiperazines is currently considered as privileged structures [43], given their ability to interact with several receptors, which may account for the diverse biological activities reported for this family of natural products. In addition, the reactivity of compounds with di(poli)sulfide bridges has been associated with a variety of biological activities, including protein cross-linking through the reaction of the disulfide bond with cysteine residues and the inactivation of thiol-containing proteins, generation of reactive oxygen species (ROS) via redox cycling, or ejection of zinc ions from some proteins [10,11,12,44,45,46][10][11][12][44][45][46].
2. Biogenesis of BPI-ETPs
Biogenetic studies of sulfur containing moieties in natural products [105][47], in particular those from marine organisms [106][48], as well as comprehensive report on the biosynthesis of pyrrolidinoindoline containing NPs, including BPI-ETPs [107][49], together with the flavoenzyme-catalyzed formation of disulfide bonds in natural products biosynthesis [108[50][51],109], have been previously covered. The biogenetic routes to these natural products are usually found in dedicated biosynthetic gene clusters [31,110,111][31][52][53]. Whereas the incorporation of sulfur to a putative dehydrodioxopiperazine was first proposed to occur immediately following the cyclodipeptide formation, N-methylation was proposed to occur after the oxidative cyclization [112][54]. Methionine, cysteine, and sodium sulfate jointly provide the sulfur connections of thiodioxopiperazines [10]. Most of the biosynthetic studies have been carried out on monomeric gliotoxin (7, Figure 1B) from Aspergillus fumigatus, in which the dioxopiperazine core is assembled by a nonribosomal peptide synthetase [113][55]. The presence in a fermentation broth of a cyclic dipeptide intermediate bound to glutathione suggested the latter to be the donor of sulfur atoms [114][56]. Gene knockout experiments revealed that gliG was responsible for encoding a glutathione sulfur transferase, namely, GliG, which incorporated the sulfur atom into the DKP framework. Prior to sulfur incorporation, the DKP should undergo an oxidation with the attachment of a hydroxyl group at the Cα-position, for which gliC was identified as the responsible P450 monooxygenase [46,113,114][46][55][56]. The biosynthesis of (−)-gliotoxin (7, Scheme 2A) in Aspergillus fumigatus was elucidated by Hertweck et al., after their discovery of the activation of DKPs by oxygenase GliC and the transfer of glutathione by a dedicated glutathione S-transferase, GliC [114[56][57],115], which led to the formation of bis(glutathione) adducts 58 from cFL-SL (57) and lately to the natural product from the dithiol intermediate 59 (Scheme 2) [114][56]. Additional insights were obtained from the large-scale fermentation of an engineered ΔgliI mutant, from which bis(cysteine) conjugates (60) were indeed isolated, en route to the dithiol intermediate 61. The step involving the dual cleavage of the C–S bonds to afford the dithiol prior to disulfide bond formation was also confirmed. The GliI C–S lyase-catalyzed reaction concomitantly forms two thiol groups, in what might reflect the dual action of the GliI homodimer [116][58].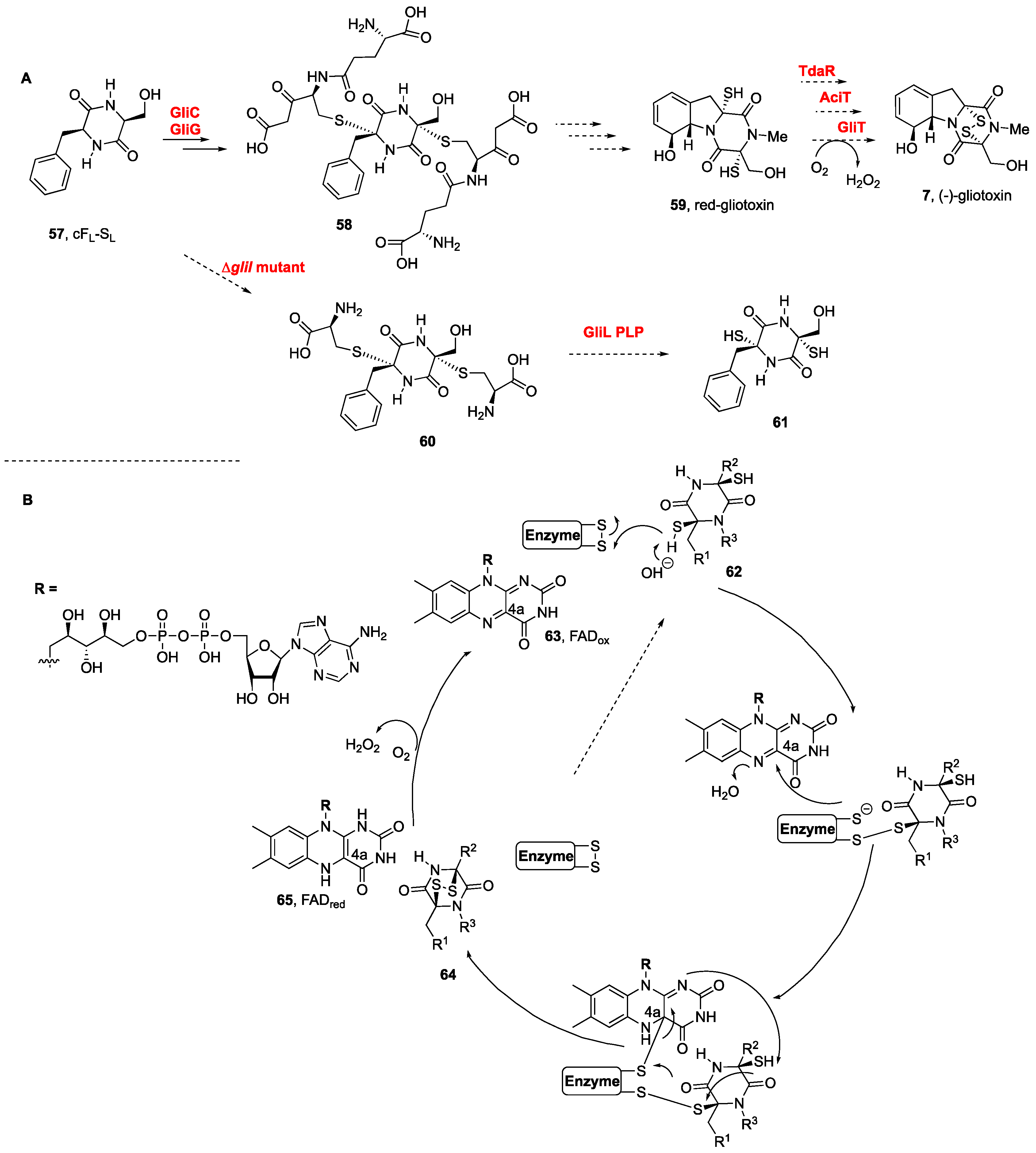
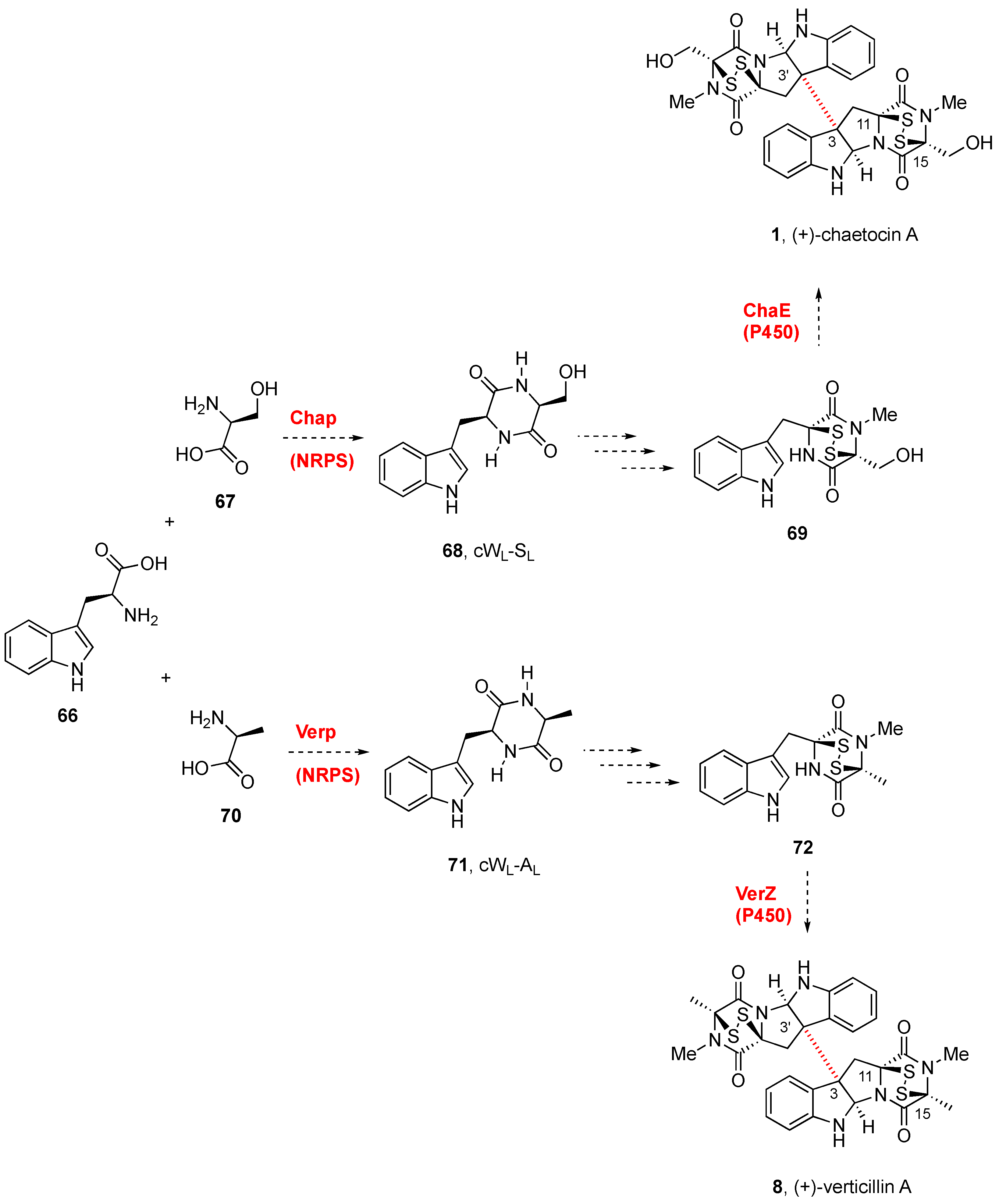
Scheme 3.
Biogenetic routes to BPI-ETP alkaloids.
References
- Wang, X.; Li, Y.; Zhang, X.; Lai, D.; Zhou, L. Structural Diversity and Biological Activities of the Cyclodipeptides from Fungi. Molecules 2017, 22, 2026.
- Bojarska, J.; Mieczkowski, A.; Ziora, Z.M.; Skwarczynski, M.; Toth, I.; Shalash, A.O.; Parang, K.; El-Mowafi, S.A.; Mohammed, E.H.M.; Elnagdy, S.; et al. Cyclic Dipeptides: The Biological and Structural Landscape with Special Focus on the Anti-Cancer Proline-Based Scaffold. Biomolecules 2021, 11, 1515.
- Ma, Z.; Zhou, A.; Xia, C. Strategies for total synthesis of bispyrrolidinoindoline alkaloids. Nat. Prod. Rep. 2022, 39, 1015–1044.
- Barrow, C.J.; Cai, P.; Snyder, J.K.; Sedlock, D.M.; Sun, H.H.; Cooper, R. WIN 64821, a new competitive antagonist to substance P, isolated from an Aspergillus species: Structure determination and solution conformation. J. Org. Chem. 1993, 58, 6016–6021.
- Borthwick, A.D. 2,5-Diketopiperazines: Synthesis, Reactions, Medicinal Chemistry, and Bioactive Natural Products. Chem. Rev. 2012, 112, 3641–3716.
- Ciarkowski, J. CNDO/2 quantum-mechanical calculations of the conformational flexibility of the diketopiperazine skeleton. Biopolymers 1984, 23, 397–407.
- Liskamp, R.M.J.; Rijkers, D.T.S.; Kruijtzer, J.A.W.; Kemmink, J. Peptides and Proteins as a Continuing Exciting Source of Inspiration for Peptidomimetics. ChemBioChem 2011, 12, 1626–1653.
- Bai, W.-J.; Wang, X. Appreciation of symmetry in natural product synthesis. Nat. Prod. Rep. 2017, 34, 1345–1358.
- Ma, Y.-M.; Liang, X.-A.; Kong, Y.; Jia, B. Structural Diversity and Biological Activities of Indole Diketopiperazine Alkaloids from Fungi. J. Agric. Food Chem. 2016, 64, 6659–6671.
- Gardiner, D.M.; Waring, P.; Howlett, B.J. The epipolythiodioxopiperazine (ETP) class of fungal toxins: Distribution, mode of action, functions and biosynthesis. Microbiology 2005, 151, 1021–1032.
- Jiang, C.-S.; Müller, W.E.G.; Schröder, H.C.; Guo, Y.-W. Disulfide- and Multisulfide-Containing Metabolites from Marine Organisms. Chem. Rev. 2011, 112, 2179–2207.
- Welch, T.R.; Williams, R.M. Epidithiodioxopiperazines. Occurrence, synthesis and biogenesis. Nat. Prod. Rep. 2014, 31, 1376–1404.
- Gomes, N.G.M.; Pereira, R.B.; Andrade, P.B.; Valentão, P. Double the Chemistry, Double the Fun: Structural Diversity and Biological Activity of Marine-Derived Diketopiperazine Dimers. Mar. Drugs 2019, 17, 551.
- Zhang, Q.; Li, H.Q.; Zong, S.C.; Gao, J.M.; Zhang, A.L. Chemical and Bioactive Diversities of the Genus Chaetomium Secondary Metabolites. Mini-Rev. Med. Chem. 2012, 12, 127–148.
- Ishikawa, Y.; Morimoto, K.; Hamasaki, T. Flavoglaucin, a metabolite of Eurotium chevalieri, its antioxidation and synergism with tocopherol. J. Am. Oil Chem. Soc. 1984, 61, 1864–1868.
- Slack, G.J.; Puniani, E.; Frisvad, J.C.; Samson, R.A.; Miller, J.D. Secondary metabolites from Eurotium species, Aspergillus calidoustus and A. insuetus common in Canadian homes with a review of their chemistry and biological activities. Mycol. Res. 2009, 113, 480–490.
- Scott, T.A.; Piel, J. The hidden enzymology of bacterial natural product biosynthesis. Nat. Rev. Chem. 2019, 3, 404–425.
- Giessen, T.W.; Marahiel, M.A. Rational and combinatorial tailoring of bioactive cyclic dipeptides. Front. Microbiol. 2015, 6, 785.
- Hauser, D.; Weber, H.P.; Sigg, H.P. Isolierung und Strukturaufklärung von Chaetocin. Helv. Chim. Acta 1970, 53, 1061–1073.
- Katagiri, K.; Sato, K.; Hakayama, S.; Matsushima, T.; Minato, H. Verticillin A, a new antibiotic from Verticillium sp. J. Antibiot. 1970, 23, 420–422.
- Weindling, R.; Emerson, O.H. The isolation of a toxic substance from the culture filtrate of Trichoderma. Phytopathology 1936, 26, 1068–1070.
- Bell, M.R.; Johnson, J.R.; Wildi, B.S.; Woodward, R.B. THE STRUCTURE OF GLIOTOXIN. J. Am. Chem. Soc. 1958, 80, 1001.
- Walsh, C.T. Insights into the chemical logic and enzymatic machinery of NRPS assembly lines. Nat. Prod. Rep. 2015, 33, 127–135.
- Walsh, C.T.; Moore, B.S. Enzymatic Cascade Reactions in Biosynthesis. Angew. Chem. Int. Ed. 2018, 58, 6846–6879.
- Gondry, M.; Sauguet, L.; Belin, P.; Thai, R.; Amouroux, R.; Tellier, C.; Tuphile, K.; Jacquet, M.; Braud, S.; Courçon, M.; et al. Cyclodipeptide synthases are a family of tRNA-dependent peptide bond–forming enzymes. Nat. Chem. Biol. 2009, 5, 414–420.
- Gondry, M.; Jacques, I.B.; Thai, R.; Babin, M.; Canu, N.; Seguin, J.; Belin, P.; Pernodet, J.-L.; Moutiez, M. A Comprehensive Overview of the Cyclodipeptide Synthase Family Enriched with the Characterization of 32 New Enzymes. Front. Microbiol. 2018, 9, 46.
- Canu, N.; Moutiez, M.; Belin, P.; Gondry, M. Cyclodipeptide synthases: A promising biotechnological tool for the synthesis of diverse 2,5-diketopiperazines. Nat. Prod. Rep. 2019, 37, 312–321.
- Shende, V.V.; Khatri, Y.; Newmister, S.A.; Sanders, J.N.; Lindovska, P.; Yu, F.; Doyon, T.J.; Kim, J.; Houk, K.N.; Movassaghi, M.; et al. Structure and Function of NzeB, a Versatile C–C and C–N Bond-Forming Diketopiperazine Dimerase. J. Am. Chem. Soc. 2020, 142, 17413–17424.
- Yao, T.; Liu, J.; Liu, Z.; Li, T.; Li, H.; Che, Q.; Zhu, T.; Li, D.; Gu, Q.; Li, W. Genome mining of cyclodipeptide synthases unravels unusual tRNA-dependent diketopiperazine-terpene biosynthetic machinery. Nature Commun. 2018, 9, 4091.
- Canu, N.; Belin, P.; Thai, R.; Correia, I.; Lequin, O.; Seguin, J.; Moutiez, M.; Gondry, M. Incorporation of Non-canonical Amino Acids into 2,5-Diketopiperazines by Cyclodipeptide Synthases. Angew. Chem. Int. Ed. 2018, 57, 3118–3122.
- Borgman, P.; Lopez, R.D.; Lane, A.L. The expanding spectrum of diketopiperazine natural product biosynthetic pathways containing cyclodipeptide synthases. Org. Biomol. Chem. 2019, 17, 2305–2314.
- García-Domínguez, P.; Areal, A.; Alvarez, R.; de Lera, A.R. Chemical synthesis in competition with global genome mining and heterologous expression for the preparation of dimeric tryptophan-derived 2,5-dioxopiperazines. Nat. Prod. Rep. 2022, 39, 1172–1225.
- Zhang, X.; Guo, J.; Cheng, F.; Li, S. Cytochrome P450 enzymes in fungal natural product biosynthesis. Nat. Prod. Rep. 2021, 38, 1072–1099.
- Liu, J.; Liu, A.; Hu, Y. Enzymatic dimerization in the biosynthetic pathway of microbial natural products. Nat. Prod. Rep. 2021, 38, 1469–1505.
- Liu, H.; Fan, J.; Zhang, P.; Hu, Y.; Liu, X.; Li, S.-M.; Yin, W.-B. New insights into the disulfide bond formation enzymes in epidithiodiketopiperazine alkaloids. Chem. Sci. 2021, 12, 4132–4138.
- Patteson, J.B.; Cai, W.; Johnson, R.A.; Maria, K.C.S.; Li, B. Identification of the Biosynthetic Pathway for the Antibiotic Bicyclomycin. Biochemistry 2017, 57, 61–65.
- Meng, S.; Han, W.; Zhao, J.; Jian, X.; Pan, H.; Tang, G. A Six-Oxidase Cascade for Tandem C−H Bond Activation Revealed by Reconstitution of Bicyclomycin Biosynthesis. Angew. Chem. Int. Ed. 2017, 57, 719–723.
- Giessen, T.W.; von Tesmar, A.M.; Marahiel, M.A. A tRNA-Dependent Two-Enzyme Pathway for the Generation of Singly and Doubly Methylated Ditryptophan 2,5-Diketopiperazines. Biochemistry 2013, 52, 4274–4283.
- Yu, H.; Xie, X.; Li, S.-M. Coupling of Guanine with cyclo-l-Trp-l-Trp Mediated by a Cytochrome P450 Homologue from Streptomyces purpureus. Org. Lett. 2018, 20, 4921–4925.
- Shi, J.; Xu, X.; Zhao, E.J.; Zhang, B.; Li, W.; Zhao, Y.; Jiao, R.H.; Tan, R.X.; Ge, H.M. Genome Mining and Enzymatic Total Biosynthesis of Purincyclamide. Org. Lett. 2019, 21, 6825–6829.
- Yu, H.; Xie, X.; Li, S.-M. Coupling of cyclo-L-Trp-L-Trp with Hypoxanthine Increases the Structure Diversity of Guanitrypmycins. Org. Lett. 2019, 21, 9104–9108.
- Liu, J.; Xie, X.; Li, S.-M. Guanitrypmycin Biosynthetic Pathways Imply Cytochrome P450 Mediated Regio- and Stereospecific Guaninyl-Transfer Reactions. Angew. Chem. Int. Ed. 2019, 58, 11534–11540.
- Yet, L. Priviledged Structures in Drug Discovery: Medicinal Chemistry and Synthesis; John Wiley & Sons, Inc.: Hoboken, NY, USA, 2018.
- Cook, K.M.; Hilton, S.T.; Mecinović, J.; Motherwell, W.B.; Figg, W.D.; Schofield, C.J. Epidithiodiketopiperazines Block the Interaction between Hypoxia-inducible Factor-1α (HIF-1α) and p300 by a Zinc Ejection Mechanism. J. Biol. Chem. 2009, 284, 26831–26838.
- Iwasa, E.; Hamashima, Y.; Sodeoka, M. Epipolithiodiketopiperazines Alkaloids: Total Synthesis and Biological Activities. Isr. J. Chem. 2011, 51, 420–433.
- Jiang, C.-S.; Wang, L.; Guo, Y.-W. Epipolythiodioxopiperazines from Fungi: Chemistry and Bioactivities. Recent Advances in Medicinal Chemistry; Atta-ur-Rahman, Choudhary, M.I., Perry, G., Eds.; Bentham eBooks: Soest, The Netherlands, 2015; Volume 2, pp. 76–106.
- Wang, N.; Saidhareddy, P.; Jiang, X. Construction of sulfur-containing moieties in the total synthesis of natural products. Nat. Prod. Rep. 2019, 37, 246–275.
- Hai, Y.; Wei, M.-Y.; Wang, C.-Y.; Gu, Y.-C.; Shao, C.-L. The intriguing chemistry and biology of sulfur-containing natural products from marine microorganisms (1987–2020). Mar. Life Sci. Technol. 2021, 3, 488–518.
- Sun, C.; Tian, W.; Lin, Z.; Qu, X. Biosynthesis of pyrroloindoline-containing natural products. Nat. Prod. Rep. 2022, 39, 1721–1765.
- Dunbar, K.L.; Scharf, D.H.; Litomska, A.; Hertweck, C. Enzymatic Carbon–Sulfur Bond Formation in Natural Product Biosynthesis. Chem. Rev. 2017, 117, 5521–5577.
- Scharf, D.H.; Groll, M.; Habel, A.; Heinekamp, T.; Hertweck, C.; Brakhage, A.A.; Huber, E.M. Flavoenzyme-Catalyzed Formation of Disulfide Bonds in Natural Products. Angew. Chem. Int. Ed. 2014, 53, 2221–2224.
- Harken, L.; Li, S.-M. Modifications of diketopiperazines assembled by cyclodipeptide synthases with cytochrome P450 enzymes. App. Microbiol. Biotechnol. 2021, 105, 2277–2285.
- Scherlach, K.; Hertweck, C. Mining and unearthing hidden biosynthetic potential. Nat. Commun. 2021, 12, 1–12.
- Pedras, M.S.C.; Séguin-Swartz, G.; Abrams, S.R. Minor phytotoxins from the blackleg fungus Phoma lingam. Phytochemistry 1990, 29, 777–782.
- Cramer, R.A.; Gamcsik, M.P.; Brooking, R.M.; Najvar, L.K.; Kirkpatrick, W.R.; Patterson, T.F.; Balibar, C.J.; Graybill, J.R.; Perfect, J.R.; Abraham, S.N.; et al. Disruption of a Nonribosomal Peptide Synthetase in Aspergillus fumigatus Eliminates Gliotoxin Production. Eukaryotic Cell 2006, 5, 972–980.
- Scharf, D.H.; Remme, N.; Habel, A.; Chankhamjon, P.; Scherlach, K.; Heinekamp, T.; Hortschansky, P.; Brakhage, A.A.; Hertweck, C. A Dedicated Glutathione S-Transferase Mediates Carbon–Sulfur Bond Formation in Gliotoxin Biosynthesis. J. Am. Chem. Soc. 2011, 133, 12322–12325.
- Davis, C.; Carberry, S.; Schrettl, M.; Singh, I.; Stephens, J.C.; Barry, S.M.; Kavanagh, K.; Challis, G.L.; Brougham, D.; Doyle, S. The Role of Glutathione S-Transferase GliG in Gliotoxin Biosynthesis in Aspergillus fumigatus. Chem. Biol. 2011, 18, 542–552.
- Scharf, D.H.; Chankhamjon, P.; Scherlach, K.; Heinekamp, T.; Willing, K.; Brakhage, A.A.; Hertweck, C. Epidithiodiketopiperazine Biosynthesis: A Four-Enzyme Cascade Converts Glutathione Conjugates into Transannular Disulfide Bridges. Angew. Chem. Int. Ed. 2013, 52, 11092–11095.
- Gerken, T.; Walsh, C.T. Cloning and Sequencing of the Chaetocin Biosynthetic Gene Cluster. ChemBioChem 2013, 14, 2256–2258.
- Wang, Y.; Hu, P.; Pan, Y.; Zhu, Y.; Liu, X.; Che, Y.; Liu, G. Identification and characterization of the verticillin biosynthetic gene cluster in Clonostachys rogersoniana. Fungal Genet. Biol. 2017, 103, 25–33.
- Guo, Z.; Hao, T.; Wang, Y.; Pan, Y.; Ren, F.; Liu, X.; Che, Y.; Liu, G. VerZ, a Zn(II)2Cys6 DNA-binding protein, regulates the biosynthesis of verticillin in Clonostachys rogersoniana. Microbiology 2017, 163, 1654–1663.
- Zhu, S.; Ren, F.; Guo, Z.; Liu, J.; Liu, X.; Liu, G.; Che, Y. Rogersonins A and B, Imidazolone N-Oxide-Incorporating Indole Alkaloids from a verG Disruption Mutant of Clonostachys rogersoniana. J. Nat. Prod. 2019, 82, 462–468.
- Wang, Y.; Ren, J.; Li, H.; Pan, Y.; Liu, X.; Che, Y.; Liu, G. The disruption of verM activates the production of gliocladiosin A and B in Clonostachys rogersoniana. Org. Biomol. Chem. 2019, 17, 6782–6785.
More
